Abstract
The aging population and the incidence of aging-related diseases such as osteoporosis are on the rise. Aging at the tissue and organ levels usually involves tissue stem cells. Human and animal model studies indicate that aging affects two aspects of mesenchymal stem cell (MSC): a decrease in the bone marrow MSC pool and biased differentiation into adipocyte at the cost of osteoblast, which underlie the etiology of osteoporosis. Aging of MSC cells is also detrimental to some non-skeletal tissues, in particular the hematopoietic system, where MSCs serve as a niche component. In addition, aging compromises the therapeutic potentials of MSC cells, including cells isolated from aged individuals or cells cultured for many passages. Here we discuss the recent progress on our understanding of MSC aging, with a focus on the effects of MSC aging on bone remodeling and hematopoiesis and the mechanisms of MSC aging.
Keywords: Mesenchymal stem cells, hematopoietic stem cell, niche, aging, biased differentiation, bone remodeling, osteoporosis
Introduction
Mesenchymal stem cells
Mesenchymal stem cells (MSCs; also known as marrow stromal cells or mesenchymal stromal cells) are mesoderm-derived multipotent cells capable of adhering to culture dishes, proliferating in vitro, and differentiating into osteoblast, chondrocyte, and adipocyte (Figure 1). They may express surface markers such as CD105, CD90, and CD73, but not CD34, CD45, CD19, or CD14. MSCs were first isolated by Friedenstein and colleagues from the bone marrow and these cells form colonies in ex vivo cultures.1,2 Later, MSCs were also isolated from fat, dental pulp, amniotic fluid, umbilical cord blood, placenta, and Wharton’s jelly.3 Mounting evidence shows that MSCs, unlike hematopoietic stem cells (HSCs), are heterogeneous populations with different origins and functions. To date, there is a lack of definite cell surface markers specific to these MSC subpopulations. Lineage tracing studies have implicated that Prx1, Dermo-1, Nestin, Gremlin 1 (a BMP antagonist), and PDGFRα can label bone marrow MSCs.
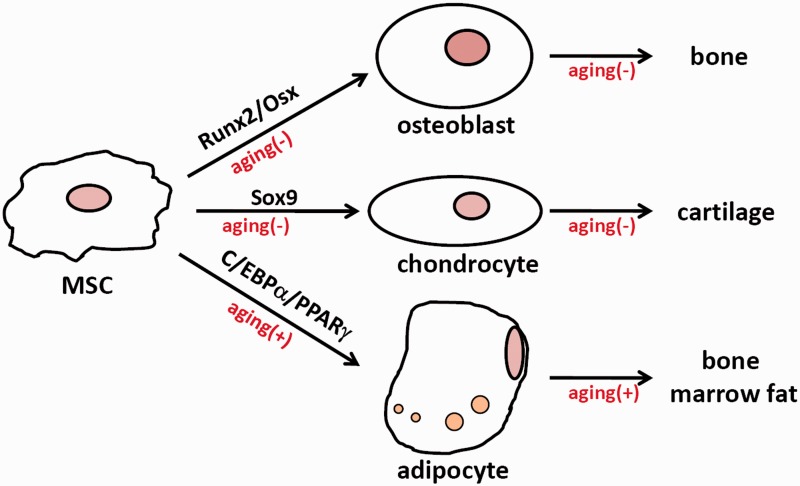
Figure 1.
The effects of aging on MSC differentiation. (+) means positive effect; (−) means negative effect. (A color version of this figure is available in the online journal.)
MSCs are tissue stem cells for the skeleton
MSCs are tissue stem cells for the skeleton, which is composed of bones and cartilages (Figure 1). MSCs, in response to growth factors such as bone morphogenetic proteins (BMPs) and Wnt molecules, express osteogenic transcription factors Runx2 and Osterix (Osx) and differentiate into bone-forming osteoblasts.4 MSCs can be induced to express Sox9 and differentiate into cartilage-forming chondrocytes.5 MSC can also be induced to express CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and differentiate into adipocytes, which form the bone marrow fat. It has also been reported that MSCs can differentiate into other cell types such as myoblast and glia under certain conditions.6
In addition to the bone-forming osteoblasts, the bone also contains bone-resorbing osteoclasts, which are derived from the HSC-monocyte lineage.7 HSCs are bone marrow resident multipotent stem cells capable of generating all the cell types of blood. HSC-derived monocytes can give rise to macrophage and granulocyte in addition to osteoclast. Osteoclasts are giant cells with numerous nuclei, which can secrete proteases to degrade bone matrix proteins such as collagen. Furthermore, osteoclasts work in synergy with osteoblasts through complicated coupling mechanisms.8 For example, MSCs and osteoblasts secrete M-CSF, RANKL, and OPG to regulate osteoclastogenesis, whereas monocytes and osteoclasts secrete a number of growth factors and cytokines to regulate osteoblastogenesis.9 Osteoblast-mediated bone formation and osteoclast-mediated bone resorption constitute the two pans of a balance. In the growth periods, bone formation outpaces bone resorption, leading to a net gain of bone mass. Afterwards, bone formation and resorption are balanced and bone mass and density remain stable until pre-menopause/andropause, when bone resorption outpaces formation and bone mass and density start to decrease, eventually leading to the development of osteoporosis. Post-menopausal osteoporosis is mainly caused by increased bone resorption due to estrogen shortage. Senile osteoporosis is mainly caused by declined bone formation, which is due to a decrease in the number of bone marrow MSCs and skewed MSC differentiation into adipocytes at the cost of osteoblasts.10 Osteoporosis is a common disease that affects 2.8 million men and 9.1 million women in the USA alone.11
The non-skeletal functions of MSCs
In addition to the critical roles played by MSCs in skeletal development and bone remodeling, recent studies have discovered non-skeletal functions for MSCs. In particular, exogenous MSC cells are able to home to damaged tissues, where they assist repairing.2,12 MSCs are isolated from bone marrow, fat, umbilical cord blood, or other sources, expanded in vitro, and then administrated locally or systematically to treat graft versus host reaction, autoimmune diseases, osteoarthritis, heart infarction, stroke, etc. MSCs exert their repairing functions by secreting growth factors, regulating immune responses, or transdifferentiating into the cell types required for tissue repair. Moreover, it has been reported that transplanting young MSC cells into the bone marrow of aged mice not only rescues bone loss,13 but also delays aging.14 Even subcutaneous implantation of MSC cells suppresses aging-related degeneration of various organs.15 These findings suggest that MSCs might be either integrated into the aging tissues or secrete microenvironmental/systematic anti-aging factors, e.g. GDF11, to rejuvenate the aged tissues.
