Abstract
Spermatogonial stem cells (SSCs) can differentiate into spermatids, reflecting that they could be used in reproductive medicine for treating male infertility. SSCs are able to become embryonic stem-like cells with the potentials of differentiating into numerous cell types of the three germ layers and they can transdifferentiate to mature and functional cells of other lineages, highlighting significant applications of human SSCs for treating human diseases. However, human SSCs are very rare and a long-term culture system of human SSCs has not yet established. This aim of study was to isolate, identify and culture human SSCs for a long period. We isolated GPR125-positive spermatogonia with high purity and viability from adult human testicular tissues utilizing the two-step enzymatic digestion and magnetic-activated cell sorting with antibody against GPR125. These freshly isolated cells expressed a number of markers for SSCs, including GPR125, PLZF, GFRA1, RET, THY1, UCHL1 and MAGEA4, but not the hallmarks for spermatocytes and spermatozoa, e.g. SYCP1, SYCP3, PRM1, and TNP1. The isolated human SSCs could be cultured for two months with a significant increase of cell number with the defined medium containing growth factors and hydrogel. Notably, the expression of numerous SSC markers was maintained during the cultivation of human SSCs. Furthermore, SMAD3 and AKT phosphorylation was enhanced during the culture of human SSCs. Collectively, these results suggest that human SSCs can be cultivated for a long period and expanded whilst retaining an undifferentiated status via the activation of SMAD3 and AKT pathways. This study could provide sufficient cells of SSCs for their basic research and clinic applications in reproductive and regenerative medicine.
Keywords: Human spermatogonial stem cells, isolation and characterization, long-term culture, expansion, SMAD3 and AKT pathways
Introduction
Spermatogenesis is an elaborate process in mammalian testis where spermatogonial stem cells (SSCs) divide and differentiate into spermatozoa. Due to the growing incidence of cancer, the morbidity of infertility resulting from the chemotherapy and/or radiotherapy becomes a rising trend.1,2 Notably, 10–15% of couples are infertile in the world, and male factor affects 40–60% of infertility.3 Transplantation of human SSCs would bring a promising treatment approach for male infertility.4,5 It has been anticipated that SSCs obtained from the patients can be cryopreserved and transplanted into the testis of survivors after treatment to restore their fertility or induction by specific culture conditions in vitro to get normal gametes for assisted reproduction technology to own their own children. We have recently shown that SSCs from cryptorchid patients can differentiate into haploid spermatids with fertilization and developmental potential.6 It could be feasible that SSCs derived from infertile patients can be induced to differentiate to spermatozoa followed by intra-cytoplasmic sperm injection (ICSI), which makes great contribution to these patients who are keen to have their own children. Therefore, human SSCs can be used in reproductive medicine for treating male infertility.
Notably, numerous studies have recently demonstrated that SSCs can be reprogrammed without gene modification to become embryonic stem (ES)-like cells with the capability of differentiating into a number of cell lineages of three germ cell layers in rodents and human.7–11 Moreover, it has been shown that SSCs from neonatal mouse testes can transdifferentiate directly to various kinds of tissues, including prostatic, uterine, and skin epithelium12 and that rat SSCs transdifferentiate to functional dopaminergic neuron-like cells.13 We have recently shown that SSCs from mouse testes are able to directly transdifferentiate into morphological, phenotypic, and functional hepatocyte-like cells when they are cultured with several growth factors in vitro.14 These studies illustrate that human SSCs can be used to generate mature and functional cells for cell therapy and tissue engineering in regenerative medicine. In addition, SSCs represent a great model for examining mechanisms underlying stem cell self-renewal versus differentiation, since SSCs divide and differentiate into the other germ cells throughout mammalian whole life.
SSCs are very rare since SSCs represent only 0.03 percentage of male germ cells in the testis.15 As such, it is essential to enrich and expand SSCs in vitro from small biopsies to provide adequate cells for their basic studies and potential applications in reproductive and regenerative medicine. SSCs are localized on the basement membrane of seminiferous tubules and they are located in a specific microenvironment or niche. The niche comprises Sertoli cells, Leydig cells, myoid cells, a number of growth factors synthesized by Sertoli cells and other somatic cells, blood vessels, and basement membrane.16,17 In rodents, long-term culture of mouse SSCs has been established and SSCs are able to proliferate for over five months.18 In human, testicular cells obtained from prostate cancer patients can be cultured for around three months.19,20 Nevertheless, the starting cells they utilized were a mixture of various kinds of male germ cells and somatic cells.19,20 We have recently shown that GPR125 is a hallmark for human SSCs and they can be cultivated for two weeks.21 However, a long-term culture system of human SSCs has not yet established. A specific culture system is critical for the expansion of mouse SSCs in vitro, which requires several growth factors, e.g. glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and leukemia inhibitory factor (LIF),18,19,22,23 and thus these growth factors were chosen in this study to expand human SSCs. Furthermore, feeder cells, including mouse embryonic fibroblasts (MEFs), facilitate the attachment and proliferation of SSCs. However, it is tedious and time-consuming to prepare mitotic inactivation MEFs, and it may result in the infection of dangerous pathogens. Therefore, the feeder-free culture system should be developed for the propagation of SSCs. In the current study, we separated GPR125+ spermatogonia from adult human testicular tissues using a two-step enzymatic digestion and magnetic-activated cell sorting (MACS). The identity and purity of freshly separated cells were evaluated by numerous markers for SSCs. Furthermore, we established a defined culture system containing certain growth factors and hydrogel to expand human SSCs for a long period whilst maintaining them in an undifferentiated status. We also demonstrated that SMAD3 and AKT pathways were activated in the proliferation of human SSCs. Collectively, our data indicate that human SSCs can be expanded and cultured for a long period via the activation of SMAD3 and AKT signaling pathways.
Materials and methods
Procurement of testicular biopsies from patients with obstructive azoospermia (OA)
Testicular biopsies were obtained from 40 patients with obstructive azoospermia (OA) (age from 22 to 35 years old) who had microdissection and testicular sperm extraction (MD-TEST) at Ren Ji Hospital affiliated to Shanghai Jiao Tong University School of Medicine. All OA patients were resulted from inflammation and vasoligation rather than by congenital absence of the vas deferens or other diseases including cancer.
Ethics statement
This study was approved by the Institutional Ethical Review Committee of Ren Ji Hospital (license number of ethics statement: 2012-01), Shanghai Jiao Tong University School of Medicine, and an informed consent of testicular tissues for research only was obtained from all subjects. All experiments were carried out according to relevant guidelines and regulation of the Institutional Ethical Review Committee of Ren Ji Hospital.
Histological examination
Testicular biopsies from five patients with OA were fixed in Bouin’s fixative for 24 h, and they were embedded in paraffin and sectioned at 5 µm thickness. Testis sections were stained with hematoxylin and eosin (H&E), and they were observed by a microscope to assess spermatogenesis status of OA patients.
