Abstract
The impact of physical activity on carcinogenesis has been demonstrated in many studies. Taking into account the discrepant results of physical exercise on the cell proliferation and apoptosis of breast cancer, we aimed to examine the impact of physical training on N-methyl-N-nitrosourea-(MNU)-induced mammary carcinogenesis. Fifty female rats were divided into four groups according to the intensity of physical activity they undertook. The number of developed tumors, tumor volume, and histopathological diagnoses were noted. Apoptosis and cell proliferation were studied by the number of TUNEL-positive and Ki-67-expressing cells. We demonstrated a statistically significant decrease in the tumor number between all trained groups and the control group. The results were most pronounced in the group with a moderate intensity of training. Moreover, we showed a decrease in tumor volume as training intensity increased, though the differences were not statistically significant. The mean number of TUNEL-positive cancer cells was significantly higher in the training groups than in the control group. These data suggest that physical training, especially of moderate intensity, may alleviate MNU-induced mammary carcinogenesis. The results could suggest that physical exercise-induced apoptosis may be a protective mechanism.
Keywords: Mammary cancer, physical training, N-methyl-N-nitrosourea
Introduction
Cancer is one of the most common diseases and a major cause of death in the world. Approximately 14.1 million new cancer cases occurred in 2012. Breast cancer is the most common cancer diagnosed in women (constituting 25.2% of all new cases) and the main cause of the death.1 It has been well demonstrated in many studies that physical activity may have a preventive effect against breast cancer. The magnitude of this effect varies between studies but can be estimated as between a 30 and 40% reduction in the risk of breast cancer.2 Physical activity has been shown to be an important agent for promoting beneficial health conditions and providing some protection against cancer,3 but the results of studies investigating its impact on patient survival have shown ambiguous results.4 Furthermore, limitations in the measurement of physical activity behaviors in human populations over the time required for cancer development (15–20 years) lead to problems in the interpretation of the results thus obtained. Epidemiological studies of the relationship between individuals’ physical activity and the prevalence of cancer and its mortality continue to be problematic. The use of animal models to investigate carcinogenesis and physical activity offers an alternative approach.5
The main animal species used in experimental studies of breast cancer are rodents (mainly rats and mice), since their glands have the most similar structure and function to human glands.6 The similarity in the basic structure of human and rodent’s mammary gland makes it possible to conduct experiments that compare the process of carcinogenesis and breast cancer development in both species.7,8
In rat mammary carcinogenesis models, as opposed to other systems, it is not possible to distinguish between tumor promotion and tumor progression. Both phases of carcinogenesis are defined to begin seven days following carcinogen administration.9 A recognized carcinogenic agent that is commonly used in rodents to induce breast cancer development is N-methyl-N-nitrosourea (MNU).10,11 The effect of physical exercise on the promotion and progression phase of mammary carcinogenesis in rats has not been adequately elucidated, and its molecular mechanisms are not fully characterized. In many studies, it has been demonstrated that influence of physical activity on carcinogenesis is dependent on the intensity of physical training. The study of Cohen demonstrated that moderate physical activity in rats had a preventive and inhibitory effect on the progression of carcinogenesis.12 Similar observations have been made in female Sprague–Dawley rats in the study of Westerlind et al., in which physical training was shown to delay cancer development and decrease tumor growth.13 On the other hand, it has been shown by other groups that moderate physical exercise does not affect the development of breast cancer following MNU administration.9,14
Nonetheless, later studies conducted by Thompson et al. have demonstrated a 37% decrease in breast cancer growth when high-intensity exercise was performed.15,16 The results of these studies show that it is the intensity, rather than the duration, of exercise that may be the crucial factor for tumor development.15,16 Studies by Cohen and S’aez et al. have shown that excessively high or low levels of intensity of physical activity are the leading factors influencing breast cancer progression.12,17 Low intensity and short duration of physical activity were observed to enhance the rate of occurrence of mammary tumors, while increasing intensity and duration of exercise resulted in a protective effect against mammary cancer development.9 However, the study of Whittal-Strange et al. has demonstrated that already developed tumors increase in weight following excessively intense exercise.18
Physical training has been shown to impact MNU-induced breast cancer development and its progression through regulating cancer cell proliferation and apoptosis.13,19 Taking into account the discrepant results obtained in the above-mentioned studies, we here aimed to examine the potential impact of physical training on MNU-induced mammary carcinogenesis in rats.
