Abstract
Based on growth or nitrogen balance, amino acids (AA) had traditionally been classified as nutritionally essential (indispensable) or non-essential (dispensable) for animals and humans. Nutritionally essential AA (EAA) are defined as either those AA whose carbon skeletons cannot be synthesized de novo in animal cells or those that normally are insufficiently synthesized de novo by the animal organism relative to its needs for maintenance, growth, development, and health and which must be provided in the diet to meet requirements. In contrast, nutritionally non-essential AA (NEAA) are those AA which can be synthesized de novo in adequate amounts by the animal organism to meet requirements for maintenance, growth, development, and health and, therefore, need not be provided in the diet. Although EAA and NEAA had been described for over a century, there are no compelling data to substantiate the assumption that NEAA are synthesized sufficiently in animals and humans to meet the needs for maximal growth and optimal health. NEAA play important roles in regulating gene expression, cell signaling pathways, digestion and absorption of dietary nutrients, DNA and protein synthesis, proteolysis, metabolism of glucose and lipids, endocrine status, men and women fertility, acid–base balance, antioxidative responses, detoxification of xenobiotics and endogenous metabolites, neurotransmission, and immunity. Emerging evidence indicates dietary essentiality of “nutritionally non-essential amino acids” for animals and humans to achieve their full genetic potential for growth, development, reproduction, lactation, and resistance to metabolic and infectious diseases. This concept represents a new paradigm shift in protein nutrition to guide the feeding of mammals (including livestock), poultry, and fish.
Keywords: Amino acids, animals, humans, nutrition, requirement
Introduction
Amino acids (AA) are utilized for the synthesis of protein and many low-molecular-weight compounds with enormous physiological importance (Figure 1).1,2 Research on AA requirements of animals and humans has spanned over a century.3–6 In 1912, Abderhalden7 reported that adult dogs could maintain a positive nitrogen balance when fed a proline-free diet but exhibited a negative nitrogen balance when fed a tryptophan-free diet. Based on this finding, he classified AA as nutritionally essential AA (EAA) or non-essential AA (NEAA). Beginning in 1924, W.C. Rose and co-workers published a series of landmark papers on AA nutrition and metabolism in rats and humans.3,4,8 They noted that: (1) the omission of alanine, arginine, aspartate, cystine, glutamate, glycine, proline, hydroxyproline, serine, or tyrosine from diets that contained EAA did not result in negative nitrogen balance in normal adult humans during a eight-day period of study or in normal adult rats during one- to five-week periods of experiment; (2) nitrogen balance was maintained in normal adult humans fed histidine-free diets for eight days; and (3) the growth of young rats was not affected when they were fed diets lacking one of these AA except arginine for three weeks. Currently, EAA are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine for humans and other animals, whereas NEAA are alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine, taurine, and tyrosine for humans and most of other animals.2,9
Figure 1.
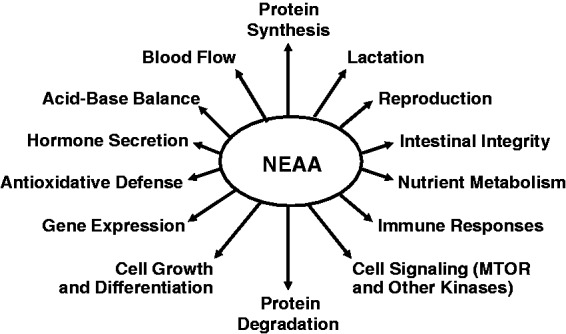
Physiological functions of NEAA in mammals, birds, and fish. NEAA displays metabolic versatility and are essential to whole-body homeostasis. Functional roles of NEAA beyond protein synthesis should be considered in defining their dietary requirements by animals and humans. Adapted from Wu.1
It had long been assumed that NEAA are synthesized sufficiently in animals and humans to meet the needs for maximal growth and optimal health.10–13 However, no experimental data substantiate this assumption.2,14–17 Selective conservation of pathways for NEAA synthesis in animals at the expense of considerable amounts of their EAA precursors and energy argues strongly that NEAA are indispensable for the metabolic needs and survival of mammals, birds, and fish. Therefore, pathways for de novo syntheses of NEAA have evolved or have been highly conserved in all vertebrates.2 The major objective of this article is to highlight emerging evidence that animals and humans have dietary requirements for NEAA to support their maximal growth, development, and lactation, as well as optimal reproduction, health, and well-being.
Metabolic functions of NEAA
Substrates for synthesis of peptides and non-peptide substances
Nutritionally significant small peptides include: (1) antibiotics produced by bacteria and the intestinal mucosa, (2) tripeptides (e.g. glutathione), (3) dipeptides (carnosine, carcinine, anserine, and balenine), and (4) physiologically important small peptides consisting of nine or 10 AA residues (e.g. oxytocin and angiotensin II).2 NEAA are substrates for synthesis of non-peptide hormones (e.g. epinephrine, norepinephrine, and thyroxine), low-molecular-weight substances (e.g. ammonia, carnitine, creatine, betaine, choline, dopamine, nucleotides, polyamines, β-alanine, d-alanine, d-aspartate, d-serine, NO, CO, and H2S).2 Of note, NO readily penetrates biological membranes to regulate smooth muscle relaxation in the artery and blood flow via the cGMP-dependent cell signaling.18 Also, polyamines are essential to DNA and protein synthesis in cells. The physiological importance of AA metabolites is epitomized by diseases resulting from inborn errors of metabolism. For example, the patients with an inborn deficiency of arginine:glycine amidinotransferase, which catalyzes the formation of guanidinoacetate and ornithine from arginine and glycine in creatine synthesis, develop mental retardation and muscular abnormalities.19 Also, patients with an inborn deficiency of glutathione synthetase exhibit oxidative stress, progressive neurologic disorders, hemolytic anemia, and metabolic acidosis.20 Thus, AA-dependent synthetic pathways are essential to whole-body homeostasis, reproduction, growth, development, and immunity.