Isolation of human GPR125-positive spermatogonia from OA patients
Testicular tissues from 3 or 4 OA patients for each isolation were washed extensively in Dulbecco modified Eagle medium (DMEM) (Gibco) containing penicillin and streptomycin (Gibco). Human seminiferous tubules were separated from testicular tissues by the first enzymatic digestion using 2 mg/ml collagenase IV (Gibco) and 1 µg/µl DNase I (Gibco) in 34℃ water bath for 15 min pursuant to the procedure as described earlier.21 Male germ cells were isolated from seminiferous tubules using a second enzymatic digestion with 4 mg/ml collagenase IV, 2.5 mg/ml hyaluronidase (Sigma), 2 mg/ml trypsin (Sigma), and 1 µg/μl DNase I and followed by differential plating.21 With regard to differential plating, cell suspension was seeded into culture plates in DMEM/F-12 supplemented with 10% fetal bovine serum (FBS) (Gibco) and incubated at 34℃ in 5% CO2 overnight. While human Sertoli cells attached to the culture plates, male germ cells remained in suspension and were collected by centrifuging at 1000 rpm for 5 min. Human GPR125-positive spermatogonia were further separated by magnetic-activated cell sorting (MACS) using an antibody against GPR125 at a dilution of 1:40. The viability of the freshly isolated cells was examined by trypan blue exclusion. The separation of GPR125-positive cells was performed at least three times from different donor testes. The proliferation potentials of human GPR125-positive spermatogonia during culture were detected by cell counting using the cytometer.
Preparation of hydrogel-coated plates
The frozen hydrogel (Stem Easy) was thawed at 4℃ overnight and diluted to the working concentration using DMEM/F-12 in the ratio of 1:20. The culture plates were coated with hydrogel and incubated overnight in 5% CO2 incubator at 37℃. The excess hydrogel was removed before the cells were seeded.
Culture of human GPR125-positive spermatogonia
Human GPR125+ spermatogonia were seeded in 96- or 24-cell plates coated with hydrogel and cultured with the conditioned medium as mentioned below. The defined culture medium comprised StemPro-34 SFM (Invitrogen) with StemPro supplement (Invitrogen), B27 supplement minus vitamin A (Invitrogen), 6 mg/ml d-(+)-glucose (Sigma), 30 mg/ml pyruvic acid (Invitrogen), 1 μl/ml DL-lactic acid (Sigma), 2.5 mg/ml lipid-rich bovine serum albumin (BSA; Invitrogen), 1% FBS (Gibco), 2 mM L glutamine (Invitrogen), 50 μM β-mercaptoethanol (Invitrogen), 1 × minimal essential medium (MEM), non-essential amino acids (Invitrogen), 1 × MEM vitamins (Invitrogen), 50 ng/ml human GDNF (Sigma), 20 ng/ml human EGF (Sigma), 10 ng/ml human bFGF (Sigma), 10 ng/ml human leukemia inhibitory factor (LIF) (Sigma) and 1 × penicillin/streptomycin (Gibco).
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the freshly separated and cultured human GPR125-positive spermatogonia and human testicular cells using Trizol (Invitrogen), and the quality and concentrations of total RNA were assessed by Nanodrop. Reverse transcription (RT) was carried out using the First Strand cDNA Synthesis Kit (Thermo Scientific), and PCR of the cDNA was conducted according to the protocol as described previously.24 The primer sequences of the chosen genes, including GPR125, GFRA1, RET, PLZF, UCHL1, MAGEA4, SYCP3, SYCP1, PRM1 (protamine 1), TNP1 (transition protein 1) and ACTB (β-actin) were designed and listed in Table 1. The PCR reaction started at 94℃ for 2 min and was performed using the follow conditions: denaturation at 94℃ for 30 s, annealing at 49–60℃ for 45 s as listed in Table 1, and elongation at 72℃ for 45 s; after 35 cycles, the PCR products were incubated for 5 min at 72℃. PCR products were separated by electrophoresis using 2% agarose gel, and they were visualized with ethidium bromide. Images were recorded and band intensities were analyzed using chemiluminescence (Chemi-Doc XRS, Bio-Rad). The expression of genes in human testicular cells was used as positive controls, whereas cDNA with PCR but without primers served as a negative control. The integrated density values (IDV) of target gene products were quantified relatively by comparing with the expression of housekeeping gene ACTB.
Table 1.
Primers of numerous genes used for RT-PCR
| Genes | Primer sequence | Product size (bp) | Tm (℃) | |
|---|---|---|---|---|
| GPR125 | Forward | TACCCTTTGGACTTGGTT | 193 | 49 |
| Reverse | CTCCATTCGTCCCATTAG | |||
| GFRA1 | Forward | CCAAAGGGAACAACTGCCTG | 121 | 50 |
| Reverse | CGGTTGCAGACATCGTTGGA | |||
| PLZF | Forward | CGGTTCCTGGATAGTTTGC | 317 | 50 |
| Reverse | GGGTGGTCGCCTGTATGT | |||
| RET | Forward | CTCGTTCATCGGGACTTG | 126 | 50 |
| Reverse | ACCCTGGCTCCTCTTCAC | |||
| UCHL1 | Forward | CCAATGTCGGGTAGATGA | 244 | 55 |
| Reverse | CAAAGTCCCTCCCACAGA | |||
| MAGEA4 | Forward | CCGAGTCCCTGAAGATG | 155 | 50 |
| Reverse | CAGGACGATTATCAGAAGG | |||
| SYCP1 | Forward | AACTACTGTCTGCAGCTTGG | 100 | 60 |
| Reverse | CATCTCTTCCAGCTCACTTGAT | |||
| SYCP3 | Forward | TGCAGGAGTAGTTGAAGATATG | 110 | 60 |
| Reverse | GCAAGAAGAGCCTTGTTAATG | |||
| PRM1 | Forward | ATAGCACATCCACCAAACTCC | 106 | 60 |
| Reverse | AGGCGGCATTGTTCCTTAG | |||
| TNP1 | Forward | CGACCAGCCGCAAATTAAAG | 105 | 60 |
| Reverse | CAGGTTGCCCTTACGGTATT | |||
| ACTB | Forward | CGCACCACTGGCATTGTCAT | 253 | 55 |
| Reverse | TTCTCCTTGATGTCACGCAC | |||
Immunocytochemistry
For immunocytochemical staining, human freshly separated and cultured GPR125-positive spermatogonia were fixed with 4% paraformaldehyde (PFA) for 30 min, washed extensively with cold phosphate-buffered saline (PBS) and permeabilized in 0.4% triton-X 100 (Sigma) for 5 min. After extensive washing with PBS, the cells were blocked in 2% BSA for 30 min and incubated with primary antibodies, including anti-GPR125 (Abcam), anti-GFRA1(Santa Cruz), anti-THY1 (Abcam), anti-PLZF (Abcam), anti-UCHL1 (AbD Serotec), anti-MAGEA4 (melanoma antigen family A, 4, a kind gift from Professor Giulio C. Spagnoli, University Hospital of Basel, Switzerland), and normal rabbit, mouse, or goat IgG at a 1:100 dilution overnight at 4℃. After extensive wash with PBS for 30 min, the cells were further incubated with the secondary antibody IgG (Sigma) conjugated with fluorescein isothiocyanate (FITC) or rhodamine at a 1:200 dilution for 1 h at room temperature. DAPI (4′,6-diamidino-2-phenylindole) was utilized to label cell nuclei, and the cells were observed for epifluorescence using a fluorescence microscope (Leica). For the expression of MAGEA4, peroxidase-conjugated goat anti-mouse IgG (Envision detection kit, DAKO) was used as the secondary antibody and examined by a light microscope. Replacement of primary antibodies with their respective isotype IgGs, including normal goat IgG and rabbit IgG, served as negative controls.