Materials and methods
Animals
Fifty female Sprague–Dawley rats (Experimental Medicine Center, Medical University of Silesia, Katowice, Poland) of 28 days of age were utilized for the experiment. The animals were kept in the animal research facility of the Department of Pathomorphology, Medical University of Wrocław, under conditions of controlled light (12 h light and 12 h dark) and temperature. Normal rat chow and water were provided ad libitum.
All experimental procedures were performed according to European Union standards and approved by the Local Bioethics Committee at the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences (accession number 37/2010).
Tumor induction and detection
Following a two-week quarantine period, the animals were intraperitoneally injected with 180 mg/kg body weight (b.w.) of MNU (Sigma-Aldrich, Munich, Germany) as described by Ko et al.10 MNU was dissolved in warm NaCl and acidified to pH 5.0 with acetic acid. Four weeks after MNU administration, the rats were observed daily and palpated three times a week in order to detect induced tumors, until the 13th week of the experiment. During the experiment, the animals were also weighed weekly. All the procedures were performed under anesthesia using a mixture of ketamine at a dose of 60 mg/kg b.w. and medetomidine at a dose of 0.5 mg/kg b.w. i.m.
Physical training
The animals were divided into four groups: the sedentary control (SC; n = 14) and the low-intensity (LIT; n = 12), moderate-intensity (MIT; n = 12), and high-intensity (HIT; n = 12) physical training groups. Immediately after MNU administration, the rats in the LIT, MIT, and HIT groups were subjected to a supervised 12-week physical training regimen (five days a week) with a three-position treadmill (Exer 3/6, Columbus, USA). The speed of the treadmill and the duration of exercise were gradually increased depending on the group’s established level of intensity (Table 1). The parameters of the low (respectively high) intensity training were 20% less than (respectively greater than) those of the moderate-intensity training.20 After the administration of MNU, the rats from the SC group did not undergo any physical training, but remained in their cages for 12 weeks, otherwise under the same conditions as rats from the training groups.
Table 1.
Moderate-intensity physical training protocol
| Period of training (week) | Speed of treadmill (km/h) | Duration (min) |
|---|---|---|
| 1 | 0.60 | 10 |
| 2 | 0.96 | 20 |
| 3 | 1.20 | 30 |
| 4 | 1.44 | 40 |
| 5 | 1.68 | 50 |
| 6 | 1.68 | 55 |
| 7 | 1.68 | 60 |
| 8 | 1.68 | 65 |
| 9–12 | 1.68 | 30 |
Tissue collection
Twelve weeks following MNU administration, the animals were euthanized by intraperitoneal injection of 200 mg/kg b.w. of pentobarbital prior to the initial anesthesia by i.m. injection of ketamine 60 mg/kg b.w. and medetomidine 0.5 mg/kg b.w., as in our previous study.11 All the tumors detected by palpation were excised and measured. Moreover, the lungs, liver, spleen, kidney, and enlarged lymph nodes were collected during the autopsy. All tissues were fixed in 4% buffered formalin, dehydrated and embedded in paraffin.
Histopathology
Histopathological examination was performed on 6-µm-thick paraffin sections stained with hematoxylin and eosin (H&E), as previously described.11 In brief, tumor sections were reviewed by two independent pathologists utilizing a double-headed BX41 microscope (Olympus, Tokyo, Japan), and were categorized as benign or malignant lesions, based on the classification of rat mammary gland tumors.21 According to these criteria, malignant lesions were characterized by the loss of the tubular–alveolar pattern, cellular pleomorphism, increased nuclear/cytoplasmic ratio, enlarged nuclei with coarse chromatin, distinct nucleoli, presence of necrosis and hemorrhage, invasion of the surrounding tissues, and metastasis. In summary, five main cancer types of the rat mammary gland can be distinguished: papillary, characterized by cancer cells situated on a fibrovascular core; cribriform, consisting of solid nests of cancer cells interrupted by various secondary lumina; solid, composed of solid nests of cancer cells without secondary lumina formations; comedo, which are multilayered epithelial structures with central necrosis; and tubular, consisting of cancer cells forming well-differentiated tubular and alveolar structures.
Tissue microarray (TMA) construction
Following the histopathological examination of the collected tumors, TMAs were prepared from selected areas of paraffin donor blocks, as in our previous studies.11,22 Three 2.0 mm core punches with potentially the highest tumor cell content were taken from each tumor using a Manual Tissue Arrayer I (Beecher Instruments Inc., Sun Prairie, WI, USA) and transferred into the recipient paraffin block.
