Abstract
The aim of this study was to investigate the role of the programmed cell death factor 4 (PDCD4)/nuclear factor-κB (NF-κB) signaling pathway in coronary micro-embolism (CME)-induced inflammatory responses and cardiac dysfunction in a porcine model. Bama miniature pigs were randomly divided into four groups (n = 5 per group). Micro-embolization balls or saline were infused through a microcatheter in the left anterior descending (LAD) artery in the CME and Sham groups, respectively. PDCD4 siRNA or control siRNA mixed with transfection reagent was infused via the LAD artery 72 h before CME induction in the CME + siRNA-PDCD4 and siRNA-control groups, respectively. Cardiac function was evaluated with ultrasound. Tissue biopsy was stained with hematoxylin–eosin (HE) and hematoxylin basic fuchsin picric acid (HBFP) to measure infarction area. Myocardial PDCD4 and tumor necrosis factor-α (TNF-α) mRNA and protein expression were analyzed by quantitative PCR and Western blotting. NF-κB activity was evaluated in gel electrophoretic mobility shift assay. Echocardiographic parameters showed that compared with the sham group, the CME group had impaired heart function, manifested as systolic dysfunction and left ventricular dilatation (reduced left ventricular ejection fraction [LVEF], left ventricular fractional shortening [FS], and cardiac output [CO] [P < 0.05] and increased left ventricular end-diastolic diameter [LVEDd] [P < 0.05]). Compared with the CME group, the CME + siRNA-PDCD4 group had attenuated CME-induced cardiac function damage (increased LVEF, FS and CO [P < 0.05] and reduced LVEDd [P < 0.05]). Compared with the sham group, the CME group had significantly increased PDCD4 and TNF-α mRNA and protein expression and increased NF-κB activity (P < 0.05). These effects were significantly inhibited in the CME + siRNA-PDCD4 group (P < 0.05). In conclusion, PDCD4/NF-κB signaling pathway activation is an important mechanism for CME-induced cardiac dysfunction, suggesting that inhibition of PDCD4/NF-κB signaling pathway may be a potential target for the prevention and treatment of CME.
Keywords: Coronary micro-embolization, vascular intervention, cardiac function, programmed cell death factor 4, nuclear factor-κB
Introduction
Coronary micro-embolism (CME) is a common and important clinical event in patients with acute coronary syndrome (ACS), and those receiving thrombolytic therapy and percutaneous coronary intervention (PCI).1,2 CME may slow down or even block blood flow, which has serious impacts on cardiac function and long-term prognosis; however, effective preventative and therapeutic approaches have yet to be identified.3 Studies have shown that CME-induced localized myocardial inflammation is the major cause of progressive cardiac dysfunction, and that tumor necrosis factor-α (TNF-α) is an important inflammatory mediator of myocardial contractile dysfunction.4 Li et al. identified nuclear factor-κB (NF-κB) activation as one of the primary factors responsible for the development of CME-induced myocarditis. Generally, NF-κB activation and the resulting massive release of inflammatory mediators, such as TNF-α, play an important role in CME-induced cardiac dysfunction and advanced heart failure. However, the detailed regulatory mechanism remains to be elucidated.5
Programmed cell death factor 4 (PDCD4) is a recently discovered tumor suppressor gene, which is not only an important regulator of programmed cell death (PCD), but is also important in the onset of inflammation.6 Sheedy et al.7 reported that PDCD4 promoted the activation of NF-κB and inhibited interleukin (IL)-10 upregulation, thus promoting inflammation. In earlier studies, we found that myocardial PDCD4 levels were significantly higher after CME,8 implicating that PDCD4-mediated signaling pathway might play an important role in CME-induced inflammation. Therefore, in this study, we used a pig CME model to investigate whether the PDCD4/NF-κB signaling pathway was activated after CME, and whether inhibition of PDCD4/NF-κB signaling pathway improved heart function. We also explored the impact of the PDCD4/NF-κB signaling pathway on the expression of TNF-α, which would enrich our understanding of the molecular mechanisms of the CME-induced inflammatory response and cardiac dysfunction.