Double immunostaining was performed with the freshly isolated and cultured human GPR125-positive spermatogonia using antibodies against GPR125 and PLZF, GFRA1 and PLZF, PLZF and phos-SMAD3 (Cell Signaling), and PLZF and phos-AKT (Cell Signaling) pursuant to the method as described earlier.21 Double immunostaining was also carried out in human Sertoli cells utilizing antibodies against PLZF and phos-SMAD3 as well as PLZF and phos-AKT.
Meiotic Spread Assays
Meiotic spread assays were carried out to check whether there was contamination of spermatocytes in the freshly isolated human GPR125-positive spermatogonia according to the method described earlier.6 Human spermatocytes were isolated by STA-PUT using 2–4% BSA according to the method as described earlier.25 The isolated human GPR125-positive spermatogonia and spermatocytes were lysed by a hypotonic solution, and they were spread evenly over slides layered with 1% PFA and 0.15% Triton X-100. The slides were dried for 24 h at room temperature within a humid chamber. The cells were treated with 0.04% photoflo for 5 min and blocked with 4% goat serum. Immunostaining was performed in the cells using rabbit polyclonal to SYCP3 (Abcam) overnight at 37℃ in a humid chamber. Goat anti-rabbit Alexa Fluor 555 (Invitrogen) was utilized at 1:1000 dilution and incubated for 90 min at 37℃. DAPI was used to label the nuclei. Cells were washed extensively with PBS, and images were captured with a fluorescence microscope (Leica).
Western blots
The cultured human GPR125-positive spermatogonia at day 0 and day 28 were lysed with RIPA buffer (Santa Cruz) for 30 min on ice. Cell lysates were cleared by centrifugation at 12,000 g, and the concentrations of proteins were measured by BCA kit (Dingguo Company, China). Western blots were performed pursuant to the protocol as described earlier,26 and the chosen antibodies include phosphorylation (phos-) of AKT (Cell Signaling), phos-Smad3 (Cell Signaling), and ACTB. The blots were detected by chemiluminescence (Chemi-Doc XRS, Bio-Rad) compared to the signals of target proteins with that of housekeeper ACTB.
Results
Isolation and morphology features of human GPR125-positive spermatogonia
We first assess the spermatogenesis status of OA patients used in this study. Histological analyses of testicular tissues revealed that there were all types of male germ cells, including several subpopulation of spermatogonia, various kinds of primary and secondary spermatocytes, and round and elongated spermatids in the testes of OA patients (Figure 1a), indicating that these patients had normal spermatogenesis.
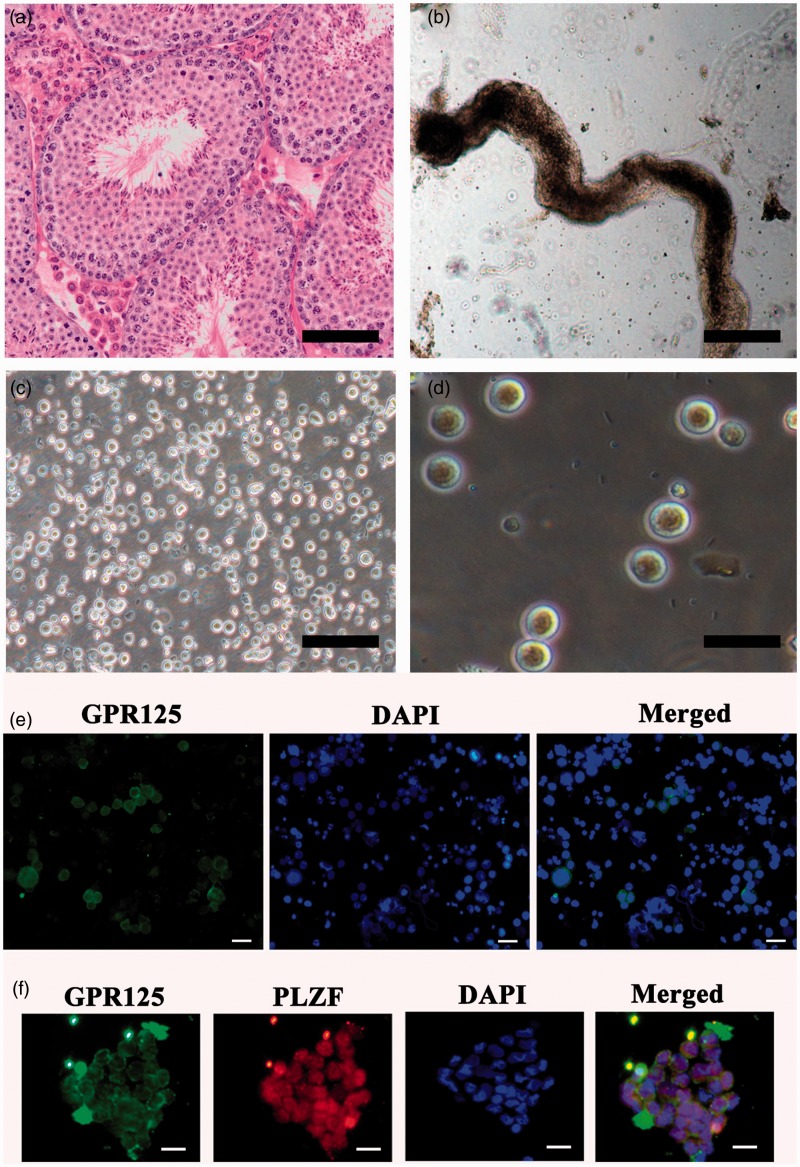
Figure 1.
Spermatogenesis status of OA patients and the isolation of human GPR125+ spermatogonia from OA patients. (a) H&E staining revealed the normal spermatogenesis status of testicular tissues from OA patients. (b) Human seminiferous tubules were obtained after the first enzymatic digestion with collagenase IV and DNase I. (c) Cell mixture of human male germ cells and Sertoli cells was isolated from seminiferous tubules using the second enzymatic digestion. (d) Human GPR125+ spermatogonia were obtained after MACS. Scale bars in A–C = 100 µm; scale bar in D = 20 µm. (e, f) Immunocytochemistry showed the expression of GPR125 in male germ cells before MACS (e) and co-expression of GPR125 and PLZF in human GPR125+spermatogonia by MACS (f). Scale bars in E–F = 20 µm. (A color version of this figure is available in the online journal.)