Proliferation and apoptosis assay
Proliferation of cancer cells
The proliferation of cancer cells was estimated using immunohistochemical (IHC) expression of Ki-67 antigen. IHC reactions were performed, as previously described, on 4-µm-thick TMA sections in an automated Autostainer Link48 staining platform (Dako, Glostrup, Denmark), in order to ensure constant reaction conditions.11 Deparaffinization, rehydratation, and antigen retrieval were performed by boiling the sections in Target Retrieval Solution buffer (Dako) using a Pre-Treatment Link Platform (Dako). The sections were then washed in a TBS/0.05% Tween buffer followed by 5 min incubation with the EnVision FLEX Peroxidase-Blocking Reagent to block the endogenous peroxidase activity. Subsequently, the sections were rinsed in the TBS/0.05% Tween buffer and incubated with a primary antibody directed against the Ki-67 antigen (MIB-5, Dako). The sections were then washed in the TBS/0.05% Tween, followed by incubation (20 min at room temperature (RT)) with EnVision FLEX/horseradish peroxidase-conjugated secondary antibodies (Dako). The substrate for peroxidase, diaminobenzidine (Dako), was then applied and the sections were incubated for 10 min at RT. Finally, the sections were rinsed and counterstained with Mayer’s hematoxylin, dehydrated in alcohol (70, 96, 99.8%) and xylene, and mounted using the SUB-X Mounting Medium (Dako).
Apoptosis detection
Apoptosis was detected with the ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA, USA), as previously described.11 Four-µm-thick TMA sections were dewaxed in xylene, rehydrated in alcohol, and rinsed in distilled water and 1 × PBS, pH 7.4. The sections were then incubated in Proteinase K (Dako) for 5 min at RT and rinsed in 1 × PBS. The endogenous peroxidase was blocked by 5 min incubation in 3% H2O2/1 × PBS. Subsequently, the sections were incubated with the Equilibration Buffer for 10 min at RT, which was followed by incubation with the TdT Enzyme and Reaction Buffer at 37℃ for 1 h. The reaction was stopped by 10 min incubation in the Stop Buffer, and the sections were rinsed in 1 × PBS. Antidioxygenin peroxidase-conjugated antibodies were then applied for 30 min at RT. Following this, sections were incubated with diaminobenzidine (Dako) for 10 min in order to visualize TUNEL-positive cell nuclei. Finally, the sections were counterstained with Mayer’s hematoxylin and, following dehydration in alcohols, mounted in the SUB-X Mounting Medium (Dako).
Evaluation of IHC reactions
The IHC sections were evaluated under a BX-41 light microscope equipped with CellD software for computer-assisted image analysis (Olympus Tokyo, Japan). For the evaluation of the Ki-67 antigen and TUNEL-positive cells in the TMA sections, three fields with the highest number of tumor cells yielding a positive reaction were selected (“hot spots”). The percentage of positive cells in each hot spot was evaluated by scoring brown-labeled nuclei of cancer cells under ×400 magnification. The average score of the three hot spots was recorded for each tumor.
Statistical analysis
Statistical analysis was performed using Prism 5.0 (Graph Pad, La Jolla. CA. USA) and Statistica 10.0 (Statsoft, Cracow, Poland). The normality of distribution was verified using the Shapiro–Wilk test and the homogeneity of variance with Levene’s test. Depending on the variables and their nature, non-parametric tests (the Mann–Whitney U-test and the Kruskal–Wallis test) or parametric tests (Student’s t-test, ANOVA with one-sided Dunnett’s test) were utilized. In addition for tumor incidence was performed Mantel Cox test.
The results were considered statistically significant when P < 0.05 in all the analyses.
Results
Tumor induction in sedentary group (SC) versus training groups
In the MIT group, two rats died during the experiment, so the analysis of results in this group involves only 10 animals. In the HIT group, five rats did not complete the physical training regimen, so the analysis was performed on seven animals. In summary, the analysis of results in these training groups (LIT, MIT, HIT) covers only 29 animals.
The body weight of rats did not differ significantly between each group in the beginning and the end of the experiment (Kruskal–Wallis test, Table 2). There were also non-significant differences in body weight when only two groups (sedentary and trained) were taken into account (Student’s t-test, Table 2).