Regulation of gene expression
Certain NEAA can regulate gene expression in animal cells, micro-RNA biogenesis, and epigenetics.21–24 For example, dietary glutamine reduces intestinal expression of genes that promote oxidative stress and immune activation, while increasing intestinal expression of genes that enhances cell growth and removal of oxidants.22 Furthermore, consistent with its antioxidative and antiobesity effects, dietary l-arginine inhibits expression of key genes responsible for fatty acid synthesis but enhances expression of key genes that are essential to fatty acid oxidation and glutathione synthesis in the white adipose tissue of rats.23 Dietary arginine also increases the expression of miRNA-15b/16 and miRNA-221/222 in the porcine umbilical vein.24 More recently, glycine has been reported to stimulate intestinal expression of glycine transporter 1, while reducing activation of the mitogen-activated protein kinase signaling pathway.25
Regulation of cell signaling pathways
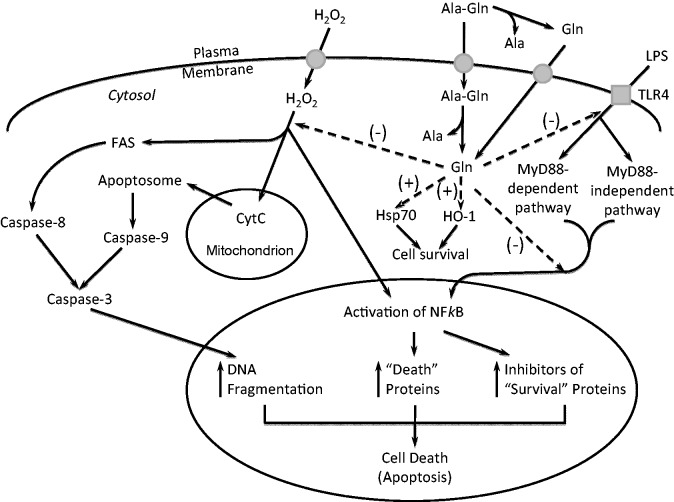
Most NEAA participate in cell signaling via kinases (e.g. mammalian target of rapamycin, AMP-activated protein kinase, cGMP-dependent kinase, cAMP-dependent kinase, and mitogen-activated protein kinase), G protein-coupled receptors, and gaseous molecules (e.g. NO, CO, and H2S) to regulate nutrient metabolism.2,18,26,27 For example, dietary arginine enhances the abundance of phosphorylated mammalian target of the rapamycin (mTOR), eukaryotic initiation factor (eIF) 4E-binding protein-1 (4E-BP1), and ribosomal protein S6 kinase 1 (S6K1), as well as the formation of the active eIF4E–eIF4G complex, but reduces the abundance of the inactive 4E-BP1–eIF4E complex in skeletal muscle, leading to increased protein synthesis and whole-body growth.28 In addition, dietary glutamine enhances intestinal integrity, cell survival, and villus height in association with activation of mTOR cell signaling, while attenuating weaning-induced reduction in occludin, claudin-1, zonula occluden (ZO)-2, and ZO-3 protein abundances.29 Figure 2 illustrates mechanisms for glutamine to protect intestinal epithelial cells from oxidant- or endotoxin-induced injury and death. Furthermore, dietary glutamate activates taste receptor signaling in the gastrointestinal tract.30 Likewise, NEAA regulate the synthesis of NO, CO, and H2S, which participate in gaseous signaling in cells through cGMP and cAMP production to enhance blood flow, nutrient transport, and immunity.18
Figure 2.
Proposed mechanisms for glutamine to prevent intestinal cells from oxidant- or lipopolysaccharide (LPS)-induced apoptosis. Exposure of intestinal epithelial cells to oxidants (e.g. H2O2) or LPS results in DNA damage and apoptosis, which are rescued by supplementation with either glutamine or its dipetide alanyl-glutamine. CytC: cytochrome C; HO-1: heme oxygenase; hsp: heat shock protein. Adapted from Haynes et al.41
Regulation of digestion and absorption of nutrients
NEAA affect digestive and absorptive function of the small intestine through: (1) the regulation of chemical sensing via the G protein-coupled receptors in the gastrointestinal tract, gastrointestinal emptying, and the motility of the small intestine; (2) formation of conjugates with bile acids (for example taurine and glycine) to facilitate lipid digestion and absorption; (3) modulation of the growth, metabolism, and population of the microbiota in the lumen of the small intestine and the large intestine.2,31–33 For example, glycine enhances water, ion, and AA transport by the pig small intestine.33 Also, glutamine reduces the net utilization of asparagine, lysine, leucine, valine, ornithine, and serine by jejunal or ileal mixed bacteria.34 Likewise, arginine decreases the net utilization of threonine, glycine, phenylalanine, and branched-chain AA by intestinal E. coli, and of lysine, threonine, isoleucine, leucine, glycine, and alanine by jejunal or ileal mixed bacteria.35 These findings indicate that glutamine and arginine exert their beneficial effects on nutrition and the metabolic status partly by regulating AA utilization and metabolism in the small-intestinal microbiota.
Regulation of energy and nutrient metabolism
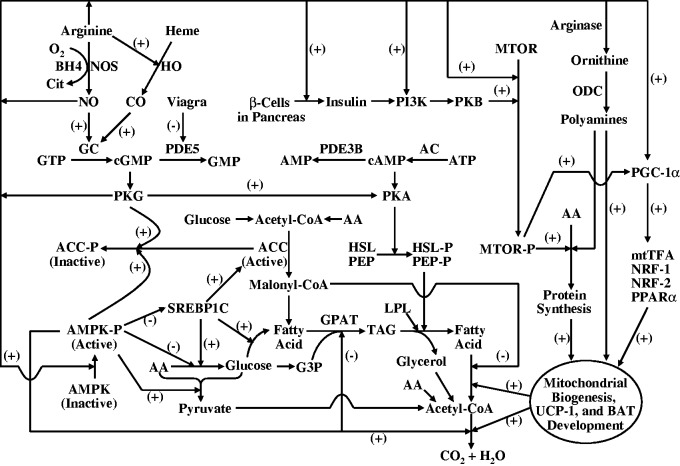
NEAA can regulate energy metabolism in a cell- and tissue-specific manner (Figure 3). Examples include: (1) stimulation of lipolysis by arginine in white adipose tissue36; activation, by arginine, of the oxidation of glucose and long-chain fatty acids to CO2 and water in skeletal muscle, liver, and white adipose tissue37; (2) inhibition, by arginine, of glucose and fatty acid synthesis in the liver and, in some species (e.g. pigs), white adipose tissue38; (3) enhancement of brown adipose tissue growth and development, as well as thermogenesis by arginine39; improvement of lean tissue gain by arginine,40 glutamine,41 glutamate,42 proline,2 and glycine33; (4) reduction, by arginine and glutamate, of white adipose tissue mass and the circulating level of triglycerides40,42; (5) improvement of bone strengths by glutamine43; participation of glycine and serine in one-carbon metabolism21; (6) control of food intake at the gastrointestinal and brain levels31; (7) provision of energy for the small intestine (glutamine, glutamate, and aspartate) and immunocytes (glutamine)2; (8) enhanced lactation (e.g. enhanced uptake of nutrients and millk synthesis by the mammary gland).17 Overall, NEAA can improve the efficiency of nutrient utilization, which is important for preventing metabolic syndrome in humans and animals and for sustaining animal agriculture to provide high-quality food protein for the growing population.
Figure 3.
Proposed mechanisms for arginine to reduce obesity in animals. Arginine activates the cGMP and AMPK signaling pathways, thereby enhancing substrate oxidation in a cell-specific manner and decreasing the accretion of white adipose tissue in the body. In some mammals, arginine also stimulates the growth of brown adipose tissue to promote the oxidation of long-chain fatty acids. AA: amino acids; AC: adenylyl cyclase; ACC: acetyl-CoA carboxylase; AMPK: AMP-activated protein kinase; BH4: tetrahydrobiopterin; Cit: citrulline; GC: guanylyl cyclase; G3P: glycerol-3-phosphate; GPAT: glycerol-3-phosphate acyltransferase; HO: heme oxygenase; HSL: hormone-sensitive lipase; LPL: lipoprotein lipase; MTOR: mammalian target of rapamycin; mtTFA: mitochondrial transcription factor A; NO: nitric oxide; NOS: nitric oxide synthase; NRF: nuclear respiration factor; ODC: ornithine decarboxylase; PDE5: phosphodiesterase 5; PDE3B: phosphodiesterase 3B; PEP: perilipins; PGC-1α: peroxisome proliferator-activated receptor γ (PPAR-γ) co-activator 1α; PKA: cAMP-dependent protein kinase A; PKG: cGMP-dependent protein kinase G; PPARα: peroxisome proliferator-activated receptor α; SREBP-1c: sterol regulatory element binding protein-1c; TAG: triacylglycerols. Taken from Wu.2
Regulation of immune function
NEAA regulates immune responses, including expression of T-cell receptors; lymphocyte proliferation; the production of cytokines and antibodies; macrophage polarization (i.e. the population of M1 and M2 cells); killing of pathogens by NO, superoxide anion, and H2O2; modulation of intestinal microbiota and its function; and prevention of infectious disease.40 For example, arginine, glutamine, and proline are essential to the functions of the innate immune system via: (1) synthesis of NO and reactive oxygen species, (2) antimicrobial activity, (3) secretion of hormones (e.g. insulin, growth hormone, prolactin, and insulin-like growth factor-I) that regulate the metabolism and activity of immunocytes, and (4) signal transduction pathways.44 In addition, these AA modulate the adaptive immune system through mechanisms that involve: (1) maturation and proliferation of T-lymphocytes and B-lymphocytes; (2) production of cytokines and specific antibodies by T-lymphocytes and B-lymphocytes, respectively; (3) circulating levels of anabolic hormones; and (4) expression of T-cell receptors.44,45 Thus, in mice overexpressing intestinal type-I arginase to hydrolyze dietary arginine, a deficiency of arginine impairs the development of progenitor-B to precursor-B lymphocytes in the bone marrow and decreases the number of B-lymphocytes in secondary lymphoid organs.46 Importantly, these immunological defects can be effectively reversed by subcutaneous administration of arginine. Additionally, dietary glutamine47 and proline48 confer immunostimulatory benefits in vaccine-immunized mice by enhancing general defense responses and decreasing expression of specific virulence factors. Thus, NEAA are considered modulators of immune responses.