Human male germ cells were separated from OA patients using a two-step enzymatic digestion and differential plating as previously described.21 Briefly, human seminiferous tubules (Figure 1b) were obtained from the testis tissues using the first enzymatic digestion and extensive washes using PBS to remove potential contamination of myoid cells and Leydig cells. Human cell mixture containing male germ cells and Sertoli cells (Figure 1c) was obtained using a second enzymatic digestion. These cells were cultured with DMEM/F12 and 10% FBS overnight for differential plating. Human Sertoli cells attached to the plates, and cell suspension containing male germ cells were collected by centrifuging at 1000 rpm. Immunocytochemistry showed that a subpopulation of male germ cells was positive for GPR125 (Figure 1e). Human GPR125-positive spermatogonia (Figure 1d) could be obtained by MACS using an antibody against GPR125. The viability of freshly isolated GPR125-positive spermatogonia was over 98%, as assayed by trypan blue exclusion (data not shown). In morphological features, human GPR125-positive spermatogonia had large nuclei, a high ratio of nucleus to cytoplasm, and few organelles in their cytoplasm. To assess the purity of these isolated cells, double immunostaining using antibodies against GPR125 and PLZF displayed that more than 95% of the freshly isolated cells were positive for both GPR125 and PLZF (Figure 1f).
Phenotype of the freshly isolated human GPR125-positive spermatogonia
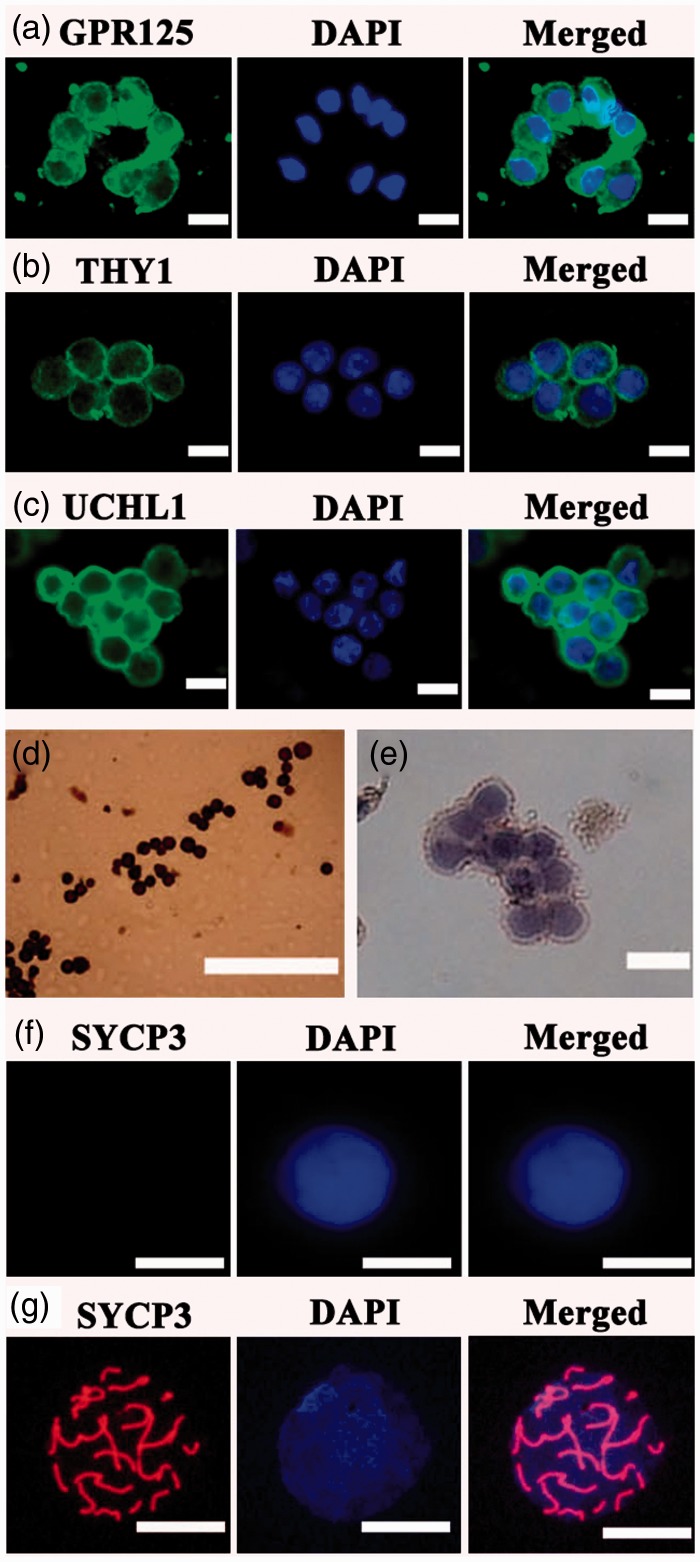
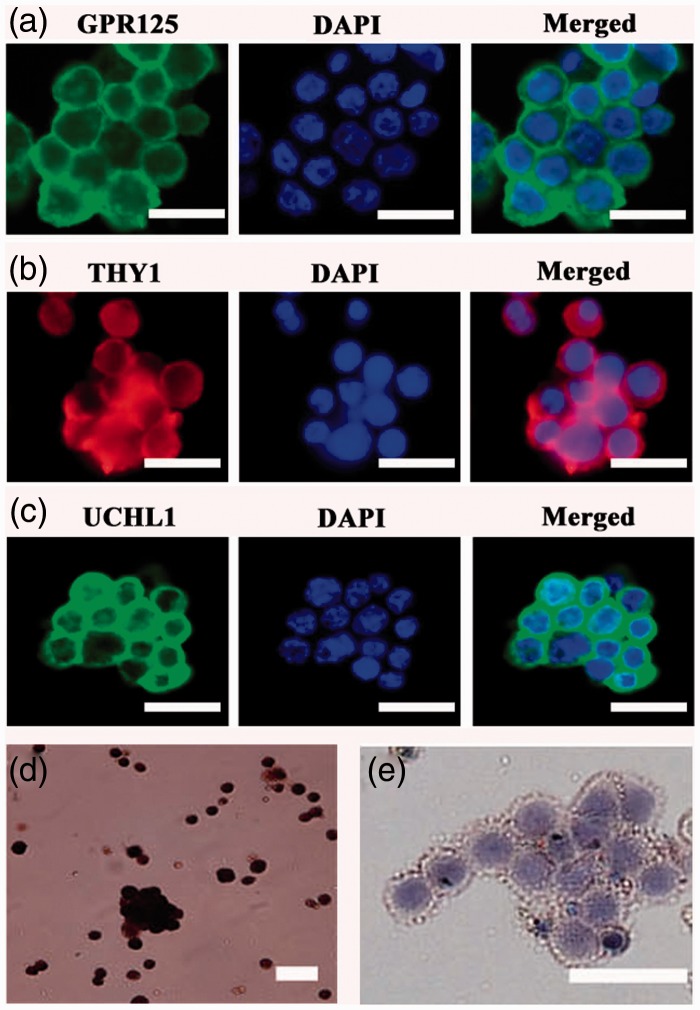
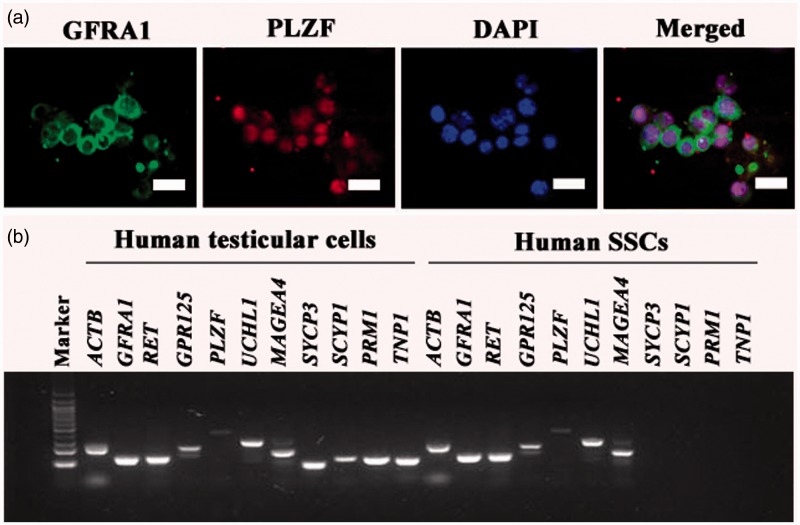
The freshly isolated human GPR125-positive spermatogonia were identified using a number of markers for human SSCs. Immunocytochemistry revealed that almost all of the isolated human GPR125-positive spermatogonia by MACS were positive for GPR125 (Figure 2a), THY1 (Figure 2b), UCHL1 (Figure 2c), and MAGEA4 (Figure 2d). Cell meiotic spread assays showed that SYCP3 was undetected in