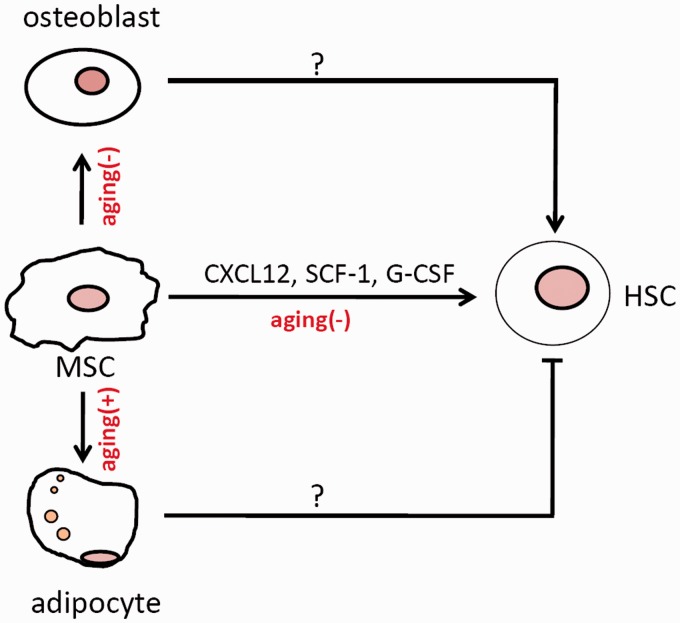
The best-studied non-skeletal function of MSCs is its niche activity for bone marrow HSCs (Figure 2). Although a substantial number of blood cells are generated from human HSCs every day, including red blood cells, lymphocyte cells, and myeloid cells, HSCs are believed to be in a relatively quiescent state, dividing once every 2–4 weeks in mouse.16 Mobilization, self-renewal, and multi-lineage differentiations of HSCs are orchestrated by their niches: the endosteal niche and the vascular niche, both of which involve MSCs or their progenies.17,18 The endosteal niche is mainly composed of stromal cells, osteoprogenitors, and osteoblasts. These stromal cells can be labeled by NG2 or Prx1, whereas osteoprogenitors and osteoblasts can be labeled by osterix or osteocalcin.19–21 The vascular niche is composed of vascular endothelial cells and stromal cells, with the latter being a heterogeneous population including cells positive for Nestin or Leptin receptor (Lepr) and CXCL12 (stromal cell-derived factor 1 (SDF-1)) abundant reticular (CAR) cells. Some of the cells such as Lepr+ cells and NG2+ cells have been proposed to be pericytes.19,22 Many of these cells can adhere to plastic surfaces, proliferate, and differentiate into the three lineages, and are thus referred to as MSCs. These cells execute their niche functions by secreting stem cell factor 1 (SCF-1, a c-Kit ligand) and CXCL12, which are essential for the maintenance of HSCs and hematopoiesis (Figure 2).
Figure 2.
MSCs and MSC-derived osteoblasts and adipocytes affect HSC self-renewal and differentiation. (+) means positive effect; (−) means negative effect. (A color version of this figure is available in the online journal.)