Regulation of animal and human reproduction
NEAA are required for optimal reproduction in animals and humans, including men and women.24,49 Concentrations of arginine, citrulline, glutamine, glutamate, ornithine, glycine, and serine are particularly high in fetal fluids (e.g. 25 mM l-glutamine and 20 mM l-serine in ovine allantoic fluid during early and late gestation, respectively) and uterine secretions (e.g. 10 mM glycine in ovine uterine fluid) during gestation.49 These AA are essential to embryonic and fetal growth, development and survival through mTOR, integrin, NO, and MAPK signaling pathways.2 In support of this view, human fetuses with a severe inborn deficiency of glutamine synthetase have intrauterine growth retardation and often die before birth.50 In males, concentrations of polyamines, which are products of arginine and proline catabolism and whose synthesis is activated by glutamine, are relatively high in seminal fluid (∼100 μM) as compared with 3–5 μM in plasma.51 NO and polyamines are essential to spermatogenesis and sperm viability. Thus, NEAA hold promise for improving fertility livestock, birds, and fish, as well as in men and women of reproductive age, particularly under stress conditions.
Regulation of endocrine status
NEAA are required to maintain the endocrine status through the synthesis and secretion of hormones, mediation of hormone actions in cells, and expression of anti-inflammatory interferons (e.g. interferon tau) and cytokines.49,52 Specifically, tyrosine is the precursor for synthesis of epinephrine, norepinephrine, dopamine, and thyroid hormones. High concentrations of arginine and glutamine are potent secretagogues for the release of insulin and growth hormone in mammals through NO- and NADH-dependent mechanisms,52 whereas glycine stimulates glucagon secretion from the pancreas via glycine-gated receptors.53 Further, dietary glutamine and arginine reduce the production of glucocorticoids and, therefore, catabolism in stress conditions.29,51 Elevated levels of these AA may partly mediate the effect of high protein intake on concentrations of hormones in plasma.52
Regulation of antioxidative and detoxification reactions
NEAA are critical for antioxidative defenses and removal of toxic substances (both xenobiotics and endogenous metabolites) through: (1) synthesis of glutathione from cysteine, glutamate, and glycine; of carnosine from β-alanine and histidine; of creatine from arginine and glycine; and of taurine from cysteine; (2) production of antioxidative enzymes (e.g. glutathione peroxidase, superoxide dismutase, and H2O2 peroxidase); (3) removal of ammonia, oxidants, and xenobiotics; and (4) anti-inflammation and regulation of apoptosis in cells.1 Glutathione plays a key role in maintaining cellular redox balance and reducing oxidative stress, thereby delaying aging and ameliorating metabolic complications of many chronic diseases.2 Thus, N-acetylcysteine, a stable and water-soluble precursor of cysteine, has been used in clinical treatment of oxidative injury to improve gut integrity and whole-body well-being.54
Regulation of neurological function
NEAA are crucial for neurological function and behavior of humans and animals through: (1) synthesis of neurotransmitters (e.g. NO, γ-aminobutyrate, serotonin, dopamine, and acetylcholine); (2) serving as agonists or co-agonists at N-methyl-d-aspartic acid receptors (e.g. glutamate, aspartate, glycine, d-aspartate, d-alanine, and d-serine); (3) production of glutathione, polyamines, agmatine, and β-alanine; and (4) neuroprotective reactions.2 Furthermore, NEAA regulate food intake through complex mechanisms involving hormonal, neuronal, and metabolic signals generated from: (1) the digestive system, (2) central nervous system, and (3) other organs (e.g. white adipose tissue) that transmit satiety signals to the brain.31,55 Furthermore, d-alanine is an agonist at the glycine site on the NMDA subtype glutamate receptor, thereby possibly affecting memory function, synaptic plasticity, as well as diurnal and nocturnal (circadian) behaviors.56 Of note, Ruzzo et al.57 recently reported that recessive mutations in the ASNS gene encoding asparagine synthetase, which catalyzes the synthesis of asparagine from glutamine and aspartate, resulted in congenital microcephaly, intellectual disability, progressive cerebral atrophy, intractable seizures, and even death in humans.
Regulation of acid–base balance
NEAA play a critical role in the regulation of acid–base balance via renal ammoniagenesis and, thus, are effective in preventing acidosis-induced muscle proteolysis and fetal growth restriction.58 In normal postabsorptive humans and rats, skeletal muscle is the major site of glutamine synthesis and release, whereas the small intestine is the primary user of glutamine in the circulation and the diet.59 During metabolic acidosis, skeletal muscle and liver release large amounts of glutamine, and the kidney becomes the major organ for extracting glutamine from the arterial blood to produce ammonia and HCO3–, thereby helping compensate the acidosis.