the isolated GPR125-positive spermatogonia (Figure 2f), whereas it was present in human spermatocytes (Figure 2g). No staining was observed when primary antibodies were replaced with isotype rabbit or goat IgGs (Figure 2e, Supplementary Figure 1(a) and (b)), reflecting the specific staining of these antibodies in the cells. Double immunostaining using antibodies against GFRA1 and PLZF revealed that the freshly isolated cells co-expressed GFRA1 and PLZF (Figure 3a), markers for human SSCs.
Figure 2.
Identification of the freshly isolated human GPR125+ spermatogonia by MACS. (a–e) Immunocytochemistry revealed the expression of GPR125 (a), THY1 (b), UCHL1 (c), MAGEA4 (d), and isotype IgG (e) in the isolated human GPR125+ spermatogonia. (f, g) Meiotic spread assays showed the expression of SYCP3 in the isolated GPR125-positive spermatogonia (f) and human spermatocytes (g). Scale bars in a–g = 20 µm. (A color version of this figure is available in the online journal.)
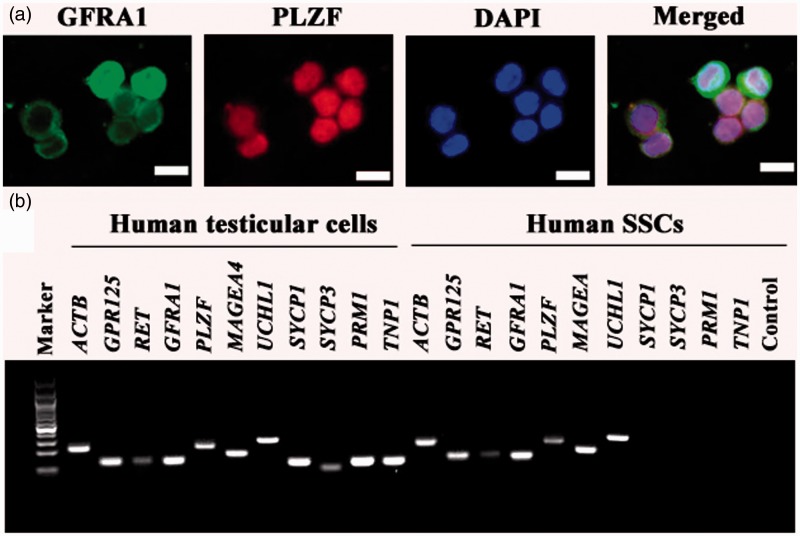
Figure 3.
Biochemical phenotype of the freshly isolated human GPR125+ spermatogonia by MACS. (a) Double immunostaining revealed the co-expression of GFRA1 and PLZF in the freshly isolated human GPR125-positive spermatogonia. (b) RT-PCR showed the transcripts of GPR125, GFRA1, RET, PLZF, UCHL1, MAGEA4, SYCP3, SYCP1, PRM1, and TNP1 in the isolated human GPR125-positive spermatogonia and human testicular cells. ACTB was used as a loading control of total RNA, while PCR without primers served as a negative control. (A color version of this figure is available in the online journal.)
RT-PCR further showed that the transcripts of GPR125, GFRA1, RET, PLZF, UCHL1, and MAGEA4 were expressed in the isolated GPR125-positive spermatogonia (Figure 3b), whereas mRNA of SYCP3, SYCP1, PRM1, and TNP1 was not detected in these cells (Figure 3b). In parallel, the expression of GPR125, GFRA1, RET, PLZF, UCHL1, MAGEA4, SYCP3, SYCP1, PRM1, and TNP1 was observed in human testicular cells (Figure 3b).