Materials and methods
Materials
Microspheres (42 µm diameter, Biosphere Medical Inc, USA); TRIzol-A + total RNA extraction reagent (KeyGEN, China); RevertAid™ FirstStrand cDNA Synthesis kit (Fermentas, Lithuania); TNF-α mRNA, PDCD4 mRNA, β-actin mRNA primer (Sagene, China), goat anti-pig PDCD4 polyclonal antibody (LifeSpan BioSciences, USA); rabbit anti-pig polyclonal TNF-α antibody (Abcam, USA); rabbit polyclonal anti-GAPDH IgG antibody (Proteintech Group, USA); PDCD4 siRNA (GenePharma, China); control siRNA (GenePharma, China); in vivo transfection reagent Entranster™-invivo (Engreen, China); gel electrophoretic mobility shift assay (EMSA) kit (Pierce, USA).
Experimental animals
Bama miniature pigs (n = 20; male or female; weight 25–30 kg) were provided by the College of Animal Science and Technology, Guangxi University (China). Experiments were approved by the Ethics Committee of Guangxi Medical University and were performed in compliance to the local animal experiment regulations.
The CME model was established according to Su et al.8 Twenty Bama miniature pigs were randomly divided into the following groups: sham, CME, CME + siRNA-PDCD4, and CME + siRNA-control (n = 5 pigs per group). Animals were anesthetized by intramuscular injection of ketamine hydrochloride (5–10 mg/kg). Anesthesia was then maintained by 0.5 mg/kg·h diazepam infusion via a 5½ needle inserted into the ear vein. The femoral artery was isolated and cannulated with a 6F vascular sheath. Heparin was bolus infused at 200 U/kg and maintained at 100 U/mg/kg·h. With the guidance of coronary angiography and a guiding catheter, a catheter was advanced to the distal end of the first diagonal branch of the left anterior descending (LAD) artery for infusion of 100,000 microspheres (suspended in 1.5 mL saline containing sodium dodecyl sulfate [SDS]). Penicillin (800,000 U) was administered to prevent infection after surgery. The sham group was treated identically with the exception that the microspheres were replaced with 1.5 mL saline.
Cardiac siRNA transfection
With reference to the method described by Wang et al.,9 we performed siRNA transfection according to the instructions of an in vivo transfection reagent (Entranster™-in vivo). PDCD4 siRNA (100 µg) was dissolved in 100 µL RNase-free water. Subsequently, either 100 µL of PDCD4 siRNA, 100 µL of control siRNA or 100 µL diluted Entranster™-in vivo was dissolved in 100 µL 10% glucose solution to give a final concentration of 5% glucose. Then, the diluted 200 µL Entranster™-in vivo was added to the diluted 200 µL PDCD4 siRNA or diluted control siRNA. The mixtures were incubated for 15 min at room temperature for intracoronary injection. PDCD4 siRNA transfection complex (400 µL) was injected into the LAD in the CME + siRNA-PDCD4 group 72 h before CME-induction using a microcatheter and 400 µL control siRNA transfection complex was injected into the CME + siRNA-control group via the same route.
Cardiac function evaluation
Nine hours after CME-induction, left ventricular ejection fraction (LVEF), increased left ventricular end-diastolic diameter (LVEDd), fractional shortening (FS), and cardiac output (CO) were evaluated as a measure of cardiac function using a 12 MHz probe. All three measurements represented the averages of three cardiac cycles.10 Echocardiographic examination was performed by an experienced echocardiography specialist.