Other functions of NEAA
NEAA are required for recovery from injury by enhancing wound healing via: (1) polyamine- and NO-dependent mechanisms; and (2) synthesis of collagen and remodeling of extracellular matrix that are greatly affected by arginine, glycine, glutamine, and proline.2 NEAA (e.g. tyrosine) are pivotal to pigmentation (skin, hair, and eyes) that has important biological, social, and cultural importance.60 Furthermore, glutamine and taurine (e.g. up to 25 and 20 mM in human skeletal muscle, respectively) contribute to the modulation of osmoregulation in diverse biological systems to maintain whole-body homeostasis.61
Dietary requirements of NEAA
Owing to technical limitations, analyses of some NEAA (e.g. free glutamine and proline, as well as glutamate, glutamine, and proline in protein) in animal tissues had been a daunting challenge until the 1970s when high-performance liquid chromatography became widely available for AA determination. In addition, research on AA biochemistry was limited primarily to EAA until 1971 when Marliss and co-workers discovered the release of glutamine from human skeletal muscle.62 Wu et al.63 proposed in 2000 that functional needs for AA beyond nitrogen balance and protein synthesis should be a major criteria with which to classify AA as EAA or NEAA in nutrition. This, along with substantial amounts of experimental evidence, led to the new nutritional concept of functional AA, which are defined as those AA that participate in and regulate key metabolic pathways to improve the health, survival, growth, development, lactation, and reproduction of animals.1 These metabolic pathways include: (1) intracellular protein turnover (synthesis and degradation) and associated events; (2) cell- and tissue-specific synthesis and catabolism of AA; (3) generation of small peptides, nitrogenous metabolites, and sulfur-containing substances (e.g. H2S); (4) urea cycle and uric acid synthesis; (5) lipid and glucose metabolism; (6) one-carbon unit metabolism and DNA synthesis; and (7) cellular redox signaling.2 Functional AA include both EAA and NEAA. Dietary NEAA requirements are affected by a plethora of nutritional, physiological, pathological, and environmental factors.1
Reevaluation of the concepts of EAA and NEAA in nutrition
Recent analysis of whole-genome sequences in a wide variety of eukaryotes reveals that deleterious mutations occurred during evolution for almost all the genes that were lost in EAA-synthetic pathways in animal cells.64 Because plants and microorganisms have conserved the capacity for the synthesis of all AA,8,65 the inability of animals to produce EAA de novo may have nutritional and physiological significance in: (1) sparing phosphoenolpyruvate, d-erythrose-4-phosphate, and acetyl-CoA for synthesis of glucose, nucleotides, and fatty acids, respectively; (2) reducing energy expenditure; and (3) minimizing the numbers of proteins and intermediary metabolites as well as metabolic complexity. Because most of the EAA can be formed from their corresponding α-ketoacids in vivo, Meister66 indicated in 1965 that it is the carbon skeletons of EAA, not EAA themselves, that cannot be synthesized in animals. To date, EAA are defined as either those AA whose carbon skeletons cannot be synthesized de novo in animal cells or those that normally are insufficiently synthesized de novo by the animal organism relative to its needs for maintenance, growth, development, and health and which must be provided in the diet to meet requirements.2 In contrast, NEAA are those AA which can be synthesized de novo in adequate amounts by the animal organism to meet requirements for maintenance, growth, development, and health and, therefore, need not be provided in the diet.2
AA-synthetic pathways are now known to be cell-, tissue-, and species-specific. Although EAA and NEAA had been described for over a century, these two terms are only a matter of definitions. It should be borne in mind that the synthesis of NEAA in the animal organism critically depends not only on energy but also on the availability of EAA that are provided from dietary protein. This macronutrient is the most expensive in feed ingredients for livestock, poultry, and fish. Because of an incomplete understanding of AA biochemistry, nutrition, and physiology, the concept of “nutritional non-essentiality” has led to the ignorance of the importance of NEAA in the practice of nutrition, particularly in animal production.2 The National Research Council13 indicated that farm animals (e.g. swine) have sufficient capacity for synthesis of all NEAA and do not require dietary NEAA. However, evidence for sufficient capacity in NEAA synthesis is lacking, and furthermore sufficient capacity does not necessarily translate into sufficient synthesis of NEAA in the livestock and poultry fed ordinary diets (e.g. diets containing virtually no excessive amounts of EAA) to minimize production costs. Similarly, while humans can consume foods of their own choice, intakes of NEAA (e.g. arginine, glutamate, glutamine, glycine, proline, and serine) should be sufficient to prevent anatomical, physiological, and biochemical abnormalities. Thus, the century-old term “NEAA” should not be used in nutritional sciences.
Insufficient synthesis of NEAA in animals and humans
There is evidence that humans cannot sufficiently synthesize NEAA to meet metabolic needs under both normal and stress conditions. For example, Holt and Albanese65 reported in 1944 that feeding an arginine-deficient diet to adult men for nine days decreased both the number and motility of sperm cells by 90% (Table 1). Further, the authors noted that although these abnormal changes could be reversed by repletion of arginine through supplementation, the full recovery to normal values took several weeks. These results indicate that men cannot synthesize sufficient arginine to maintain their reproductive function. The striking findings also underline a critical role for arginine in spermatogenesis and maintenance of sperm quality, and further argues that functional needs beyond nitrogen balance should be considered in determining dietary requirements of AA. In support of this view, Tanimura67 reported that oral administration of l-arginine–HCl (0.5 g/day) to infertile men for 6–8 weeks markedly increased sperm counts and motility in most infertile patients and resulted in successful pregnancies. Furthermore, Meléendez-Hevia et al.68 estimated that endogenous synthesis of glycine in healthy humans can satisfy at most only 30% of the metabolic needs for the synthesis of proteins (e.g. collagens). Similarly, humans, young or adult, cannot synthesize a sufficient quantity of proline to repair wound tissues.2 More recently, Sevastiadou et al.69 found that preterm infants cannot synthesize enough glutamine, as oral administration of glutamine improves intestinal integrity, while reducing the overall incidence of necrotizing enterocolitis and septicemia in these neonates.
Table 1.
Effects of dietary arginine deficiency on seminal plasma in adult men
| Group | Sperm counts (× 106/mL) | Non-motile sperm cells (%) | White blood cells (× 106/mL) |
|---|---|---|---|
| Normal average values | 120 | 5–10 | None |
| Arginine-deficient subjects* | 14.7 ± 1.5 | 95.0 ± 2.9 | 16.7 ± 1.7 |
| Arginine-repleted subjects† | 94.0 ± 9.7 | 15, 20 | 4.7 ± 0.3 |
Adapted from Holt and Albanese.65
Values are means ± SEM, n = 3. Values on non-motile sperm cells were reported for two of the three arginine-supplemented subjects.
Adult men were fed an arginine-deficient diet for nine days.
Adult men were fed an arginine-deficient diet for nine days and then repleted with arginine through dietary supplementation for several weeks (the exact length was not reported in the original paper).
Studies with laboratory and farm animals have also revealed that endogenous synthesis of NEAA is insufficient to meet their physiological needs.17 For example, a deficiency of dietary arginine in young male rats over a period of two months resulted in progressive damage to testes, absence of sperm production, as well as the filling of the lumina of the tubules with cellular debris, leukocytes, and macrophages.65 Also, Harper and other investigators70–74 reported in the 1960s and 1970s that the absence of NEAA from chicken and rat diets prevented maximal growth in these animals. This conclusion was consistent with our own findings (Table 2). Specifically, although rats can synthesize arginine, glutamine, and glutamate,2,75 the omission of each of these AA from the purified diet reduces growth rate, with the most severe growth retardation being observed for the absence of arginine. Clearly, these three AA cannot be synthesized sufficiently in young rats and, therefore, must be present in diets to sustain maximum growth of the animals. Although glutamate was frequently used to prepare isonitrogenous diets in the previous nutritional studies,6 none of the investigators considered that humans or animals have a dietary requirement of glutamate for the maintenance of intestinal function, optimal whole-body growth, or, in the case of farm animals, production performance.
Table 2.
Growth of rats fed purified diets lacking l-arginine, l-glutamine, or l-glutamate.