Recent studies suggest that the vascular niche, which is composed of endothelial cells and MSC cells, plays a more important role than the endosteal niche in hematopoiesis. Most HSC cells are in contact with Nestin+ cells or CAR cells,18,23,24 and they, together with Lepr+ cells, secrete CXCL12 to regulate HSC retention and maintenance.25 Ablation of SCF-1 or CXCL12 from these cells or depletion of these cells with the inducible diphtheria toxin system reduces the number of bone marrow HSCs and lessens the regenerative activity of HSCs. On the other hand, ablation of SCF-1 or CXCL12 from osteoblasts or depletion of osteoblasts displayed a minimal effect on HSCs.26,27 Yet, there is evidence that the endosteal niche may regulate HSC differentiation to restricted lineages such as B cells.20
Therefore, MSCs and their progenies, such as osteoprogenitors and osteoblasts, act as important niche components for bone marrow HSCs. They regulate HSC homeostasis and regeneration by synthesizing and secreting signaling molecules such as SCF-1, CXCL12, and angiopoietin-1. Besides, MSCs may modulate hematopoiesis via its progeny adipocytes.28 Alteration in the number of adipocytes under certain conditions may disrupt the HSC niche anatomy or alter the synthesis and secretion of signaling molecules required for HSC turnover.20,28
Aging of MSCs
Tissue/organ aging is associated with functional deterioration and diminished regenerative capacity, both of which are generally believed to involve tissue stem cells. In some tissues, aging is accompanied by a decrease in the number of stem cells, due to compromised renewal, cell senescence, apoptosis, or premature differentiation. In other tissues, aging is accompanied by skewed differentiation. For example, aged HSC cells tend to differentiate into myeloid cells rather than lymphocytes. To date, little is known about what triggers the aging of various tissue stem cells.
Although there are several studies suggesting that aged MSC cells may not differ much from young MSCs,29,30 a large body of evidence shows that bone marrow MSCs do age in elderly human and model animals, premature aging patients and mouse models, and in vitro cultures.31,32 MSC aging is reflected by a decrease in the number of bone marrow MSCs, which is assayed by colony forming unit-fibroblast (CFU-F), and biased differentiation to adipocytes at the cost of osteoblasts. The increase in bone marrow fat gives the bone marrow a yellowish look in aged model animals and human beings.32–34 As such, aging compromises the yield of functional osteoblasts and osteocytes, leading to a decrease in bone formation and the development of osteoporosis (Figure 3). In mice, aging also leads to a defect in the healing of bone fractures due to a decrease in chondrogenesis, bone formation, and vascularization, a process that requires Cox2-controlled production of prostaglandin E2. Interestingly, Cox2 expression was found to be reduced in aged mice.35
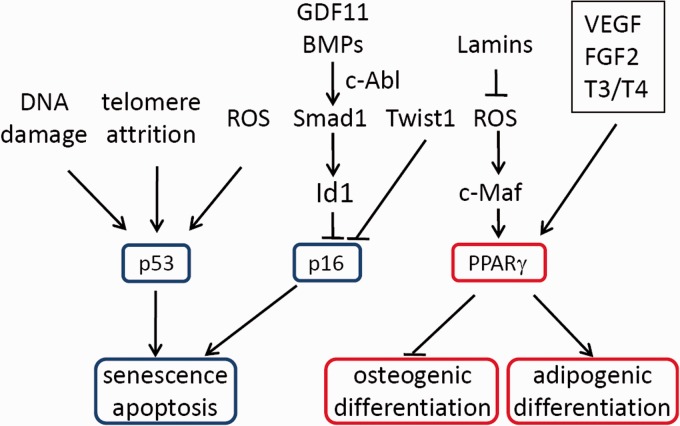
Figure 3.
Extrinsic and intrinsic factors that regulate MSC aging. p53 and p16 control MSC senescence and apoptosis, and PPARγ regulates the biased differentiation. (A color version of this figure is available in the online journal.)
Osteogenic differentiation and adipogenic differentiation from MSCs are two competitive processes. MSC aging promotes adipogenesis at the cost of osteogenesis. This results in an accumulation of bone marrow fat. Bone marrow fat accounts for 7% of the total fat in adults. Although it is not directly correlated to body mass index or body fat,30,36 bone marrow fat is inversely related to bone volume/mass.37,38 Bone marrow fat content can be regulated by estrogen, transforming growth factor β (TGFβ), and insulin-like growth factor 1 (IGF1).39,40 In aged animals or humans, enhanced adipogenic differentiation may further inhibit osteogenic differentiation by secreting adiponectin and releasing free fatty acids.36
Bone marrow or fat-derived MSC cells need to be extensively expanded in order to obtain sufficient cells for clinical use. It has been reported that cultured human and murine MSC cells undergo replicative senescence, which is characterized by cell enlargement, increased transparency, increased SA-β-Gal activity, and cell cycle arrest. Aging has been shown to compromise the therapeutic activity of MSCs.41 For example, aged MSC cells fail to alleviate autoimmune phenotypes in mouse models, a phenomenon that is likely due to reduced migration ability and reduced expression of anti-inflammatory cytokines.42,43 Aged MSCs also show reduced ability to repair bone fracture and decreased response to PTH(1-34) treatment.44 Furthermore, aging makes MSC cells more susceptible to stress-induced senescence.45 Finally, aged MSCs tend to transform and form fibrosarcoma.46 Thus, it is essential to maintain the stemness of MSCs and prevent MSCs from aging during in vitro expansion.