Long-term culture of GPR125-positive spermatogonia
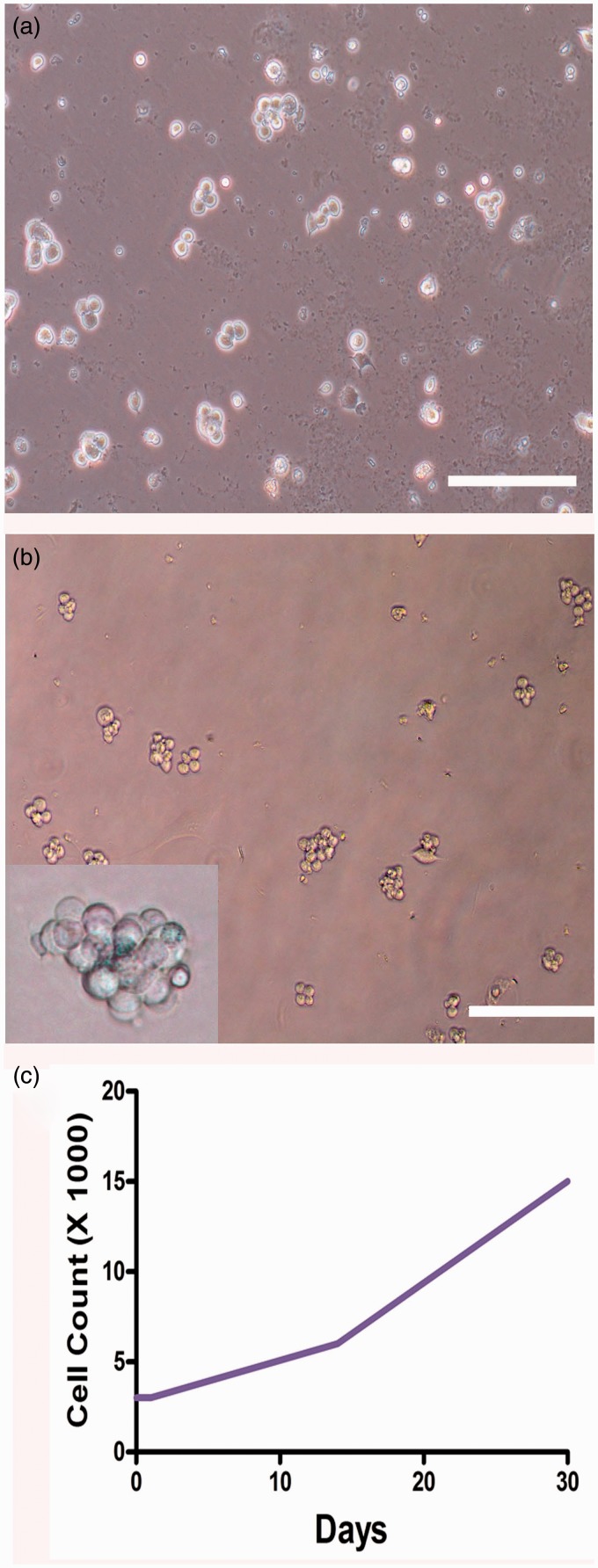
Human GPR125-positive spermatogonia were seeded in plates coated with hydrogel, and they were cultured in the defined medium with StemPro-34 SFM supplemented with several growth factors, including GDNF, EGF, bFGF, and LIF. The colonies containing several cells were formed in human GPR125-positive spermatogonia at day 14 after being plated (Figure 4a), and larger colonies with a dozen of cells were observed when they were cultured for 30 days (Figure 4b). Colonies with tens of cells were seen in human GPR125-positive spermatogonia after culture for two months (Supplementary Figure 3, lower panel). Cell proliferation assay showed that cell number was significantly increased from 3000 to 15,000 during the culture of one month (Figure 4c). In contrast, human GPR125-positive spermatogonia cultured without growth factors in the uncoated plates underwent apoptosis after culture for two or three days (data not shown).
Figure 4.
Colony formation and proliferation assay of human GPR125+ spermatogonia in vitro. (a, b) Phase-contrast microscope displayed the colony formation of human GPR125-positive spermatogonia when cultured for 14 days (a) and 30 days (b). Scale bars in A–B = 100 µm. (c) Cell proliferation assay showed the number increase of human GPR125-positive spermatogonia when cultured for one month. (A color version of this figure is available in the online journal.)
Biochemical characteristics of the cultured GPR125-positive spermatogonia
To check whether the cultured human GPR125-positive spermatogonia maintained gene expression and phenotypic characteristics of human SSCs, we carried out immunocytochemical analysis and RT-PCR. Immunocytochemistry showed that the cultured GPR125-positive spermatogonia were positive for GPR125 (Figure 5a), THY1 (Figure 5b), UCHL1 (Figure 5c), and MAGEA4 (Figure 5d). No immunostaining was seen when primary antibodies were replaced with isotype rabbit or goat IgGs (Figure 5e, Supplementary Figure 2(a) and (b)), thus verifying the specific staining of these antibodies in the cells. Double immunostaining using antibodies against GFRA1 and PLZF revealed that the cultured GPR125-positive spermatogonia were positive for both GFRA1 and PLZF (Figure 6a).
Figure 5.
Phenotype of human GPR125-positive spermatogonia after 30 days of culture. (a–e) Immunocytochemistry revealed the expression of GPR125 (a), THY1 (b), UCHL1 (c), MAGEA4 (d), and isotype IgG (e) in human GPR125-positive spermatogonia after culture for one month. Scale bars in A–E = 20 µm. (A color version of this figure is available in the online journal.)
Figure 6.
Gene and protein characteristics of human GPR125-positive spermatogonia when cultured for 1 month. (a) Double immunostaining revealed the co-expression of GFRA1 and PLZF in human GPR125-positive spermatogonia after 30 days of culture. (b) RT-PCR showed the expression of GPR125, GFRA1, RET, PLZF, UCHL1, MAGEA4, SYCP3, SYCP1, PRM1, and TNP1 in human GPR125-positive spermatogonia when cultured for 30 days and human testicular cells. ACTB was used as a loading control of total RNA. (A color version of this figure is available in the online journal.)
RT-PCR further revealed that the mRNA of GFRA1, GPR125, RET, PLZF, UCHL1, and MAGEA4 was expressed in the cultured GPR125-positive spermatogonia (Figure 6b); in contrast, the transcripts of SYCP3, SYCP1, PRM1, and TNP1 were undetectable in these cells (Figure 6b), suggesting that these cells did not differentiate into spermatocytes or spermatids. The transcripts of GPR125, GFRA1, RET, PLZF, UCHL1, MAGEA4, SYCP3, SYCP1, PRM1, and TNP1 were observed in human testicular cells (Figure 6b). In addition, immunostaining demonstrated that the expression of GPR125 was maintained in human GPR125-positive spermatogonia after culture for 1 month and 2 months (Supplementary Figure 3, middle and lower panels).