| Group | Body weight at d 0 (g) | Body weight at d 21 (g) | Body weight gain between d 0–21 (g) | Food intake between d 0–21 (g/kg BW) |
|---|---|---|---|---|
| Complete diet (C) | 95.8 ± 2.3 | 226.4 ± 4.2 | 130.7 ± 2.2 | 9.98 ± 0.41 |
| C—Glutamate | 96.0 ± 2.6 | 204.8 ± 3.9 | 108.8 ± 1.6 | 9.96 ± 0.34 |
| C—Glutamine | 95.6 ± 2.7 | 193.6 ± 3.5 | 98.1 ± 2.0 | 10.1 ± 0.38 |
| C—Arginine | 95.4 ± 2.5 | 131.4 ± 3.1 | 36.1 ± 1.3 | 10.2 ± 0.37 |
Values are means ± SEM, n = 8. Male Sprague–Dawley rats were fed, between 30 and 51 days of age, purified diets containing all proteinogenic amino acids (complete diet, C) or the complete diet without l-glutamate (C—glutamate), l-glutamine (C—glutamine), or l-arginine (C—arginine). The composition of the complete diet (g/kg det) was: cornstarch, 545.5; maltodextrin, 10, 125; cellulose, 50; corn oil, 50; salt mix, 35; sodium bicarbonate, 7.5; vitamin mix, 10; choline bitartrate, 2; l-alanine, 10; l-arginine (free base), 10; l-asparagine-H2O, 5; l-aspartate, 10; l-cystine, 4; l-glutamate, 20; l-glutamine, 20; glycine, 10; l-histidine–HCl–H2O, 6; l-isoleucine, 8; l-leucine, 12; l-lysine–HCl, 14; l-methionine, 6; l-phenylalanine, 8; l-proline, 5; l-serine, 5; l-threonine, 8; l-tryptophan, 2; l-tyrosine, 4; and l-valine, 8. Diets were made isonitrogenous and isocaloric with l-alanine and cornstarch. The salt mix and the vitamin mix were the same as described by Wu et al.45 Rats in the “C”, “C—glutamate”, and “C—glutamine” groups were individually pair-fed their respective diets based on food intake by rats in the “C—arginine” group per kg body weight. Means for body weight (P < 0.05) or body weight gain (P < 0.01) differ among the four groups of rats, as analyzed by one-way analysis of variance and the Student-Newman-Keuls multiple comparison test (SAS Institute Inc., Cary NC, USA).
d 0 = 30 days of age; d 21 = 51 days of age.
Young pigs grow rapidly and are sensitive to the provision of dietary NEAA.75 In addition, milk production by sows respond readily to dietary intakes of protein and AA.76 Thus, these animals are very useful to evaluate their ability for NEAA synthesis. Deng et al.77 have reported that, in weanling pigs fed diets containing the same amount of EAA, reduced dietary content of NEAA limits tissue protein synthesis and growth performance (Table 3). Furthermore, results of recent studies indicate that: (1) diets must contain sufficient amounts of arginine and glutamine to support optimal fetal, neonatal, and postweaning growth in pigs; (2) diets must provide sufficient proline, glutamate, and glycine to sustain maximal growth performance and feed efficiency in early weaned pigs; and (3) diets must supply adequate arginine and glutamate to maximize milk production by lactating sows.14,17
Table 3.
Deficiency of NEAA limits tissue protein synthesis and growth of piglets (days 25–39) fed a low-protein diet.†
| Dietary protein content |
|||
|---|---|---|---|
| 20.7% | 12.7% + EAA‡ | Pooled SEM | |
| Protein synthesis (%/day) | |||
| Longissimus muscle | 11.8 | 7.1* | 0.8 |
| Liver | 83.5 | 63.0* | 4.2 |
| Kidney | 36.1 | 24.1* | 1.6 |
| Pancreas | 76.4 | 62.7* | 2.3 |
| Feed intake (g/day) | 432 | 455 | 50 |
| Body-weight gain (g/day) | 299 | 264* | 10 |
| Feed:gain ratio (g/g) | 1.44 | 1.72* | 0.02 |
Adapted from Deng et al.77 Beginning at 25 days of age, postweaning pigs were fed a corn- and soybean meal-based diet for 14 days. Data are means ± SEM, n = 6. * P < 0.05 versus the control (20.7% protein) group.
EAA (Lys, Met, Thr, Trp, Leu, Ile, and Val) were added to the low-protein diet, so that both diets had the same amounts of all EAA. However, the low-protein diet provided less amounts of NEAA than the 20.7% crude-protein diet.
Criteria for assessing dietary requirements of NEAA
Determination of dietary requirements of EAA for animals is simpler than that for NEAA because the organisms usually respond more sensitively and more rapidly to a deficiency of an EAA in the diet.2 Wu et al.14 have proposed that the end points for determining dietary EAA requirements by animals (e.g. mortality, morbidity, food intake, growth, lactation, and reproductive performance of animals) can be used to estimate their dietary requirements of NEAA. Additional criteria can also be helpful and include assessments of physiological parameters (e.g. concentrations of hormones, hemoglobins, ammonia, urea, AA, nitrogenous metabolites, lipids, and glucose in plasma, as well as concentrations of neurotransmitters, glutathione, creatine, and polyamines in tissues).17 Furthermore, dietary NEAA requirements can be based on any abnormal values for anatomical, physiological, biochemical, and immunological indices, including (1) abnormalities in small intestinal morphology, mass, absorptive capacity, and integrity; (2) imbalances among AA and high concentrations of ammonia in plasma and urine; (3) dysfunctional regulation of nutrient metabolism to reduce muscle protein gain, promote white-fat accretion, and cause metabolic syndrome in the body; (4) impaired response of peripheral lymphocytes to stimulation by mitogens; (5) abnormal blood chemistry; and (6) abnormal organ function (e.g. impaired vision, skin lesions, and skeletal muscular weakness).17 Therefore, multiple variables can be used to define dietary requirements of NEAA by animals and humans, and the choices should depend on species and goals.
Methods for determining dietary requirements of NEAA
Feeding experiments have traditionally been employed to determine nitrogen balance in animals and humans, and the results provide a basis for assessing both qualitative and quantitative requirements of dietary NEAA by animals.3,4 Minimal requirements of AA can also be estimated using the factorial analysis, which involves the measurements of the loss of nitrogen by animals fed a nitrogen-free diet via urine, feces, gas emission, and other routes (namely maintenance) + AA deposited in animals + AA excreted as animal products (e.g. milk, egg, wool, and fetus).17 Over the past three decades, studies involving radioactive and stable AA tracers have been used along with the nitrogen balance technique to determine dietary requirements for EAA by humans and farm animals.5 These more modern methods require the use of direct and indirect indicators of AA oxidation during a period of several hours. Examples are: (1) measurement of rates of phenylalanine oxidation in animals fed diets containing graded levels of phenylalanine to indicate optimal dietary requirement of phenylalanine (direct indicator method); and (2) measurement of rates of phenylalanine oxidation in animals fed diets containing graded levels of lysine to indicate optimal dietary requirement of lysine (indirect indicator method). For yet unknown reasons, the AA oxidation methods generally yielded much higher values of dietary EAA requirements by humans than the nitrogen balance studies.2 At present, little is known about dietary requirements for NEAA by mammals, birds, or fishes.
While nitrogen balance studies are relatively simple and have many advantages, this approach may not be sufficiently sensitive to evaluate the needs of animals and humans for EAA and NEAA. Let us use histidine and arginine as examples. First, feeding healthy adult subjects a histidine-free diet for eight days did not result in a negative nitrogen balance, leading to an erroneous conclusion that histidine was synthesized in the body and humans did not have a dietary requirement of histidine.3,4 However, there is no metabolic pathway for histidine synthesis in animal cells.2 Hydrolysis of histidine-containing dipeptides in tissues (primarily skeletal muscle) and of histidine-rich proteins (e.g. hemoglobins) can provide a sufficient amount of endogenous histidine to maintain nitrogen balance in adult humans for eight days. Second, nitrogen balance studies failed to detect a dietary requirement of arginine by adult men for sustaining reproductive function.65 Specifically, feeding an arginine-deficient diet to adult men for nine days did not induce negative nitrogen balance but did greatly impair their reproductive function (Table 1). These results supports the view that the composition of synthetic and semi-synthetic diets for animals needs to be recalculated to correct for the amounts of so-called NEAA needed for optimum growth and reproduction in the body.