Biopsy collection and processing
After the evaluation of cardiac function under anesthesia, animals were killed by ear vein infusion of 10% KCl. The hearts were collected and the ventricles were cut into six sections (thickness, 5–8 mm) in parallel to the atrioventricular groove. The left ventricular anterior wall was fixed in 4% neutralized formalin for hematoxylin–eosin (HE) and hematoxylin basic fuchsin picric acid (HBFP) staining to evaluate myocardial infarction size.11
Myocardial infarction evaluation
HBFP staining is an important diagnostic method for early myocardial ischemia. Ischemic myocardium and red blood cells were stained red, while the cytoplasm and nuclei in the normal myocardium were stained blue. A DMR + Q550 pathological image analyzer was used to evaluate the stained sections. Five areas of HBFP-stained sections were randomly selected (100×) to measure the infarcted area using Leica Qwin software. Data were expressed as the average of five measurements of percentage of the total section area.12
Evaluation of PDCD4 and TNF-α mRNA expression by quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from left ventricular myocardium using TRIzol according to the manufacturer’s instructions. The concentration of total RNA was measured by Nanodrop before being subjected to cDNA reverse transcription. The fluorescent dye, SYBR Green Ι, was used to detect PCR products according to the manufacturer’s instructions. The following primers were used: forward PDCD4: 5′-GCTACGGTGCTCCTGAGTAT-3′, reverse PDCD4 primer: 5′-GGCAATGTTCAGCTTCCGAT-3′; forward TNF-α primer: 5′-GGCCCAAGGACTCAGATCAT-3′, reverse TNF-α primer: 5′-GCATACCCACTCTGCCATTG-3′; forward β-actin primer: 5′-CACCTTCTACAACGAGCTGC-3′, reverse β-actin primer: 5′-TCATCTTCTCACGGTTGGCT-3′. Each sample was set up in duplicate along with negative controls. PCR products were confirmed by sequencing and the 2−ΔΔCT method was used for relative quantitation.
Myocardial expression of PDCD4 and TNF-α protein detected by Western blot
Total myocardium protein was extracted from tissue lysates. Protein concentrations were determined using the Lowry method. Protein sample (50 µg) was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and wet transferred onto polyvinylidene fluoride (PVDF) membranes at 100 mA for 2 h. The membrane was blocked for 1 h at room temperature and washed with TBST before incubation for 2 h at room temperature with goat anti-pig polyclonal PDCD4 antibody (1:1000) or rabbit anti-pig polyclonal TNF-α antibody (1:200) using rabbit polyclonal anti-GAPDH IgG antibody (1:2000) as the internal control. After washing, the membrane was incubated with the appropriate secondary antibodies for 1 h. Antigen–antibody complex was visualized using the enhanced chemiluminescence method. Immunoblots were then exposed to X-ray film and images were taken with a digital camera. Integrated absorbance (IA = average absorbance × area) of the target band was determined using the Gel Doc2000 gel image analysis system and software (Bio-Rad). The relative expression of PDCD4 and TNF-α (represented by IA values) were normalized against GAPDH expression and presented as PDCD4 and TNF-α IA/GAPDH IA.
Evaluation of NF-κB activity by EMSA
Cardiomyocyte nucleoproteins were extracted using a nuclear protein extraction kit according to the manufacturer’s instructions. The oligonucleotides containing NF-κB binding sites were biotin-labeled at the 5′ end: 5′-AGTTGAGGGGACTTTCCCAGGC-3′, 3′-TCAACTCCCCTGAAAGGGTCCG-5′ (Shanghai Biological technology Co., Ltd.). Nucleoproteins were incubated with the probe for 20 min at room temperature before being separated by electrophoresis using a 6.5% polyacrylamide gel. The absorbance value (A value) measured using the gel imaging analysis system represented the NF-κB activity.
Statistical analysis
All data were analyzed using SPSS16.0 statistical software and expressed as mean ± standard deviation (mean ± SD). Paired t-test was used to compare two groups and analysis of variance (ANOVA) with least significant difference (LSD) test was used to compare differences among groups. P < 0.05 was considered as statistically significant.
Results
Changes in cardiac function
LVEF, FS, CO, and LVEDd at 9 h post-surgery were evaluated with echocardiography (Table 1). Compared with the sham group, the CME and CME + siRNA-control groups had significantly impaired heart function manifested as systolic dysfunction and left ventricular dilatation (decreased LVEF, FS and CO [P < 0.05] and increased LVEDd [P < 0.05]). Compared with the CME group, the CME + siRNA-PDCD4 group had attenuated cardiac dysfunction manifested as increased LVEF, FS, CO (P < 0.05), and reduced LVEDd (P < 0.05).
Table 1.
Changes in cardiac function (mean ± SD)
| Group | n | LVEF (%) | LVFS (%) | CO (L/min) | LVEDd (mm) |
|---|---|---|---|---|---|
| Sham | 5 | 68.39 ± 2.44 | 41.17 ± 5.81 | 3.86 ± 0.73 | 3.51 ± 0.34 |
| CME | 5 | 51.03 ± 3.41a | 27.17 ± 7.84a | 2.92 ± 0.83a | 4.96 ± 0.58a |
| CME + siRNA-PDCD4 | 5 | 65.47 ± 2.71b | 41.92 ± 5.63b | 3.85 ± 0.89b | 3.72 ± 0.34b |
| CME + siRNA-control | 5 | 49.71 ± 3.75a | 26.14 ± 8.07a | 2.86 ± 0.77a | 5.11 ± 0.68a |
LVEF: left ventricle ejection fraction; CO: cardiac output; LVEDd: left ventricular end-diastolic diameter; LVFS: left ventricle fractional shortening.