Aging mechanisms of MSCs
Like many other tissue stem cells, MSC aging is caused by intrinsic and/or extrinsic factors.47 Intrinsically, accumulation of DNA damage, reactive oxygen species, and damaged proteins may promote aging.3 In vitro cell senescence can be caused by telomere attrition, oxidative stress, genotoxic stress, and oncogene activation, all of which activate the DNA damage response Atm/Atr-p53 and Atm/Atr-Chk2/Chk1 pathways. Activation of these pathways induce cell cycle arrest or cell senescence by increasing the expression of cyclin-dependent kinase (CDK) inhibitor p21 and by inhibiting CDK activity through CDC25-mediated dephosphorylation, and cell death by inducing the expression of Bax and Puma.48,49 Ablation of p53 leads to spontaneous cell immortalization and promotes transformation.50
However, what triggers stem cell aging in vivo remains unclear. Systematic growth factors including Wnt molecules and GDF11 have been found to decline with aging.51,52 The microenvironment for MSC cells may also change. For example, the composition of niche cell types and the growth factors/cytokines that are secreted by the niche cells may change with aging. In particular, an increase in bone marrow adipocytes may disrupt the niche structure for HSCs.
Telomere attrition and aging
Most of the primary cells have a finite lifespan. Once their replication capacity is exhausted, cells will undergo replicative senescence. Telomere shortening is believed to be a major cause of replicative senescence in human cells due to their short telomere length. Telomere attrition is sensed by the cells as a type of DNA damage, which activates the DNA damage response and up-regulates p53 and p21, leading to cell cycle arrest, cell senescence, and/or apoptosis (Figure 3). Comparison of the telomere length did not reveal a significant difference between human MSC cells isolated from the young and aged individuals.53 Yet, enforced telomere dysfunction caused by ablation of Terc, an essential component of the telomerase complex, leads to reduced Runx2 expression, compromised osteogenic differentiation, and increased expression of p53 and p16.54 These results suggest that telomere shortening can indeed induce MSC aging, yet human MSC cells manage to maintain their telomere integrity during aging. This implies that mechanisms other than telomere attrition must be involved in MSC aging. Another explanation is that MSCs are not fast turnover cells, so that telomere erosion may occur at a rather slow pace.
DNA damage/DNA damage response and aging
Cell senescence can also be induced in young cells by stress, especially DNA damage and oxidative stress, which mainly activates the p53 pathway to cause cell cycle arrest and senescence via its target gene p21 (Figure 3).55 Indeed, a study showed that knockdown of p21 was able to increase proliferation and osteogenic differentiation of aged MSCs.56 Consistent with this notion, aged MSC cells usually show an increase in DNA damage and reactive oxygen species (ROS).57,58
Moreover, mutations in genes involved in DNA damage response and DNA repair have been found to cause premature aging in human and mouse models. Trichothiodystrophy, a premature aging model, is caused by mutations in TTD, a component of the NER repair protein complex. These patients show a decrease in MSC pool and in bone nodule formation rate.59 Werner syndrome, another premature aging model, is caused by mutations in the WRN gene, which encodes a DNA helicase essential for DNA repair and replication. Wrn−/− mice also showed premature aging phenotypes including osteoporosis, which is caused by decreased bone formation. MSC cells isolated from Wrn−/− mice showed a reduced lifespan and an osteogenic differentiation defect.54 Wrn deficiency-induced MSC aging can be rescued by expression of telomerase or deletion of p53.60
In addition, ablation of Atm or Atr, two Ser/Thr kinases thought to be the sensor of DNA damage, also leads to premature aging and osteoporosis.48,61 MSC cells deficient in Atm showed defective osteogenic differentiation and premature senescence.62,63 The osteogenic differentiation defect could be caused by compromised BMP-Smad1 signaling, and premature senescence could be caused by increased p21 expression.64
On the other hand, ablation of p53 leads to the development of osteosclerosis in mice, which also rescues the osteoporotic phenotype of mice deficient for Mdm2, a negative regulator of p53.65,66 p53−/− MSCs can be passaged continuously and are spontaneously immortalized. Moreover, p53−/− MSCs or osteoprogenitors showed an accelerated osteogenic differentiation. These results suggest that p53 plays a role in the aging of MSCs.65,67 The accelerated differentiation was due to increased expression of Smad1 and enhanced activation of the BMP-Samd1 pathway.64
p16 and aging
Another CDK inhibitor, p16, has been reported to accumulate in aged tissue stem cells and contribute to aging of stem cells and the tissues. Aging of these stem cells and tissues can be reversed by ablation of p16. Human MSC aging is associated with an increase in p16 expression, an increase in secretion of IL8, IL2, GRO, and MDC, and altered morphological alteration via CK2.68 MSC cells isolated from old mice also showed an increase in the expression of p16.69,70 Moreover, p16−/− mice showed an increase in the number of MSC cells at advanced age compared to control mice. These findings suggest that p16 plays an important role in the aging of murine MSCs.70