Animal studies to determine dietary requirements of NEAA
Much of our current knowledge about dietary requirements of NEAA has been built from studies with economically important animals and laboratory animals. They include: (1) swine, an excellent animal model for human nutrition research78; (2) rats and mice; (3) chickens and other poultry species; and (4) fish.2 In published studies, animals were fed typical or conventional diets that were supplemented with or without an NEAA.17,79 Criteria that have been used to assess dietary requirements of NEAA include litter size, fetal growth, milk production, postnatal growth, skeletal muscle gain, reduction of white adipose tissue, digestive function and intestinal integrity, immunity, feed efficiency, and meat quality.14
Recommendations of dietary NEAA requirements for animals depend on the expected levels of their growth, optimal reproduction, optimal health, and in the case of livestock, poultry, and fish, also production performance. Recent advances in the analysis of glutamate, glutamine, aspartate, and asparagine in food and animal-tissue proteins have made it possible to quantify dietary intakes of these four AA by animals and humans.80,81 Based on published studies, Wu79 recently proposed Texas A&M University’s optimal ratios of all AA (including NEAA) in typical corn- and soybean meal-based diets for pigs and chickens at various stages of growth and production gestating. The values for gestating, lactating, and growing-finishing swine are provided in Table 4. This example can provide a model for estimating dietary requirements of NEAA by other livestock species and humans. Note that the recommended values for dietary AA requirements are expressed per true ileal digestibilities of dietary AA and can be converted to percentages of total AA in the diet (g/100 g diet). Likewise, dietary AA requirements of humans may be expressed in terms of total dietary AA content or true ileal digestibility of dietary AA (e.g. 85–90% depending on the type of food). Supplemental AA can be either synthetic AA or complex food proteins that are excellent sources of both EAA and NEAA.
Table 4.
Texas A&M University’s optimal ratios of true digestible amino acids for swine diets.*
| AA | Growing pigs (kg)† |
Gestating pigs‡ |
Lactating sows† | ||||
|---|---|---|---|---|---|---|---|
| 5–10 | 10–20 | 20–50 | 50–110 | d 0–90 | d 90–114 | ||
| % of diet (as-fed basis) | |||||||
| Alanine | 1.14 | 0.97 | 0.80 | 0.64 | 0.69 | 0.69 | 0.83 |
| Arginine | 1.19 | 1.01 | 0.83 | 0.66 | 1.03 | 1.03 | 1.37 |
| Asparagine | 0.80 | 0.68 | 0.56 | 0.45 | 0.50 | 0.50 | 0.66 |
| Aspartate | 1.14 | 0.97 | 0.80 | 0.64 | 0.61 | 0.61 | 0.94 |
| Cysteine | 0.32 | 0.28 | 0.24 | 0.20 | 0.19 | 0.19 | 0.26 |
| Glutamate | 2.00 | 1.70 | 1.39 | 1.12 | 0.89 | 0.89 | 1.81 |
| Glutamine | 1.80 | 1.53 | 1.25 | 1.00 | 1.00 | 1.60 | 1.38 |
| Glycine | 1.27 | 1.08 | 0.89 | 0.71 | 0.48 | 0.48 | 0.75 |
| Histidine | 0.46 | 0.39 | 0.32 | 0.26 | 0.29 | 0.29 | 0.39 |
| Isoleucine | 0.78 | 0.66 | 0.54 | 0.43 | 0.45 | 0.45 | 0.66 |
| Leucine | 1.57 | 1.33 | 1.09 | 0.87 | 1.03 | 1.03 | 1.41 |
| Lysine | 1.19 | 1.01 | 0.83 | 0.66 | 0.51 | 0.51 | 0.80 |
| Methionine | 0.32 | 0.28 | 0.24 | 0.20 | 0.16 | 0.16 | 0.25 |
| Phenylalanine | 0.86 | 0.73 | 0.60 | 0.48 | 0.54 | 0.54 | 0.77 |
| Proline | 1.36 | 1.16 | 0.95 | 0.76 | 0.89 | 0.89 | 1.24 |
| Serine | 0.70 | 0.60 | 0.49 | 0.39 | 0.45 | 0.45 | 0.74 |
| Threonine | 0.74 | 0.65 | 0.55 | 0.46 | 0.41 | 0.41 | 0.56 |
| Tryptophan | 0.22 | 0.19 | 0.17 | 0.14 | 0.11 | 0.11 | 0.18 |
| Tyrosine | 0.67 | 0.57 | 0.46 | 0.37 | 0.40 | 0.40 | 0.62 |
| Valine | 0.85 | 0.72 | 0.59 | 0.47 | 0.55 | 0.55 | 0.72 |
Adapted from Wu.79
Except for glycine, all amino acids are l-isomers. Values are based on true ileal digestible amino acids. Crystalline amino acids (e.g. feed-grade arginine, glutamate, glutamine, and glycine), whose true ileal digestibility is 100%, can be added to a diet to obtain their optimal ratios. The molecular weights of intact amino acids were used for all the calculations. The content of dry matter in all the diets is 90%. The content of metabolizable energy in the diets of growing pigs, gestating pigs, and lactating pigs is 3330, 3122, and 3310 kcal/kg diet, respectively.
Fed ad libitum (90% dry matter).
Fed 2 kg/day on days 0–90, and 2.3 kg/day on days 90–114 (90% dry matter).
“Synthesizable AA” should not be considered as “nutritionally non-essential AA” in animal or human nutrition. Rather, these NEAA should be regarded as nutritionally essential for maximum growth and lactation, as well as optimal health, well-being, and reproduction. NEAA may also regulate epigenetics,16,24 thereby possibly affecting the growth, development, and health of many generations of offspring. To date, the concept of dietary requirements of NEAA by animals and humans is still in its infancy. Recommended values for requirements of all AA (including NEAA) will need to be revised as new experimental data become available. Nonetheless, our new initiative should provide a much-needed framework for further studies to evaluate dietary requirements for NEAA by humans, livestock, poultry, and fish. Establishment and adoption of these values can beneficially reduce dietary protein content; reduce excretion of nitrogen to the environment; and improve the efficiency of nutrient utilization, growth, and production in farm animals.17 The concept of dietary requirements for NEAA also has important implications for preventing both chronic and infectious diseases, maximizing growth, and optimizing health in humans.14,82
Conclusion
The traditional classification of AA as EAA or NEAA was solely based on growth or nitrogen balance of animals and humans. Large amounts of emerging evidence indicate that this century-old concept has major limitations in protein nutrition, such that efficiencies of nutrient utilization in farm animals and humans remain relatively low despite much effort on establishing dietary requirements of EAA. While all organisms are known to have metabolic needs for all proteinogenic and other physiologically important AA, the needs of dietary NEAA for animals and humans have largely been ignored in animal production and human health. Based on new developments of AA biochemistry and nutrition, we propose that mammals, birds, and fish have dietary needs of all NEAA for optimal growth, development, lactation, reproduction, and health. This new paradigm shift in nutrition has now led to the recognition of dietary essentiality of “nutritionally non-essential AA” for animals and humans.
Authors’ contributions
GW conceived this project. YH and GW wrote the manuscript. YY contributed to the discussion and revision of the article. GW had the primary responsibility for the content of the paper. All authors read and approved the final version of the manuscript.
Acknowledgements
Work in our laboratories were supported by the National Basic Research Program of China (no. 2012CB126305), the National Natural Science Foundation of China (no. 31330075, 31372319 and 31110103909), the Hubei Provincial Research and Development Program (no. 2010BB023), the Hubei Hundred Talent program, the Natural Science Foundation of Hubei Province (no. 2013CFA097, 2013CFB325, 2012FFB04805, and 2011CDA131), Agriculture and Food Research Initiative Competitive Grants (no. 2011-67015-20028, 2014-67015-21770 and 2015-67015-23276) from the USDA National Institute of Food and Agriculture, and a Hatch project from Texas A&M AgriLife Research (H-8200). The authors declare no conflict of interest.