P < 0.05, compared with the sham group.
P < 0.05, compared with CME group.
CME pathology
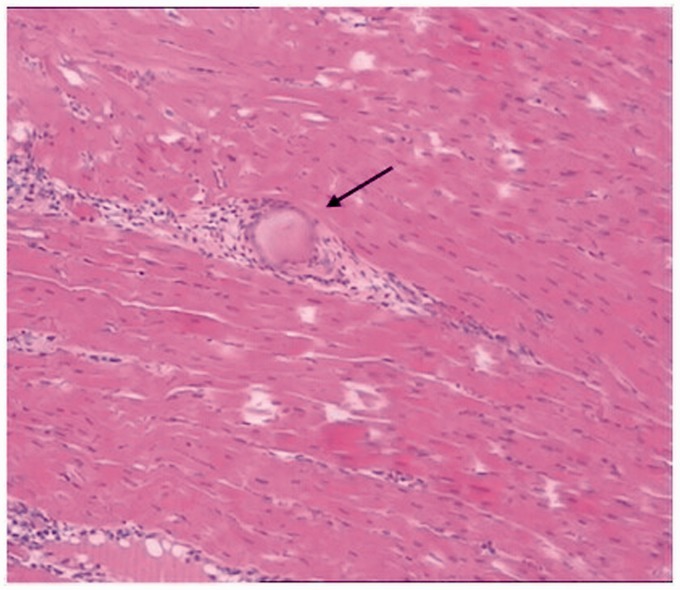
HE and HBFP staining showed occasional subendocardial ischemia in the sham group without obvious infarction. The CME, CME + siRNA-PDCD4, and CME + siRNA-control groups had multiple micro-infarctions, mostly apparent as a wedge shape with focal distribution in the endocardium and left ventricle (Figure 1). At infarction sites, HE staining showed karyolysis and hypochromatosis, red-stained cytoplasm, surrounding myocardial edema and degeneration, peripheral inflammatory cell infiltration and erythrocyte leakage as well as microspheres in the microarteries (Figure 2). Infarction sizes in the CME, CME + siRNA-PDCD4, and CME + siRNA-control groups were 9.52 ± 3.69%, 9.17 ± 3.34%, and 9.22 ± 3.31%, respectively. There was no significant difference between the groups (P > 0.05), indicating that inhibition of PDCD4 expression did not affect the infarction size caused by CME.
Figure 1.
HBFP staining of cardiac infarction sites (200×). a–d, sham, CME, CME + siRNA-PDCD4, and CME + siRNA-control groups, respectively. Ischemic myocardium was stained red. Arrows indicate microinfarct sites. (A color version of this figure is available in the online journal.)
Figure 2.
HE staining of microinfarct sites after CME (400×). Arrow indicates a microsphere. (A color version of this figure is available in the online journal.)