What controls the expression of p16 is still unclear. Cakouros et al. reported that transcription factor Twist1 suppresses the expression of E47, a transcription activator of p16, and promotes the expression of EZH2, a suppressor of p16 expression.71 As a consequence, Twist1 extends the lifespan of MSC cells by down-regulating p16.71 Our studies show that c-Abl, a proto-oncogene for BCR-ABL, could modulate the canonical and non-canonical signaling pathways of BMPs to regulate p16 expression and MSC senescence.70 BMPs are abundant in the bone and are critical in bone development, bone remodeling, and bone repair. We found that c-Abl deficiency led to a reduced number of bone marrow MSCs, compromised osteogenic differentiation, premature senescence of osteoprogenitor cells, and increased sensitivity to oxidative stress. The premature senescence phenotype is associated with an increase in p16 but not p53 or p21.70 Ablation of p16 in c-Abl−/− mice rescued MSC premature aging phenotypes and bone formation defects.70
Lamin, Sirt proteins, and aging
Lamins are intermediate filament proteins that form the nuclear lamina underneath the nuclear envelope. Mutations in Lamin genes in humans cause Hutchinson-Gilford Progeria syndrome (HGPS), with clinical symptoms including muscular dystrophy, lipodystrophy, premature aging, and osteoporosis, all of which are recapitulated in Lamin deficient mouse models.72,73 Knockdown of Lamin A/C in human MSCs led to biased differentiation into adipocyte while sacrificing osteoblast and chondrocyte.74,75 Ablation of ZMPSTE24, an enzyme required for Lamin maturation, causes aging and osteoporosis. ZMPSTE24 deficient osteoblasts showed an increase in PPARγ and a decrease in Runx2.76 ZMPSTE24 can be regulated by miRNA-141-3p, which increases the levels of pre-Lamin A and induces MSC senescence.77
The skewed differentiation of aged MSCs could also be related to lower Sirt1 expression.78 Sirt1 is a NAD-dependent deacetylase and an important anti-aging protein that can be activated by Lamin A and resveratrol. MSC-specific deletion of Sirt1 led to a defect in osteogenic differentiation due to lack of deacetylation and nuclear exit of β-Catenin.79 In addition, miR-486-5p can down-regulate Sirt1 expression and induce MSC aging.80
Niche/systematic factors and aging
Like other tissue stem cells, MSCs reside in a microenvironment called the niche, which is composed of various types of cells, extracellular matrix (ECM), and growth factors/cytokines secreted by the niche cells. ECM is found to play a role in the maintenance of MSCs. It was reported that young ECM can rejuvenate aged MSCs, likely via inhibiting production of ROS.81 In addition, senescent MSCs secrete increased levels of IGFBP4 and 7, which may further enhance aging of the tissue.82 Another study showed that follistain, an inhibitor of TGFβ family members such as BMPs and Activins, is greatly increased in human MSC cells isolated from aged donors,83 while GDF11, a member of the TGFβ family and an important anti-aging molecule,84,85 is decreased in the serum of aged mice, which mediates decreased MSC proliferation and osteogenesis. GDF11 may promote MSC osteogenic differentiation by inhibiting PPARγ expression.51 In addition, deletion of FGF2 or thyroid hormone receptors also increases MSC adipogenic differentiation but decreases osteogenic differentiation, suggesting that FGF2 and thyroid hormone (T3 and T4) are also involved in regulating the balance of MSC osteogenic and adipogenic differentiation.86,87
Transcription factor PPARγ and aging
The crucial factor that underlies the skewed differentiation of MSC seems to be PPARγ (Figure 3). The levels of PPARγ2, a transcription factor required for adipogenesis, are increased with aging.88 PPARγ2 may also inhibit osteogenesis by suppressing the expression of BMP2 and BMP4.88 Inhibition of PPARγ is reported to increase osteoblastogenesis and bone mass.89 A recent study suggests that PPARγ may inhibit osteogenesis with a novel mechanism. It has been reported that PPARγ similarly increases osteoblastogenesis and adipogenesis, yet it promotes apoptosis of osteoblasts but not adipocytes.90
One important regulator of PPARγ expression is c-Maf, a large Maf family member that contains the bZIP domain. c-Maf is down-regulated during MSC aging and in response to ROS. Down-regulation of c-Maf compromises osteoblast differentiation as c-Maf partners with Runx2 to regulate osteoblast-specific genes. c-Raf down-regulation also leads to an increase in PPARγ expression and adipogenesis. Antioxidants reverse the adipogenic phenotype by increasing c-Raf expression, which leads to increased bone formation.91 These results suggest that the accumulation of ROS may cause skewed MSC differentiation via the c-Maf-PPARγ pathway.
Another related finding is that autocrine vascular endothelial growth factor (VEGF) controls MSC osteogenic and adipogenic differentiation by regulating transcription factors Runx2 and PPARγ2 (Figure 3). Ablation of VEGF in osteoprogenitors led to development of osteoporosis-like phenotypes and an increase in bone marrow fat. Reduced VEGF expression in MSC favors adipogenesis at the cost of osteogenesis. More importantly, VEGF expression is affected by Lamin A/C.92 These results suggest that Lamin A/C may regulate MSC aging by controlling the VEGF-PPARγ pathway.