SMAD3 and AKT pathways were activated during the culture of human GPR125-positive spermatogonia
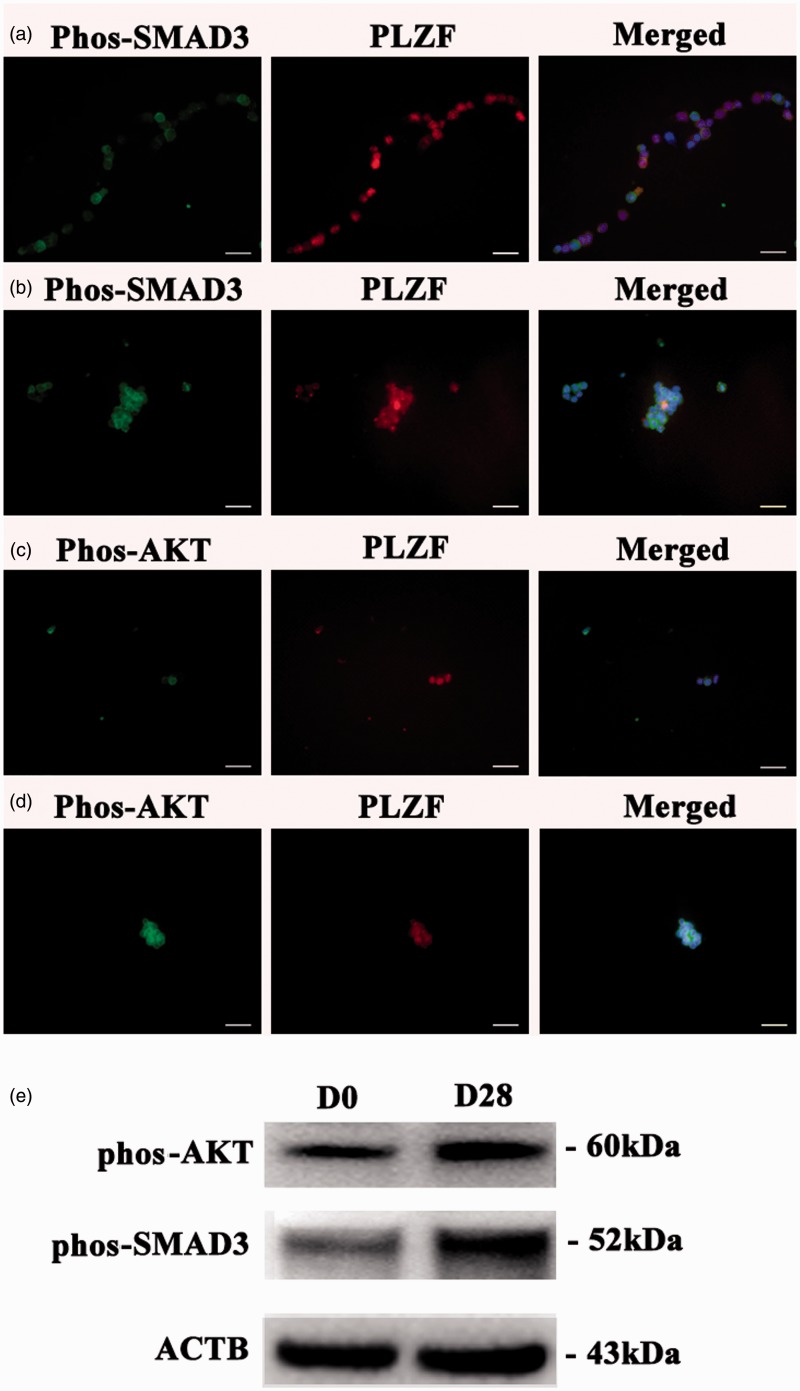
We also examined which signaling pathways were activated during the culture of human GPR125-positive spermatogonia. Immunocytochemistry showed that the percentages of phos-SMAD3-positive and phos-AKT-positive cells were enhanced significantly by 4.2 fold and 3.5 fold, respectively, in the cultured human GPR125-positive spermatogonia (Figure 7b and d) compared to the freshly isolated cells (Figure 7a and c). To verify the specific staining of these antibodies, double immunostaining displayed that phos-ATK was expressed, whereas phos-SMAD3 was undetected in human Sertoli cells (Supplementary Figure 4a and b). Western blots further revealed that the expression of phos-AKT and phos-Smad3 was enhanced in the cultured human GPR125-positive spermatogonia when compared with the freshly isolated cells (Figure 7e). Together, these data suggest that the AKT and SMAD3 pathways were activated in the culture of human GPR125-positive spermatogonia.
Figure 7.
The expression of phos-SMAD3 and phos-AKT pathways in the freshly isolated and cultured human GPR125-positive spermatogonia. (a–d) Immunocytochemistry displayed the expression of phos-SMAD5 and phos-AKT in the freshly human GPR125-positive spermatogonia (a and c) and human GPR125-positive spermatogonia cultured for 30 days (b and d). (e) Western blots showed the expression of phos-AKT and phos-Smad3 in the cultured human GPR125-positive spermatogonia after culture for 0 day and 28 days. (A color version of this figure is available in the online journal.)
Discussion
SSCs are one of the important stem cells due to their unique properties. First, SSCs are the only stem cells that transmit genetic information to subsequent generations. Second, SSCs were previously believed to be unipotent stem cells since they can only produce sperm in vivo; however, this viewpoint has recently been changed. Numerous studies have shown that rodent and human SSCs under appropriate culture conditions can acquire pluripotency to become ES-like cells capable of differentiating into all cell lineages of three germ cell layers.7–11 Notably, it has been clearly shown by peers and us that SSCs can transdifferentiate directly into a number of mature and functional cells.12–14 Thus, SSCs have great applications in both regenerative and reproductive medicine. Nevertheless, very little information is available on human SSC biology and long-term culture of these cells has not yet been established, because it is rather hard to obtain testis tissues from donors. In this study, we obtained human testis biopsies from OA patients who underwent MD-TEST. These patients had normal spermatogenesis, and thus they were used to isolate and culture human SSCs.
We have recently identified GPR125 as a hallmark for human SSCs based upon our observations that GPR125 is present in a subpopulation of human spermatogonia along the basement membrane of the seminiferous tubules.21 Of note, only one or two spermatogonia are GPR125-positive in each cross section of seminiferous tubule,21 suggesting that these cells are indeed the stem cells in human testis. In this study, we isolated GPR125-positive spermatogonia with a high viability from OA patients by MACS using the antibody against GPR125. The purity of the isolated cell is pretty high, since more than 95% of the cells were positive for GPR125, as indicated by both single and double immunostaining. The isolated cells were stained positively for a number of markers for rodent and human SSCs, including GFRA1, THY1, PLZF, UCHL1, and MAGEA4.21 GFRA1 and THY1 have been found to be markers for rodent and monkey SSCs,21,26,27 reflecting that these markers are conserved in non-human primates and humans. UCHL1 is a marker for pig and human spermatogonia.21,28 Notably, double immunostaining revealed that the isolated cells were co-expressed GFRA1 and PLZF, a hallmark for rodent and human SSCs,21,29,30 reflecting that the isolated cells were actually human SSCs. Moreover, the GPR125-positive spermatogonia were negative for markers of spermatocytes and spermatids, e.g. SYCP1, SYCP3, PRM1, and TNP1,31 excluding the contamination of these differentiated germ cells in the isolated cells.