PDCD4 and TNF-α mRNA expression
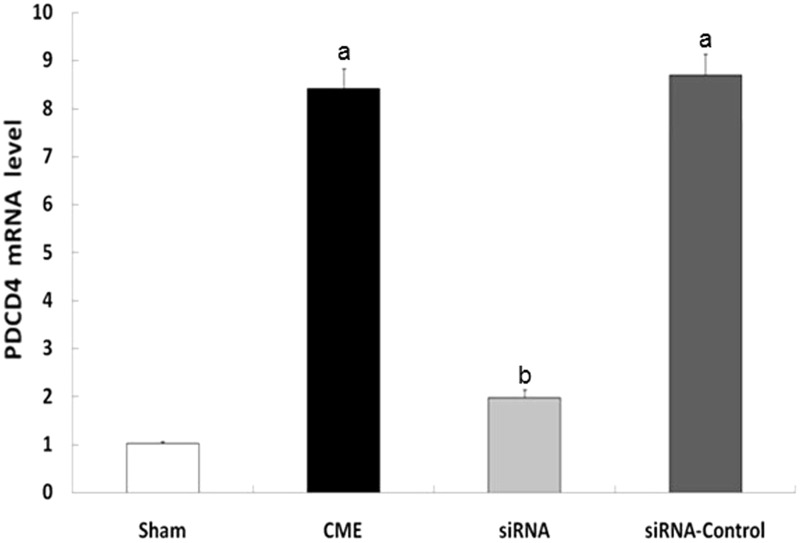
Compared with the sham group, the CME and CME + siRNA-control groups had significantly increased PDCD4 and TNF-α mRNA expression in cardiomyocytes (P < 0.05). Compared with the CME group, the CME + siRNA-PDCD4 groups had lower PDCD4 and TNF-α mRNA expression (P < 0.05), illustrating a decreased expression of TNF-α following suppression of PDCD4 mRNA expression (Figures 3 and 4).
Figure 3.
Quantitative PCR detecting the expression of PDCD4 mRNA. aP < 0.05 compared with the sham group; bP < 0.05, compared with CME group
Figure 4.
Quantitative PCR detecting the expression of TNF-α mRNA. aP < 0.05, compared with sham group; bP < 0.05, compared with CME group
PDCD4 and TNF-α expression detected by Western blot
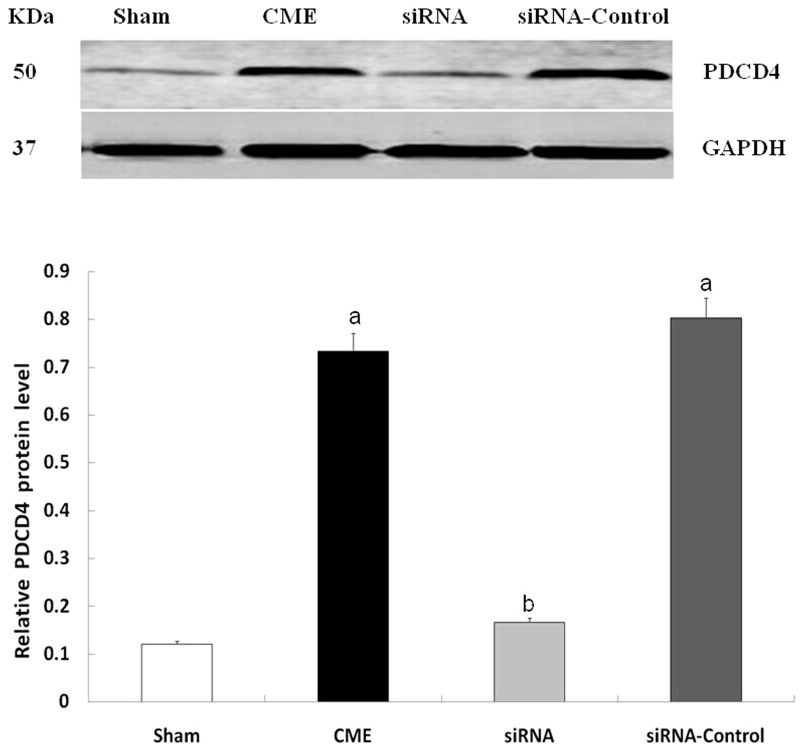
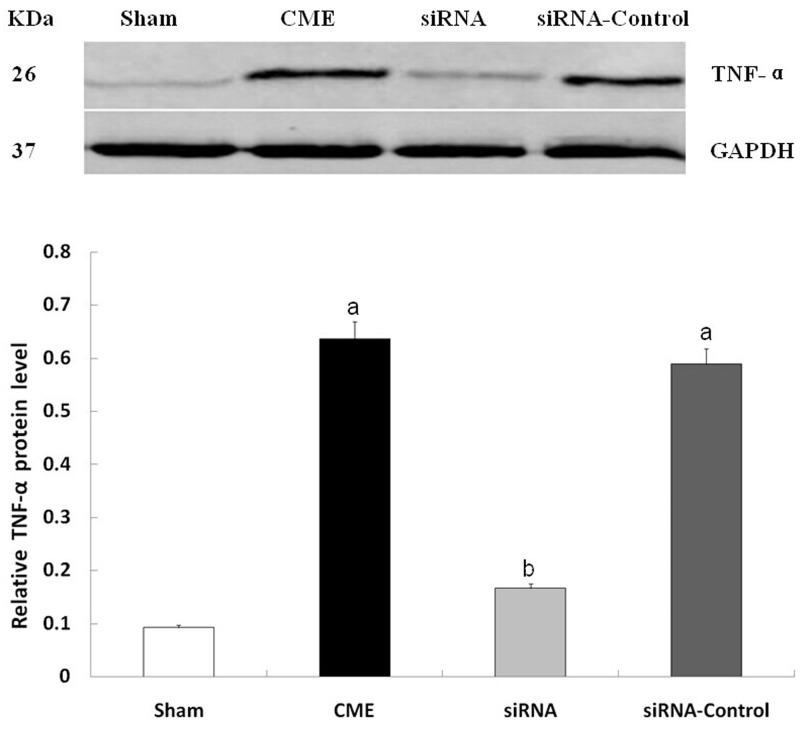
Compared with the sham group, the CME and CME + siRNA-control groups had significantly increased PDCD4 and TNF-α expression in cardiomyocytes (P < 0.05). Compared with the CME group, the CME + siRNA-PDCD4 group had lower PDCD4 and TNF-α expression (P < 0.05), indicating that inhibition of PDCD4 expression resulted in decreased TNF-α expression (Figures 5 and 6).
Figure 5.
Western blot detecting the expression of PDCD4 protein. aP < 0.05, compared with sham group; bP < 0.05, compared with CME group
Figure 6.
Western blot detecting the expression of TNF-α protein. aP < 0.05, compared with sham group; bP < 0.05, compared with CME group
NF-κB activity
Compared with the sham group, the CME and CME + siRNA-control groups had significantly increased NF-κB activity (P < 0.05). Compared with the CME group, the CME + siRNA-PDCD4 group had lower NF-κB activity (P < 0.05), indicating that inhibition of PDCD4 expression resulted in reduced NF-κB activity (Figure 7).
Figure 7.
EMSA detecting NF-κB activity. aP < 0.05, compared with sham group; bP < 0.05, compared with CME group