mTOR pathway and MSC aging
Inhibition of mechanistic target of rapamycin (mTOR) by rapamycin and caloric restriction are the only two strategies available to extend lifespan in higher organisms and delay the onset and progression of aging-related diseases.90,91,93,94 HSC aging is accompanied by an increase in mTOR activation, which could reactivate quiescent HSC. Forced mTOR activation also led to p16 up-regulation and increased levels of ROS, which appears to be responsible for the defect in HSC self-renewal.95
It has been reported that treatment with mTOR inhibitor rapamycin delays MSC aging and corrects the skewed MSC differentiation. The proposed mechanism is that inhibition of mTOR could up-regulate Oct4 and reduce DNA damage.96 In addition, a recent study shows that HMGA2 regulates human MSC aging by controlling activation of mTOR signaling.97
MSC aging and non-skeletal tissues
MSC aging not only affects osteogenic differentiation and bone formation, but also has an influence on hematopoiesis, as MSCs are important niche components of HSCs (Figure 2). Telomere shortening in Terc−/− mice impedes B lineage differentiation but promotes myeloid differentiation. It seems that the MSC niche also plays a role, as Terc−/− MSC cells showed decreased ability to maintain HSC. This is due to altered expression of cytokines, especially G-CSF. Inhibition of G-CSF could partially rescue HSC engraftment in aged Terc−/− mice.98 Another study showed that aged MSCs deficient for SHIP1 stimulate HSC expansion as well as myeloid differentiation, which might involve G-CSF secretion as well.99 In addition, increased bone marrow adipocytes, due to MSC aging, impair HSC maintenance in addition to negatively regulating MSC osteogenic differentiation.28
Conclusions and perspectives
Aging is associated with a decrease in the MSC pool and biased MSC differentiation. As a result, there is a decrease in the supply of osteoblast progenitors and osteoblasts, and this eventually results in a decrease in bone formation and the development of osteoporosis. On the other hand, an increase in adipogenic differentiation may cause accumulation of bone marrow fat, which may show lipotoxic effects on MSCs and HSCs. Yet the molecular mechanisms by which MSC cells age remain elusive. Future studies may identify the extrinsic factors, including the systematic factors and the niche molecules, and the intrinsic factors, including the sensors of environmental cues, the signaling pathways, and the effectors molecules, that control the aging process of MSCs. Only after we have connected the dots and established the network that controls MSC aging we can develop strategies to prevent and/or delay MSC aging. In particular, caloric restriction, inhibition of the mTOR pathway, and administration of active vitamin D3 may be considered as interventions for healthy aging of MSCs.
Acknowledgment
We would like to apologize to the researchers whose work cannot be cited due to space limit. The work in the authors’ laboratory is supported by grants from the National Key Scientific Program (2012CB966901 and 2014CB942900), Natural Science Foundation of China (81130039 and 81421061), and Program of Shanghai Subject Chief Scientist (13XD1401900).
Author contributions
This work was conceived by HJL, XCX, and BJL. Literature search was conducted by HJL, XCX, and BJL. BJL contributed to the writing of the manuscript and HJL contributed to production of the figures.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976; 4: 267–74. [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell 2012; 10: 709–16. [DOI] [PubMed] [Google Scholar]
- 3.Yu KR, Kang KS. Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology 2013; 59: 557–63. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet 2008; 9: 183–96. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Zhu H, Liang C, Li W, Xing G, Ma L, Ding L, Zhang Y, He F, Zhang L. CKIP-1 suppresses the adipogenesis of mesenchymal stem cells by enhancing HDAC1-associated repression of C/EBPalpha. J Mol Cell Biol 2014; 6: 368–79. [DOI] [PubMed] [Google Scholar]
- 6.Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 2012; 8: 215–25. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol 2011; 7: 235–43. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen K, Karsdal MA, Martin TJ. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int 2014; 94: 88–97. [DOI] [PubMed] [Google Scholar]
- 9.Crane JL, Cao X. Function of matrix IGF-1 in coupling bone resorption and formation. J Mol Med (Berl) 2014; 92: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011; 377: 1276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res 2011; 469: 1900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajek A, Czerwinski M, Olkowska J, Gurtowska N, Kloskowski T, Drewa T. Does aging of mesenchymal stem cells limit their potential application in clinical practice? Aging Clin Exp Res 2012; 24: 404–11. [DOI] [PubMed] [Google Scholar]
- 13.Singh L, Brennan TA, Kim JH, Egan KP, Mcmillan EA, Chen QJ, Hankenson KD, Zhang Y, Emerson SG, Johnson FB, Pignolo RJ. Brief report: long-term functional engraftment of mesenchymal pogenitor cells in a mouse model of accelerated aging. Stem Cells 2013; 31: 607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen JH, Tsai YT, DiMarco NM, Long MA, Sun XK, Tang LP. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci Rep 2011; 1: 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaza T, Miura Y, Akiyama K, Bi YM, Sonoyama W, Gronthos S, Chen W, Le A, Shi S. Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood 2009; 113: 2595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 2008; 132: 631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 2013; 31: 285–316. [DOI] [PubMed] [Google Scholar]
- 18.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol 2014; 35: 32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrouahen BS, Al-Hijji I, Tabrizi AR. Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia. Stem Cells Int 2011:375857. [DOI] [PMC free article] [PubMed]