To establish a stable and suitable culture system supporting the division and expansion of SSCs in vitro is essential to obtain adequate cells for basic studies and applications of SSCs. The niche of SSCs in the testis, which is composed of Sertoli cells, growth factors, peritubular cells, and extra cellular matrix (ECM), plays a critical role in regulating the proliferation and differentiation of male germline stem cells.32 Human testicular peritubular cells have been found to synthesize ECM proteins, including collagens, laminin, and fibronectin.33 In healthy men, ECM proteins are the main components that build the wall of the seminiferous tubules and SSCs require the basement membrane to maintain their stem cell properties both in vivo and in vitro.34 The long-term survival and expansion of SSCs in vitro depend upon feeder cells (e.g. the mitotically arrested MEFs).18 However, it is time-consuming to prepare the feeder cells and it may bring contamination of pathogens. Meanwhile, these cells may be inefficient for the difference from batch to batch. In human, a feeder-free culture system containing growth factors has been reported to culture testicular cells.19,20 However, human SSCs were not separated and the starting cells were a cell mixture including human testicular cells,19,20 which results in the fact that somatic cells may easily overgrow compared to SSCs. Recently, a novel feeder-free culture system using the matrigel as the matrix has been developed for culturing mouse SSCs. In this study, we chose hydrogel as a better matrix for the maintenance and expansion of human SSCs, and the cells were cultured with several growth factors, including GDNF, EGF, FGF, and LIF, which are required for the survival and expansion of mouse SSCs,18,19,22,23 for two months and retained high levels of numerous genes and proteins for SSCs, including GPR125, THY1, UCHL1, and MAGEA4. Double immunostaining further showed that the cultured GPR125-positive spermatogonia were co-expressed GFRA1 and PLZF. Moreover, the transcripts of SYCP3, SYCP1, PRM1, and TNP1 were undetected in human SSCs after culture for 30 days. Taken together, these data suggest that the cultured human SSCs were maintained in an undifferentiated status. In contrast, apoptosis of human SSCs occurred when they were cultured without matrix or growth factors.
It remains unknown which signaling pathways regulate the fate determinations of human SSCs. We observed that the percentages of phos-SMAD3+ and phos-AKT+ cells and the expression of phos-SMAD3 and phos-AKT were enhanced significantly during the culture of human GPR125-positive spermatogonia, implicating that AKT and SMAD3 signaling pathways are activated during the culture of human SSCs.
In summary, we have successfully isolated human SSCs with high viability and purity using MACS. We developed a feeder-free culture system using hydrogel and certain growth factors to culture and expand human SSCs while maintaining their stem cell characteristics, which could provide enough cells of human SSCs for basic study and clinic applications in treating human diseases. We also demonstrated that human SSCs proliferate via the activation of AKT and SMAD3 signaling pathways, which sheds a novel insight into molecular mechanisms underlying the division and differentiation of human SSCs.
Acknowledgements
We thank Professor Giulio C. Spagnoli, University Hospital of Basel, Switzerland, for providing the antibody to MAGEA4. This study was supported by the grants from National Natural Science Foundation of China (31230048, 31171422, 31401250) and Chinese Ministry of Science and Technology (2014CB943101), The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (2012.53), a key grant from the Science and Technology Commission of Shanghai Municipality (12JC1405900), Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning, and Shanghai Pujiang Program (11PJ1406400).
Authors' contributions
YG, LL, MS, and YH conducted the experiments, ZL provided the testis biopsies, ZH designed the experiments, and YG and ZH wrote the manuscript and interpreted the data.
Conflicts of interest
The authors indicate no potential conflicts of interest.
References
- 1.Brougham MF, Wallace WH. Subfertility in children and young people treated for solid and haematological malignancies. Br J Haematol 2005; 131: 143–55. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell RT, Saunders PT, Sharpe RM, Kelnar CJ, Wallace WH. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr Dev 2009; 15: 101–34. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN. Evaluation of male infertility. Minerva Ginecologica 2009; 61: 261–83. [PubMed] [Google Scholar]
- 4.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994; 91: 11298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 1994; 91: 11303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Ping P, Ma M, Li P, Tian R, Yang H, Liu Y, Gong Y, Zhang Z, Li Z, He Z. Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Reports 2014; 3: 663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature 2008; 456: 344–9. [DOI] [PubMed] [Google Scholar]
- 8.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell 2004; 119: 1001–12. [DOI] [PubMed] [Google Scholar]
- 9.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, Valenzuela DM, Hobbs RM, Pandolfi PP, Rafii S. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 2007; 449: 346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izadyar F, Pau F, Marh J, Slepko N, Wang T, Gonzalez R, Ramos T, Howerton K, Sayre C, Silva F. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction 2008; 135: 771–84. [DOI] [PubMed] [Google Scholar]
- 11.Streckfuss-Bomeke K, Vlasov A, Hulsmann S, Yin D, Nayernia K, Engel W, Hasenfuss G, Guan K. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res 2009; 2: 139–54. [DOI] [PubMed] [Google Scholar]
- 12.Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, Hofmann MC, Cooke PS. Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells 2009; 27: 1666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Gong Z, Hou L, Huang Y. The induction of rat spermatogonial stem cells into neuronal-like cells and behavioral recovery following transplantation in a rat Parkinson's disease model. Int J Mol Med 2012; 29: 239–44. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Gong Y, Guo Y, Hai Y, Yang H, Yang S, Liu Y, Ma M, Liu L, Li Z, Gao WQ, He Z. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. Cell Commun Signal 2013; 11: 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776–98. [PubMed] [Google Scholar]
- 16.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol 2008; 288: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Techniq 2009; 72: 580–5. [DOI] [PubMed] [Google Scholar]
- 18.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003; 69: 612–6. [DOI] [PubMed] [Google Scholar]
- 19.Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, de Rooij DG, Repping S, van Pelt A. Propagation of human spermatogonial stem cells in vitro. JAMA-J Am Med Assoc 2009; 302: 2127–34. [DOI] [PubMed] [Google Scholar]
- 20.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA-J Am Med Assoc 2011; 305: 2416–8. [DOI] [PubMed] [Google Scholar]
- 21.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod 2010; 82: 363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287: 1489–93. [DOI] [PubMed] [Google Scholar]
- 23.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA 2004; 101: 16489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Jiang J, Kokkinaki M, Tang L, Zeng W, Gallicano I, Dobrinski I, Dym M. MiRNA-20 and mirna-106 a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells 2013; 31: 2205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Niu M, Yao C, Hai Y, Yuan Q, Liu Y, Li Z, He Z. Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci Rep 2015; 5: 8084–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Z, Jiang J, Hofmann MC, Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod 2007; 77: 723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 2007; 25: 2330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev 2006; 73: 1531–40. [DOI] [PubMed] [Google Scholar]
- 29.Piazza F, Costoya JA, Merghoub T, Hobbs RM, Pandolfi PP. Disruption of PLZP in mice leads to increased T-lymphocyte proliferation, cytokine production, and altered hematopoietic stem cell homeostasis. Mol Cell Biol 2004; 24: 10456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647–52. [DOI] [PubMed] [Google Scholar]
- 31.West JA, Park IH, Daley GQ, Geijsen N. In vitro generation of germ cells from murine embryonic stem cells. Nat Protoc 2006; 1: 2026–36. [DOI] [PubMed] [Google Scholar]
- 32.Hai Y, Hou J, Liu Y, Yang H, Li Z, He Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol 2014; 29: 66–5. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht M, Ramsch R, Kohn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocr Metab 2006; 91: 1956–60. [DOI] [PubMed] [Google Scholar]
- 34.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001; 414: 98–104. [DOI] [PubMed] [Google Scholar]