Correlation of cardiomyocyte PDCD4 and TNF-α expression and LVEF after CME induction
PDCD4 expression in cardiomyocytes was negatively correlated with LVEF (r = −0.603, P < 0.0001). PDCD4 expression was positively correlated with TNF-α expression (r = 0.704, P < 0.0001).
Discussion
CME is defined as coronary microcirculation thrombosis and microinfarction caused by atherosclerotic plaque rupture or microemboli caused by PCI treatment.13 Unlike epicardial proximal vessel occlusion, CME-induced left ventricular function impairment is not closely related to areas with myocardial perfusion defects,14 and cannot be explained simply by regional myocardial perfusion or microinfarction size. Studies have shown that regional myocardial inflammation is an important cause of left ventricular dysfunction after CME, in which NF-κB activation and the resulting massive release of inflammatory mediators such as TNF-α play important roles in the onset and progress of cardiac dysfunction. Furthermore, CME-induced regional myocardial inflammation was significantly reduced when NF-κB activity was specifically inhibited by pyrrolidine dithiocarbamate (PDTC), and cardiac function was significantly improved as a consequence.5 However, the specific gene regulation and molecular mechanism involved in these processes are unclear.
In previous studies, we found that myocardial TNF-α expression peaked 9 h after CME-induction; therefore, in this experiment, we evaluated cardiac function at this time-point. We found that myocardial NF-κB activity was significantly increased after CME-induction, which promoted the release of TNF-α and damaged myocardial contractile function. This further confirmed the important role of NF-κB activation in the onset and development of CME-induced cardiac dysfunction. Because NF-κB is pivotal to the transduction of a variety of inflammatory signals, studies of its upstream regulatory mechanisms could help to identify potential new targets for the prevention and treatment of CME.