Table 2.
Weight and MNU doses injected into rats of the experimental groups; data were analyzed using the Kruskal–Wallis test for the analysis in particular groups and Student’s t-test to compare the sedentary group (SC) with the training groups (LIT + MIT + HIT)
| Variables | SC group (n = 14) | LIT group (n = 12) | MIT group (n = 10)* | HIT group (n = 7)† | P | Training Groups LIT + MIT + HIT (n = 29) | SC vs. LIT + MIT + HIT P |
|---|---|---|---|---|---|---|---|
| Average weight at MNU (g) | 105.50 ± 11.12 | 114.16 ± 27.12 | 113.00 ± 35.60 | 98.85 ± 11.49 | 0.44 | 110.06 ± 27.68 | 0.55 |
| Average dose of MNU (mL/kg) | 1.90 ± 0.21 | 2.10 ± 0.44 | 2.08 ± 0.54 | 1.77 ± 0.22 | 0.34 | 2.01 ± 0.45 | 0.40 |
| Average weight at necropsy (g) | 287.50 ± 23.40 | 279.58 ± 20.93 | 289.50 ± 25.10 | 297.14 ± 18.81 | 0.40 | 287.24 ± 22.41 | 0.97 |
Two rats died during the experiment.
Five rats did not complete the physical training regimen.
At the end of the experiment, the autopsy revealed a total of 22 tumors in nine out of 14 rats (64%) in the control sedentary group (SC group), 13 tumors in eight out of 12 rats (67%) in the LIT group, four tumors in four out of 10 rats (40%) in the MIT group, and three tumors in three out of seven rats (43%) in the HIT group (Table 3).
Table 3.
Tumor development characteristics in relation to the level of physical training; Symbols indicate type of test used the analysis; significant P values are bolded
| Variables | SC group (n = 14) | LIT group (n = 12) | MIT group (n = 10) | HIT group (n = 7) | P | Training Groups LIT + MIT + HIT (n = 29) | SC vs. LIT + MIT + HIT P |
|---|---|---|---|---|---|---|---|
| Number of tumors | 22 | 13 | 4 | 3 | 0.16* | 20 | 0.20† |
| Number of affected rats | 9 (64%) | 8 (67%) P = 0.81‡ | 4 (40%) P = 0.28‡ | 3 (43%) P = 0.38‡ | 0.50* | 15 (52%) | 0.44§ |
| Number of tumors per rat | 1.57 | 1.1 P = 0.36‡ | 0.4 P = 0.04‡ | 0.4 P = 0.08‡ | 0.12* | 0.68 | 0.04§ |
Kruskal–Wallis test.
Mann–Whitney test.
Dunnett’s test post hoc analysis.
Unpaired Student’s t-test
Since the Kruskal–Wallis non-parametric test yielded a chi square value of 7.464, p = 0.059, a parametric analysis of variance with Dunnett’s one-tailed post hoc test was employed, even though the normal distribution assumption was not met (Table 3). The mean number of tumors per rat was 1.6 (±1.98) in the SC group, 1.1 (±0.99) in the LIT group, 0.4 (±0.51) in the MIT group, and 0.4 (±0.53) in the HIT group. A statistically significant difference in the number of tumors per rat was noted only between the SC group and the MIT group (P = 0.04, Dunnett’s test; Table 3). Accordingly, at the end of the experiment, the autopsy revealed a total of 22 tumors in nine out of 14 rats (64%) in SC group, and a total of 20 tumors in 15 out of 29 rats (52%) in the animals that underwent training (LIT + MIT + HIT; Table 3). The mean number of tumors per rat was 1.57 (±1.98) in the control group and 0.68 (±0.80) in the training group. There was a statistically significant difference in the number of tumors per rat between the control and all trained groups (P = 0.04, Student’s t-test; Table 3).
The rats of the MIT and HIT groups developed only solitary tumors (40 and 43%, respectively). The rats of the SC and LIT group were identified as having multiple tumors (from two to seven tumors per animal in 64% of rats in SC group and from two to three tumors per animal in 67% of rats in LIT group).
The highest total tumor volume was found in the LIT group, and the lowest in the HIT group, though the differences were not statistically significant (Kruskal–Wallis test; Table 4).
Table 4.