- 20.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505: 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013; 502: 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. All MSCs are pericytes? Cell Stem Cell 2008; 3: 229–30. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–88. [DOI] [PubMed] [Google Scholar]
- 24.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495: 227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495: 231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481: 457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol 2014; 10: 737–48. [DOI] [PubMed] [Google Scholar]
- 29.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology 2013; 14: 573–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res 2001; 16: 1120–9. [DOI] [PubMed] [Google Scholar]
- 31.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 1999; 14: 1115–22. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008; 7: 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou GD, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 2008; 9: 60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell 2006; 5: 213–24. [DOI] [PubMed] [Google Scholar]
- 35.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O'Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res 2009; 24: 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardouin P, Pansini V, Cortet B. Bone marrow fat. Joint Bone Spine 2014; 81: 313–9. [DOI] [PubMed] [Google Scholar]
- 37.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2006; 2: 35–43. [DOI] [PubMed] [Google Scholar]
- 38.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone–new perspectives. J Clin Endocrinol Metab 2013; 98: 935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 2009; 10: 747–55. [DOI] [PubMed] [Google Scholar]
- 40.Marie PJ. Bone cell senescence: mechanisms and perspectives. J Bone Miner Res 2014; 29: 1311–21. [DOI] [PubMed] [Google Scholar]
- 41.Duscher D, Rennert RC, Januszyk M, Anghel E, Maan ZN, Whittam AJ, Perez MG1, Kosaraju R, Hu MS, Walmsley GG, Atashroo D, Khong S, Butte AJ, Gurtner GC. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014; 4: 7144–7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scruggs BA, Semon JA, Zhang XJ, Zhang SJ, Bowles AC, Pandey AC, Imhof KM, Kalueff AV, Gimble JM, Bunnell BA. Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model. Stem Cells Transl Med 2013; 2: 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N, Mora AL, Rojas M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med 2014; 189: 787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yukata K, Xie C, Li TF, Takahata M, Hoak D, Kondabolu S, Zhang X, Awad HA, Schwarz EM, Beck CA, Jonason JH, O'Keefe RJ. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone 2014; 62: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009; 27: 1288–97. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, Rogers AB, Montrose M, Houghton J. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res 2007; 67: 10889–98. [DOI] [PubMed] [Google Scholar]
- 47.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006; 5: 91–116. [DOI] [PubMed] [Google Scholar]
- 48.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009; 461: 1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zannini L, Delia D, Buscemi G. CHK2 kinase in the DNA damage response and beyond. J Mol Cell Biol 2014; 6: 442–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol 2014; 6: 214–30. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Shao J, Wang Z, Yang T, Liu S, Liu Y, Fan X, Ye W. Growth differentiation factor 11 is a protective factor for osteoblastogenesis by targeting PPARgamma. Gene 2015; 557: 209–14. [DOI] [PubMed] [Google Scholar]
- 52.L'Episcopo F, Tirolo C, Caniglia S, Testa N, Morale MC, Serapide MF, Pluchino S, Marchetti B. Targeting Wnt signaling at the neuroimmune interface for dopaminergic neuroprotection/repair in Parkinson's disease. J Mol Cell Biol 2014; 6: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roura S, Farre J, Soler-Botija C, Llach A, Hove-Madsen L, Cairo JJ, Gòdia F, Cinca J, Bayes-Genis A. Effect of aging on the pluripotential capacity of human CD105(+) mesenchymal stem cells. Eur J Heart Fail 2006; 8: 555–63. [DOI] [PubMed] [Google Scholar]
- 54.Pignolo RJ, Suda RK, McMillan EA, Shen J, Lee SH, Choi Y, Wright AC, Johnson FB. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell 2008; 7: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M, Nakayama T, Nakamura T, Toguchida J. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 2007; 25: 2371–82. [DOI] [PubMed] [Google Scholar]
- 56.Yew TL, Chiu FY, Tsai CC, Chen HL, Lee WP, Chen YJ, Chang MC, Hung SC. Knockdown of p21(Cip1/Waf1) enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells. Aging Cell 2011; 10: 349–61. [DOI] [PubMed] [Google Scholar]
- 57.Almeida M, OBrien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci 2013; 68: 1197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 2006; 7: 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diderich KEM, Nicolaije C, Priemel M, Waarsing JH, Day JS, Brandt RMC, Schilling AF, Botter SM, Weinans H, van der Horst GT, Hoeijmakers JH, van Leeuwen JP. Bone fragility and decline in stem cells in prematurely aging DNA repair deficient trichothiodystrophy mice. Age 2012; 34: 845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung HH, Liu XZ, Canterel-Thouennon L, Li L, Edmonson C, Rennert OM. Telomerase protects Werner Syndrome lineage-specific stem cells from premature aging. Stem Cell Rep 2014; 2: 534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007; 1: 113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hishiya A, Ito M, Aburatani H, Motoyama N, Ikeda K, Watanabe K. Ataxia telangiectasia mutated (Atm) knockout mice as a model of osteopenia due to impaired bone formation. Bone 2005; 37: 497–503. [DOI] [PubMed] [Google Scholar]