References
- 1.Wu G. Functional amino acids in growth, reproduction and health. Adv Nutr 2010; 1: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu G. Amino acids: biochemistry and nutrition, Boca Raton, FL: CRC Press, 2013. [Google Scholar]
- 3.Rose WC. The amino acid requirements of adult man. Nutr Abstr Rev Ser Hum Exp 1957; 27: 631–47. [PubMed] [Google Scholar]
- 4.Rose WC. The sequence of events leading to the establishment of the amino acid needs of man. Am J Public Health 1968; 58: 2020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeds PJ. Dispensable and indispensable amino acids for humans. J Nutr 2000; 130: 1835S–40S. [DOI] [PubMed] [Google Scholar]
- 6.Baker DH. Advances in protein-amino acid nutrition of poultry. Amino Acids 2009; 37: 29–41. [DOI] [PubMed] [Google Scholar]
- 7.Abderhalden E. Experiment on the feeding with completely degraded nutrition substances. Z Physiol Chem 1912; 77: 22–58. [Google Scholar]
- 8.Rose WC, Cox GJ. The relation of arginine and histidine to growth. J Biol Chem 1924; 61: 747–73. [Google Scholar]
- 9.Institute of Medicine. Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, proteins, and amino acids, Washington, DC: The National Academies Press, 2005. [Google Scholar]
- 10.Morrison FB. Feeds and feeding, 22nd ed Ithaca, NY: The Morrison Publishing Company, 1956. [Google Scholar]
- 11.Maynard LA, Loosli JK, Hintz HF, Warner RG. Animal nutrition, 7th ed New York: McGraw-Hill Book Company, 1979. [Google Scholar]
- 12.Crampton EW, Harris LE. Applied animal nutrition, 2nd ed San Francisco: W.H. Freeman and Company, 1969. [Google Scholar]
- 13.National Research Council. Nutrient requirements of swine, Washington, DC: National Academy Press, 2012. [Google Scholar]
- 14.Wu G, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang JJ, Yin YL. Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 2013; 44: 1107–13. [DOI] [PubMed] [Google Scholar]
- 15.Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. J Biol Chem 2012; 287: 19786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phang JM, Liu W, Hancock C. Bridging epigenetics and metabolism: role of non-essential amino acids. Epigenetics 2013; 8: 231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2014; 2: 387–417. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Bazer FW, Gao H, Jobgen W, Johnson GA, Li P, McKnight JR, Satterfield MC, Spencer TE, Wu G. Amino acids and gaseous signaling. Amino Acids 2009; 37: 65–78. [DOI] [PubMed] [Google Scholar]
- 19.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007; 27: 241–61. [DOI] [PubMed] [Google Scholar]
- 20.Dahl N, Pigg M, Ristoff E, Gali R, Carlsson B, Mannervik B, Larsson A, Board P. Missense mutations in the human glutathione synthetase gene result in severe metabolic acidosis, 5-oxoprolinuria, hemolytic anemia and neurological dysfunction. Hum Mol Genet 1997; 6: 1147–52. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Wu ZL, Li DF, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G. Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal 2012; 17: 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JJ, Chen LX, Li P, Li XL, Zhou HJ, Wang FL, Li DF, Yin YL, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 2008; 138: 1025–32. [DOI] [PubMed] [Google Scholar]
- 23.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 2009; 37: 187–98. [DOI] [PubMed] [Google Scholar]
- 24.Liu XD, Wu X, Yin YL, Liu YQ, Geng MM, Yang HS, Blachier F, Wu GY. Effects of dietary L-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 2012; 42: 2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang WW, Wu ZL, Lin G, Hu SD, Wang B, Dai ZL, Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small-intestinal epithelial cells. J Nutr 2014; 144: 1540–8. [DOI] [PubMed] [Google Scholar]
- 26.Rhoads JM, Niu XM, Surendran S, Liu YY, Wu G. Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and p70s6k signaling. J Nutr 2008; 138: 1652–7. [DOI] [PubMed] [Google Scholar]
- 27.Wang XQ, Frank JW, Xu J, Dunlap KA, Satterfield MC, Burghardt RC, Romero JJ, Hansen TR, Wu G, Bazer FW. Functional role of arginine during the peri-implantation period of pregnancy. II. Consequences of loss of function of nitric oxide synthase NOS3 mRNA in ovine conceptus trophectoderm. Biol Reprod 2014; 91: 1–10. [DOI] [PubMed] [Google Scholar]
- 28.Yao K, Yin YL, Chu WY, Liu ZQ, Deng D, Li TJ, Huang RL, Zhang JS, Tan BE, Wang W, Wu G. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr 2008; 138: 867–872. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Zhang C, Wu G, Sun YL, Wang B, He BB, Dai ZL, Wu ZL. Glutamine enhances tight-junction protein expression and modulates CRF signaling in the jejunum of weanling piglets. J Nutr 2015; 145: 25–31. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Yin YL, Shu XG, Li TJ, Li FN, Tan BE, Wu ZL, Wu G. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 2013; 45: 1169–77. [DOI] [PubMed] [Google Scholar]
- 31.San Gabriel A, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids 2013; 45: 451–61. [DOI] [PubMed] [Google Scholar]
- 32.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 2011; 16: 1768–86. [DOI] [PubMed] [Google Scholar]
- 33.Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014; 46: 2037–45. [DOI] [PubMed] [Google Scholar]
- 34.Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013; 45: 501–12. [DOI] [PubMed] [Google Scholar]
- 35.Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Regulatory role for L-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 2012; 43: 233–44. [DOI] [PubMed] [Google Scholar]
- 36.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 2005; 135: 714–21. [DOI] [PubMed] [Google Scholar]
- 37.McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids 2010; 39: 349–57. [DOI] [PubMed] [Google Scholar]
- 38.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 2006; 17: 571–88. [DOI] [PubMed] [Google Scholar]
- 39.Wu ZL, Satterfield MC, Bazer FW, Wu G. Regulation of brown adipose tissue development and white fat reduction by L-arginine. Curr Opin Clin Nutr Metab Care 2012; 15: 529–38. [DOI] [PubMed] [Google Scholar]
- 40.Tan B, Yin YL, Liu ZQ, Li XG, Xu HJ, Kong XF, Huang RL, Tang WJ, Shinzato I, Smith SB, Wu G. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 2009; 37: 169–75. [DOI] [PubMed] [Google Scholar]
- 41.Haynes TE, Li P, Li XL, Shimotori K, Sato H, Flynn NE, Wang JJ, Knabe DA, Wu G. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 2009; 37: 131–42. [DOI] [PubMed] [Google Scholar]
- 42.Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 2013; 44: 911–23. [DOI] [PubMed] [Google Scholar]
- 43.Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest 2015; 125: 551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li P, Yin YL, Li DF, Kim SW, Wu G. Amino acids and immune function. Br J Nutr 2007; 98: 237–52. [DOI] [PubMed] [Google Scholar]
- 45.Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr 1999; 129: 1347–54. [DOI] [PubMed] [Google Scholar]