Tumor volume in relation to the training intensity (Kruskal–Wallis test)
| Variables | SC group (n = 14) | LIT group (n = 12) | MIT group (n = 10) | HIT group (n = 7) | P |
|---|---|---|---|---|---|
| Total tumor volume (Σ) (mm3) | 8 241.0 | 10 683.5 | 3 576.5 | 1 897.0 | 0.45 |
| Average volume of tumor (per rat) (mm3) | 588.64 ± 775.98 | 890.29 ± 1 386.73 | 357.65 ± 644.42 | 271.00 ± 600.55 |
Of the analyzed tumors (n = 42), cribriform carcinoma (n = 23) and papillary carcinomas (n = 15) were the most frequent. The tubular carcinoma developed in four rats.
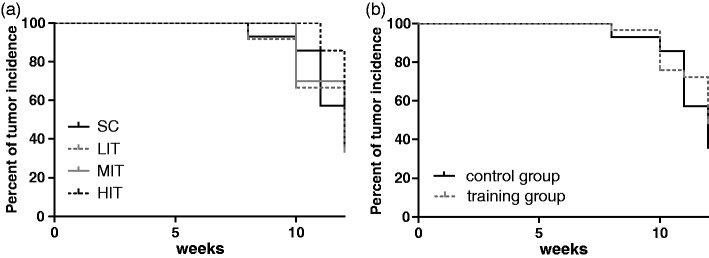
Tumor incidence was not significantly different (Figure 1(a) and (b)).
Figure 1.
Tumor incidence in particular study groups (a) and the combined training groups (b)
Results of Ki-67 expression and the TUNEL
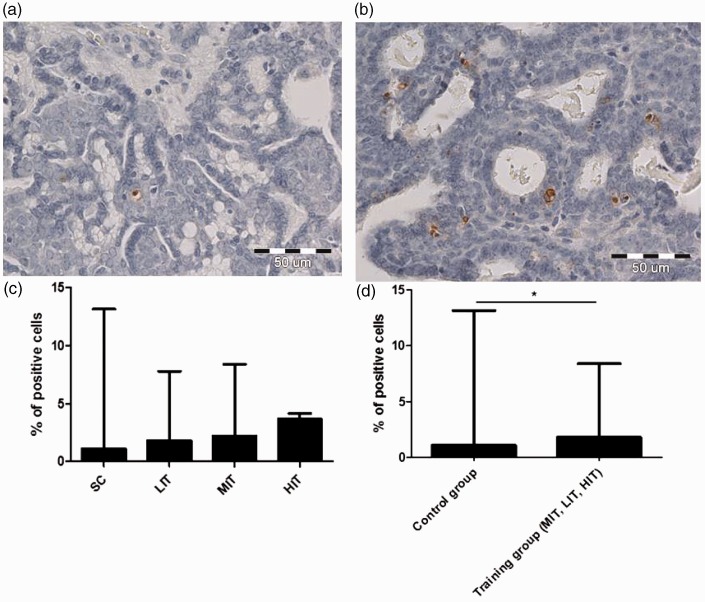
The Ki-67 antigen expression was observed in cancer cells of all in the analyzed tumors. The presence of TUNEL-positive cells was noted in the majority of the cases, though in four tumors of the SC group, no apoptotic cells were observed. An increasing number of TUNEL-positive cells were observed in parallel with the increase in training intensity in all study groups (Figure 2(a) and (b)). Increasing trend was demonstrated in the distribution of the median. The lowest number of TUNEL-positive cells was noted in the SC group (1.09) and was increased in each training group: LIT (1.78), MIT (2.17), and HIT (3.65; Figure 2(c)). When the analysis was performed between two groups: trained (which includes LIT + MIT + HIT) versus control group (SC) the number of TUNEL-positive cells was significantly higher in the trained group (Figure 2(d)).
Figure 2.
Low (a) and high (b) number of TUNEL-positive cells in the analyzed tumor samples; number of TUNEL-positive cells in particular study groups (c) and the combined training groups (d). *P < 0.05; Mann–Whitney test
No significant differences were noted in regard to the expression of the Ki-67 antigen in parallel with the intensity of training. Moreover, the number of Ki-67 and TUNEL-stained cells did not differ between tumor types, and no correlation between the two parameters was noted.