PDCD4 is a recently discovered tumor suppressor that initially attracts much attention because of its important role in a variety of human tumors. Recent studies show that PDCD4 promotes inflammation. Sheedy et al.7 reported that PDCD4 promoted NF-κB activation, and inhibited IL-10 upregulation to promote inflammation. Hilliard et al.6 reported increased resistance to inflammation, significantly increased lymphocytes secretion of inflammatory cytokines such as IL-10 and IL-4, and inhibited TNF-α production in PDCD4 gene knockout mice. In addition, Su et al.15 found that PDCD4 expression was significantly upregulated, which elevated TNF-α levels and then mediated myocardial inflammatory responses in patients undertaken PCI for unstable angina. These observations indicated the importance of PDCD4 in the onset and development of inflammation. We also found previously that PDCD4 expression levels were significantly elevated after CME-induction, and its expression was significantly negatively correlated with changes in cardiac function. Therefore, we hypothesized that the PDCD4-mediated inflammatory signaling pathway was involved in the CME-mediated cardiac function damage via the NF-κB/TNF-α pathway.
In this study, we found that coronary injection of a PDCD4 siRNA transfection complex 72 h before CME-induction effectively inhibited myocardial expression of PDCD4 and improved heart function after CME. These observations support our hypothesis that a PDCD4-mediated inflammatory signaling pathway is involved in the CME-induced damage of cardiac function. We also found that down-regulation of myocardial PDCD4 was associated with inhibition of NF-κB activation and significantly decreased TNF-α expression. These observations indicate that the PDCD4/NF-κB signaling pathway plays an important role in CME-induced damage of cardiac function. Thus, it can be speculated that blocking the PDCD4 signaling pathway will reduce the production and release of TNF-α and attenuate the direct damage of cardiomyocytes mediated by TNF-α via inhibition of NF-κB activation, thereby improving cardiac function after CME induction.
Previous studies suggested that PDCD4 signaling pathway activation was involved in the onset and development of cardiovascular diseases mainly through the pro-apoptotic effects and that inhibiting PDCD4 expression significantly reduced myocardial apoptosis, thereby protecting cardiac function. Dong et al.16 found that, in a rat model of acute myocardial infarction, inhibiting PDCD4 expression reduced the area of myocardial infarction and that this cardioprotective effect was correlated with the inhibition of the pro-apoptotic effects of PDCD4. In the present study we found that, although inhibiting PDCD4 expression attenuated cardiac function after CME, the microinfarct size was not significantly altered. This suggests that inhibition of the PDCD4 signaling pathway protects cardiac function and reduces the infarct size after CME mainly through inhibiting myocardial NF-κB activation and reducing TNF-α expression and release, rather than through suppressing apoptosis. The discrepancy in these results may be due to essential differences in CME between acute myocardial infarction and an animal ischemia–reperfusion model. The latter two were more severe myocardial damage caused by blood supply reassuming after complete coronary blockage induced myocardial ischemia or infarction, whereas the former one was CME-induced myocardial microcirculation obstacles without blocking the blood flow through the coronary arteries. The pathophysiological mechanisms are also different in these cases. In myocardial infarction or ischemia–reperfusion, myocardial apoptosis and necrosis are the main mechanisms leading to myocardial damage, and the myocardial damage induced by PDCD4 is mainly related to its pro-apoptotic effects.17 However, the main mechanism of CME-mediated myocardial injury is myocardial inflammatory responses, while myocardial apoptosis and necrosis are not the primary pathogenic mechanisms.18 Therefore, in this study, the improvements in cardiac function after CME mediated by inhibition of PDCD4 expression were achieved mainly through the inhibition of regional inflammatory responses.
The limitations of this study should be noted. In clinical practice, CME is induced by biologically active particles (such as platelets and red blood cells) that are rich in embolic materials and are products of atherosclerotic plaque rupture. We used plastic microspheres for CME modeling, which may cause different pathophysiological changes compared with those induced by actual CME. Moreover, this is a randomized controlled study with a small population, and conclusions drawn require confirmation in larger scale randomized controlled studies.
In summary, the PDCD4/NF-κB/TNF-α signaling pathway plays an important role in CME-induced cardiac function damage. Reducing myocardial PDCD4 expression blocks NF-κB activation, thereby inhibiting TNF-α expression and alleviating inflammatory responses. Therefore, intervention in the PDCD4/NF-κB/TNF-α signaling pathway may help to reduce cardiomyocyte damage and improve heart function after CME; thus, the PDCD4/NF-κB/TNF-α signaling pathway is implicated as a new target for the prevention and treatment of CME-induced cardiac damage.