- 46.De Jonge WJ, Kwikkers KL, Te Velde AA, Van Deventer SJH, Nolte MA, Mebius RE, Ruijter JM, Lamers MC, Lamers WH. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. J Clin Invest 2002; 110: 1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren WK, Liu SP, Chen S, Zhang FM, Li NZ, Yin J, Peng YY, Wu L, Liu G, Yin YL, Wu G. Dietary L-glutamine supplementation increases Pasteurella multocida burden and expression of major virulence factors in mice. Amino Acids 2013; 45: 947–55. [DOI] [PubMed] [Google Scholar]
- 48.Ren WK, Zou LX, Ruan Z, Li NZ, Wang Y, Peng Y, Liu G, Yin YL, Huang RL, Hou YQ, Wu G. Dietary L-proline supplementation confers immuno-stimulatory effects on inactivated Pasteurella multocida vaccine immunized mice. Amino Acids 2013; 45: 555–61. [DOI] [PubMed] [Google Scholar]
- 49.Bazer FW, Wu G, Johnson GA, Wang XQ. Environmental factors affecting pregnancy: endocrine disrupters, nutrients and metabolic pathways. Mol Cell Endocrinol 2014; 398: 53–68. [DOI] [PubMed] [Google Scholar]
- 50.Häberle J, Görg B, Rutsch F, Schmidt E, Toutain A, Benoist JF, Gelot A, Suc AL, Höhne W, Schliess F, Häussinger D, Koch HG. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med 2005; 353: 1926–33. [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, Yin YL. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009; 37: 153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newsholme P, Abdulkader F, Rebelato E, Romanatto T, Pinheiro CH, Vitzel KF, Silva EP, Bazotte RB, Procopio J, Curi R, Gorjao R, Pithon-Curi TC. Amino acids and diabetes: implications for endocrine, metabolic and immune function. Front Biosci 2011; 16: 315–39. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, Zhang T, Nissim I, Daikhin Y, Stokes D, Yudkoff M, Bennett MJ, Stanley CA, Matschinsky FM, Naji A. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J Biol Chem 2013; 288: 3938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou YQ, Wang L, Yi D, Wu G. N-acetylcysteine and intestinal health: a focus on mechanisms of its actions. Front Biosci 2015; 20: 872–91. [DOI] [PubMed] [Google Scholar]
- 55.Tsurugizawa T, Uneyama H, Torii K. Brain amino acid sensing. Diabetes Obes Metab 2014; 16: 41–8. [DOI] [PubMed] [Google Scholar]
- 56.Billard JM. D-Amino acids in brain neurotransmission and synaptic plasticity. Amino Acids 2012; 43: 1851–60. [DOI] [PubMed] [Google Scholar]
- 57.Ruzzo EK, Capo-Chichi JM, Ben-Zeev B, Chitayat D, Mao H, Pappas AL, Hitomi Y, Lu YF, Yao X, Hamdan FF, Pelak K, Reznik-Wolf H, Bar-Joseph I, Oz-Levi D, Lev D, Lerman-Sagie T, Leshinsky-Silver E, Anikster Y, Ben-Asher E, Olender T, Colleaux L, Décarie JC, Blaser S, Banwell B, Joshi RB, He XP, Patry L, Silver RJ, Dobrzeniecka S, Islam MS, Hasnat A, Samuels ME, Aryal DK, Rodriguiz RM, Jiang YH, Wetsel WC, McNamara JO, Rouleau GA, Silver DL, Lancet D, Pras E, Mitchell GA, Michaud JL, Goldstein DB. Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron 2013; 80: 429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 1995; 15: 133–59. [DOI] [PubMed] [Google Scholar]
- 59.Taylor L, Curthoys NP. Glutamine metabolism: role in acid-base balance. Biochem Mol Biol Educ 2004; 32: 291–304. [DOI] [PubMed] [Google Scholar]
- 60.Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet 2009; 18: R9–17. [DOI] [PubMed] [Google Scholar]
- 61.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 1998; 78: 247–306. [DOI] [PubMed] [Google Scholar]
- 62.Marliss EB, Aoki TT, Pozefsky T, Most AS, Cahill GF., Jr Muscle and splanchnic glutamine and glutamate metabolism in postabsorptive and starved man. J Clin Invest 1971; 50: 814–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu G, Meininger CJ, Knabe DA, Bazer FW, Rhoads JM. Arginine nutrition in development, health and disease. Curr Opin Clin Nutr Metab Care 2000; 3: 59–66. [DOI] [PubMed] [Google Scholar]
- 64.Guedes RLM, Prosdocimi F, Fernandes GR, Moura LK, Ribeiro HAL, Ortega JM. Amino acids biosynthesis and nitrogen assimilation pathways: a great genomic deletion during eukaryote evolution. BMC Genomics 2011; 12: S2–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holt LE, Jr, Albanese AA. Observations on amino acid deficiencies in man. Trans Assoc Am Physicians 1944; 58: 143–56. [Google Scholar]
- 66.Meister A. Biochemistry of amino acids, New York: Academic Press, 1965. [Google Scholar]
- 67.Tanimura J. Studies on arginine in human semen. Part II. The effects of medication with L-arginine-HCl on male infertility. Bull Osaka Med School 1967; 13: 84–9. [PubMed] [Google Scholar]
- 68.Meléndez-Hevia E, De Paz-Lugo P, Cornish-Bowden A, Cárdenas ML. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci 2009; 34: 853–72. [DOI] [PubMed] [Google Scholar]
- 69.Sevastiadou S, Malamitsi-Puchner A, Costalos C, Skouroliakou M, Briana DD, Antsaklis A, Roma-Giannikou E. The impact of oral glutamine supplementation on the intestinal permeability and incidence of necrotizing enterocolitis/septicemia in premature neonates. J Matern Fetal Neonatal Med 2011; 24: 1294–300. [DOI] [PubMed] [Google Scholar]
- 70.Rogers QR, Chen DM, Harper AE. The importance of dispensable amino acids for maximal growth in the rat. Proc Soc Exp Biol Med 1970; 134: 517–22. [DOI] [PubMed] [Google Scholar]
- 71.Breuer LH, Jr, Pond WG, Warner RG, Loosli JK. The role of dispensable amino acids in the nutrition of the rat. J Nutr 1964; 82: 499–506. [DOI] [PubMed] [Google Scholar]
- 72.Womack M. Amino acid feeding studies: effects of various nonessential nitrogen sources and of added water. Proc Soc Exp Biol Med 1969; 131: 977–9. [DOI] [PubMed] [Google Scholar]
- 73.Stucki WP, Harper AE. Importance of dispensable amino acids for normal growth of chicks. J Nutr 1961; 74: 377–83. [Google Scholar]
- 74.Maruyama K, Sunde ML, Harper AE. Is L-glutamic acid nutritionally a dispensable amino acid for the young chick? Poultry Sci 1976; 55: 45–60. [DOI] [PubMed] [Google Scholar]
- 75.Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. Important roles for L-glutamine in swine nutrition and production. J Anim Sci 2011; 89: 2017–30. [DOI] [PubMed] [Google Scholar]
- 76.Lei J, Feng DY, Zhang YL, Zhao FQ, Wu ZL, San Gabriel A, Fujishima Y, Uneyama H, Wu G. Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 2012; 17: 2725–39. [DOI] [PubMed] [Google Scholar]
- 77.Deng D, Yin YL, Chu WY, Yao K, Li TJ, Huang RL, Liu ZQ, Zhang JS, Wu G. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem 2009; 20: 544–52. [DOI] [PubMed] [Google Scholar]
- 78.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Nutr Metab Care 2009; 12: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 2014; 5: 34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li XL, Rezaei R, Li P, Wu G. Composition of amino acids in feed ingredients for animal diets. Amino Acids 2011; 40: 1159–68. [DOI] [PubMed] [Google Scholar]
- 81.Dai ZL, Wu ZL, Jia SC, Wu G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B 2014; 964: 116–27. [DOI] [PubMed] [Google Scholar]
- 82.Hou Y, Wang L, Zhang W, Yang ZG, Ding BY, Zhu HL, Liu YL, Qiu YS, Yin YL, Wu G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012; 43: 1233–42. [DOI] [PubMed] [Google Scholar]