Discussion
Epidemiological investigations provide strong evidence that individuals who are in the highest quartile of physical activity have a lower incidence of breast cancer and lower mortality rates than those who are in the lowest quartile.23–26 Furthermore, it has been shown that moderate or higher intensity of physical activity seems to exert a more pronounced protective effect against cancer development.26
In the present study, the incidence of breast cancer after the administration of MNU was more than twice as low in rats subjected to physical training than in the SC group. Similarly, the number of tumors that developed was lower in all trained groups than in the SC group. A tendency toward a decrease in the number of tumors with the increase in training intensity was observed. Low-intensity physical training led to a diminution in tumor development as compared to the control group, although the differences were not statistically significant. The results of studies investigating the impact of low-intensity physical training are the most equivocal, as both inhibition and enhancement of the carcinogenic process have been reported.27 In an early study of Thompson et al., the low-intensity exercise led to enhanced tumorigenesis.28,29 However, in our study, the low-intensity level was sufficient to reduce breast cancer development. The number of tumors was about 50% lower than in the sedentary control group, although this finding is non-significant.
Many studies have demonstrated the impact of moderate physical activity on carcinogenesis.5,14,27 Our results are in accordance with the previous study of Thompson et al., who demonstrated that health benefits result mainly from moderate-intensity physical activity.27 The positive influence of moderate exercise training on mammary tumor development has also been shown in other studies, in which delayed and diminished tumor growth rates in trained groups have been observed.5,14
Our results show a statistically significant difference between the sedentary and the moderate-intensity trained groups. We have also observed a significantly lower number of induced mammary tumors in the MIT group than in other groups, which suggests the beneficial influence of this level of exercise. It is well accepted that moderate-intensity exercise has an important influence on the immune system.30–32 The link between exercise and the immune system includes the metabolic approach involving amino acid, carbohydrate, and lipid metabolism changes in the neuroendocrine system.32,33 Furthermore, some studies have shown that lipid peroxidation and free radicals increase in humans and animals after physical activity.34,35 It is known that lipid peroxides inhibit tumor cell growth, and this might play a role in the anticancer effect of exercise on tumor growth.32,35–37
The results obtained in our study did not confirm the observations of S’aez et al., who demonstrated a higher rate of breast cancer growth in animals subjected to very intense physical exercise.17 These authors suggested that the increased release of stress hormones during physical exercise could be responsible for the development of malignant tumors.
In our study, the distribution of tumors in individuals was dependent on the training intensity. The rats subjected to moderate- or high-intensity physical training developed solitary, isolated tumors, whereas multiple tumors (ranging from two to seven tumors per animal) were observed in the rats from the sedentary and LIT groups. This tendency may account for the lack of statistical significance of the number of tumors across the groups and, at the same time, confirm the protective effect of moderate- and high-intensity training, indicating that the intensity of physical exercise may be the decisive variable. Previously, Thompson et al. showed that the degree of protection against cancer was proportional to the intensity of physical activity.16
Most malignant lesions induced in this model were carcinomas of the cribriform and papillary types; we noted similar incidences in the histological types of malignant epithelial lesions, as observed in earlier studies with rat strains susceptible to chemical carcinogenesis.38–40
We have not shown significant differences in tumor volume between the control group and the groups subjected to physical training, regardless of the training intensity. Some other authors have observed changes in tumor volume triggered by physical training. Steiner et al. demonstrated that voluntary wheel running reduced the mean tumor volume, although this activity was associated with an increased number of tumors developing in mice.41 On the contrary, Whittal-Strange et al. found an increase in tumor weight under the influence of physical training, especially in the course of high-intensity exercise.18 These findings indicate that the risk of breast cancer development increases as a consequence of physical exercise, therefore pointing to its negative impact in cancer patients.
The total tumor volume is correlated with the apoptosis and proliferation of the tumor’s cells. We did not observe any changes in cell proliferation in the studied groups, as shown by the Ki-67 antigen expression. On the contrary, Westerlind demonstrated an increase in the proliferation of mammary tumor cells of all exercised animals.13 This may be a result of using different marker of proliferation in our study (Ki-67) as compared to PCNA antigen, which was used in study of Westrelind. Our results could indicate that physical exercise did not elevate the risk of cell proliferation or the subsequent increase in tumor volume. By contrast, the tumor volume could decrease by apoptosis of tumor cells. In our study, a significant difference was observed between the number of apoptotic cells in the control and the trained groups. Moreover, the mean number of apoptotic cells increased with training intensity. The highest percentage of apoptotic cells was observed in the tumors of the moderate- and high-intensity trained rats. Since now few studies showed the influence of physical exercise on the induction of the apoptotic process in skeletal muscles and in kidney cells.42–44 These findings are in line with the observations of others who have demonstrated that apoptosis may be one of the mechanisms that slows the progression of malignancy under the influence of physical training.45,46
The increased number of apoptotic cells in Walker 256 mammary-gland-derived tumors of rats subjected to physical exercise has also been shown in the study of De Lima et al.35 Similar results describing the increased apoptosis of tumor cells in prostate and lung cancer in rodents subjected to physical exercise have been observed by other authors.47,48 All these results suggest that physical exercise is associated with the disruption of the balance between apoptosis and cell proliferation.