ACKNOWLEDGMENT
This study was supported by National Natural Science Foundation of China (Grant No. 81260042) and Youth Science Foundation of Guangxi Medical University (Grant No. GXMUYSF201321).
This manuscript has not been published elsewhere in whole or in part. All authors have read and approved the content, and agree to submit for consideration for publication in the journal. There is no ethical/legal conflicts involved in the article.
Author contributors
Qiang Su proposed and wrote the paper. All authors contributed to editing the final manuscript for content and style.
References
- 1.Higuchi Y, Iwakura K, Okamura A, Date M, Nagai H, Ozawa M, Ito H, Fujii K. Effect of embolic particles during coronary interventional procedures on regional wall motion in patients with stable angina pectoris. Am J Cardiol 2012; 109: 1142–7. [DOI] [PubMed] [Google Scholar]
- 2.Bose D, von Birgelen C, Zhou XY, Schmermund A, Philipp S, Sack S, Konorza T, Möhlenkamp S, Leineweber K, Kleinbongard P, Wijns W, Heusch G, Erbel R. Impact of atherosclerotic plaque composition on coronary microembolization during percutaneous coronary interventions. Basic Res Cardiol 2008; 103: 587–97. [DOI] [PubMed] [Google Scholar]
- 3.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000; 36: 1202–9. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Li L, Zhao X, Huang W, Wen W. Beta blocker metoprolol protects against contractile dysfunction in rats after coronary microembolization by regulating expression of myocardial inflammatory cytokines. Life Sci 2011; 88: 1009–15. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Zhong S, Zeng K, Luo Y, Zhang F, Sun X, Chen L. Blockade of NF-kappaB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous microthrombi in rats. Basic Res Cardiol 2010; 105: 139–50. [DOI] [PubMed] [Google Scholar]
- 6.Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W, Göke R, Chen YH. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol 2006; 177: 8095–102. [DOI] [PubMed] [Google Scholar]
- 7.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 2010; 11: 141–7. [DOI] [PubMed] [Google Scholar]
- 8.Su Q, Li L, Zhou Y, Wang J, Liu Y, Ma G. Induction of myocardial PDCD4 in coronary microembolization-related cardiac dysfunction: evidence from a large-animal study. Cell Physiol Biochem 2014; 34: 533–42. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Li L, Su Q, Zhou Y, Chen H, Ma G, Liu T, Tang Z, Liu Y. The involvement of phosphatase and tensin homolog deleted on chromosome ten (PTEN) in the regulation of inflammation following coronary microembolization. Cell Physiol Biochem 2014; 33: 1963–74. [DOI] [PubMed] [Google Scholar]
- 10.Su Q, Li L, Liu YC, Zhou Y, Lu YG, Wen WM. Effect of metoprolol on myocardial apoptosis and caspase-9 activation after coronary microembolization in rats. Exp Clin Cardiol 2013; 18: 161–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Su Q, Wang Y, Dai R, Lu Y, Su B, Zhao Y. Effect of atorvastatin (Lipitor) on myocardial apoptosis and caspase-8 activation following coronary microembolization. Cell Biochem Biophys 2011; 61: 399–406. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Li DH, Qu N, Wen WM, Huang WQ. The role of ERK1/2 signaling pathway in coronary microembolization-induced rat myocardial inflammation and injury. Cardiology 2010; 117: 207–15. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation 2009; 120: 1822–36. [DOI] [PubMed] [Google Scholar]
- 14.Malyar NM, Lerman LO, Gossl M, Beighley PE, Ritman EL. Relation of nonperfused myocardial volume and surface area to left ventricular performance in coronary microembolization. Circulation 2004; 110: 1946–52. [DOI] [PubMed] [Google Scholar]
- 15.Su Q, Li L, Liu Y, Zhou Y, Wang J, Sun Y. Effect of intensive atorvastatin therapy on periprocedural PDCD4 expression in CD4+ T lymphocytes of patients with unstable angina undergoing percutaneous coronary intervention. Cardiology 2014; 127: 169–75. [DOI] [PubMed] [Google Scholar]
- 16.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem 2009; 284: 29514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 2010; 87: 431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol 2002; 34: 51–62. [DOI] [PubMed] [Google Scholar]