- 63.Rasheed N, Wang X, Niu QT, Yeh J, Li B. Atm-deficient mice: an osteoporosis model with defective osteoblast differentiation and increased osteoclastogenesis. Hum Mol Genet 2006; 15: 1938–48. [DOI] [PubMed] [Google Scholar]
- 64.Ma G, Li L, Hu Y, Chau JF, Au BJ, Jia D, Liu H, Yeh J, He L, Hao A, Li B. Atypical Atm-p53 genetic interaction in osteogenesis is mediated by Smad1 signaling. J Mol Cell Biol 2012; 4: 118–20. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 2006; 172: 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zambetti GP, Horwitz EM, Schipani E. Skeletons in the p53 tumor suppressor closet: genetic evidence that p53 blocks bone differentiation and development. J Cell Biol 2006; 17: 795–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang HT, Chen QJ, Lee SH, Choi Y, Johnson FB, Pignolo RJ. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell 2012; 11: 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang DJ, Jang DJ. Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res 2009; 69: 8200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008; 129: 163–73. [DOI] [PubMed] [Google Scholar]
- 70.Kua HY, Liu H, Leong WF, Li L, Jia D, Ma G, Hu Y, Wang X, Chau JF, Chen YG, Mishina Y, Boast S, Yeh J, Xia L, Chen GQ, He L, Goff SP, Li B. c-Abl promotes osteoblast expansion by differentially regulating canonical and non-canonical BMP pathways and p16INK4a expression. Nat Cell Biol 2012; 14: 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cakouros D, Isenmann S, Cooper L, Zannettino A, Anderson P, Glackin C, Gronthos S. Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Mol Cell Biol 2012; 32: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang JQ, Lian QZ, Zhu GL, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, Stewart CL, Colman A. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 2011; 8: 31–45. [DOI] [PubMed] [Google Scholar]
- 73.Mounkes LC, Kozlov S, Hernandez L, Sullivan T, Stewart CL. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 2003; 423: 298–301. [DOI] [PubMed] [Google Scholar]
- 74.Mateos J, De la Fuente A, Lesende-Rodriguez I, Fernandez-Pernas P, Arufe MC, Blanco FJ. Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res 2013; 11: 1137–48. [DOI] [PubMed] [Google Scholar]
- 75.Akter R, Rivas D, Geneau G, Drissi H, Duque G. Effect of Lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res 2009; 24: 283–93. [DOI] [PubMed] [Google Scholar]
- 76.Rivas D, Li W, Akter R, Henderson JE, Duque G. Accelerated features of age-related bone loss in zmpste24 metalloproteinase-deficient mice. J Gerontol A Biol Sci Med Sci 2009; 64: 1015–24. [DOI] [PubMed] [Google Scholar]
- 77.Yu KR, Lee S, Jung JW, Hong IS, Kim HS, Seo Y, Shin TH, Kang KS. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J Cell Sci 2013; 126: 5422–31. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, He X, Li YL, He JX, Anderstam B, Andersson G, Lindgren U. Nicotinamide Phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: a possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J Bone Miner Res 2011; 26: 2656–64. [DOI] [PubMed] [Google Scholar]
- 79.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med 2013; 5: 430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim YJ, Hwang SH, Lee SY, Shin KK, Cho HH, Bae YC, Jung JS. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev 2012; 21: 1749–60. [DOI] [PubMed] [Google Scholar]
- 81.Sun Y, Li WP, Lu ZD, Chen R, Ling J, Ran QT, Jilka RL, Chen XD. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J 2011; 25: 1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 2013; 4: e991–e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alves H, van Ginkel J, Groen N, Hulsman M, Mentink A, Reinders M, van Blitterswijk C, de Boer J. A mesenchymal stromal cell gene signature for donor age. PLoS One 2012; 7: e42908–e42908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013; 153: 828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014; 344: 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kindblom JM, Gevers EF, Skrtic SM, Lindberg MK, Gothe S, Tornell J, Vennström B, Ohlsson C. Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone 2005; 36: 607–16. [DOI] [PubMed] [Google Scholar]
- 87.Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010; 47: 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004; 3: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol 2008; 20: 429–34. [DOI] [PubMed] [Google Scholar]
- 90.Bruedigam C, Eijken M, Koedam M, van de Peppel J, Drabek K, Chiba H, van Leeuwen JP. A New concept underlying stem cell lineage skewing that explains the detrimental effects of thiazolidinediones on bone. Stem Cells 2010; 28: 916–27. [DOI] [PubMed] [Google Scholar]
- 91.Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, Kodama T, Yamaguchi A, Owen MJ, Takahashi S, Takayanagi H. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest 2010; 120: 3455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 2012; 122: 3101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013; 493: 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460: 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 2008; 205: 2397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gharibi B, Farzadi S, Ghuman M, Hughes FJ. Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells 2014; 32: 2256–66. [DOI] [PubMed] [Google Scholar]
- 97.Yu KR, Park SB, Jung JW, Seo MS, Hong IS, Kim HS, Seo Y, Kang TW, Lee JY, Kurtz A, Kang KS. HMGA2 regulates the in vitro aging and proliferation of human umbilical cord blood-derived stromal cells through the mTOR/p70S6K signaling pathway. Stem Cell Res 2013; 10: 156–65. [DOI] [PubMed] [Google Scholar]
- 98.Ju ZY, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nature Med 2007; 13: 742–7. [DOI] [PubMed] [Google Scholar]
- 99.Iyer S, Brooks R, Gumbleton M, Kerr WG. SHIP1-expressing mesenchymal stem cell regulate hematopoietic stem cell homeostasis and lineage choice during aging. Stem Cells Dev 2015; 24: 1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]