Conclusions
These data suggest that physical training reduces the number of tumors that develop in rats following carcinogenic MNU administration. Our results indicate that the most efficient protective effect is produced by moderate training. Furthermore, one of the protective mechanisms of exercise in breast cancer might be the activation of apoptosis by physical exercise.
Authors’ Contributions
IM, KS, PD, MPO, MW designed, IM, KS, BP, CK, DH, UP, MC conducted the experiment, IM wrote the manuscript, IM, PD, MPO, MW reviewed of the manuscript, all authors approved the final version.
Acknowledgements
The authors thank Ms Renata Brykner, Mr Jedrzej Grzegrzolka, Ms Aleksandra Jethon, Mrs Łucja Cwynar-Zając, Mrs Aleksandra Piotrowska, Mrs Bożena Przygodzka, Mrs Agnieszka Barańska, Mrs Elżbieta Polejko, and Mr Mateusz Olbromski for their technical assistance. This work was funded by the grant of Polish Ministry of Science N N404 088240 “Impact of physical training on the carcinogenesis and progression of rat mammary glands.” The authors declare that there is no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0 Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 (Internet), Lyon, France, International agency for Research on Cancer, http://globocan.iarc.fr (2013, accessed 18 December 2013).
- 2.Friedenreich CM, Courneya KS, Bryant HE. Influence of physical activity in different age and life periods on the risk of breast cancer. Epidemiology 2001; 12: 604–12. [DOI] [PubMed] [Google Scholar]
- 3.Fong D, Ho J, Hui B, Lee A, Macfarlane D, Leung S, Cerin E, Chan W, Leung I, Lam S, Taylor A, Cheng K. Physical activity for cancer survivors: meta-analysis of randomized controlled trials. Br Med J 2012; 344: e70–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 2014; 25: 1293–311. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Jiang W, McGinley JN, Thompson HJ. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J Appl Physiol 2009; 106: 911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardiff RD, Wellings SR. The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 1999; 4: 105–22. [DOI] [PubMed] [Google Scholar]
- 7.Niemiec J, Ryś J. Morfologia i immunocharakterystyka raka piersi w świetle nowych poglądów na temat karcinogenezy (In Polish). Pol J Pathol 2009; l: 1–9. [PubMed] [Google Scholar]
- 8.Wang M, Yu B, Westerlind K, Strange R, Khan G, Patil D, Boeneman K, Hilakivi-Clarke LM. Prepubertal physical activity up-regulates estrogen receptor b, BRCA1 and p53 mRNA expression in the rat mammary gland. Breast Cancer Res Treat 2009; 115: 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res Suppl 1994; 1: 1960s–3s. [PubMed] [Google Scholar]
- 10.Ko EY, Lee SH, Kim HH, Kim SM, Shin MJ, Kim N, Gong G. Evaluation of tumor angiogenesis with a second-generation US contrast medium in a rat breast tumor model. Korean J Radiol 2008; 9: 243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pula B, Malicka I, Pawlowska K, Paslawska U, Cegielski M, Podhorska-Okolow M, Dziegiel P, Wozniewski M. Immunohistochemical characterization of N-Methyl-N-nitrosourea-induced mammary tumours of Sprague–Dawley rats. In Vivo 2013; 27: 793–802. [PubMed] [Google Scholar]
- 12.Cohen LA. Physical activity and cancer. Cancer Prev 1991; 26: 1–10. [Google Scholar]
- 13.Westerlind KC, McCarty HL, Gibson KJ, Strange R. Effect of exercise on the rat mammary gland: implications for carcinogenesis. Acta Physiol Scand 2002; 175: 147–56. [DOI] [PubMed] [Google Scholar]
- 14.Westerlind KC, McCarty HL, Schultheiss PC, Story R, Reed AH, Baier ML, Strange R. Moderate exercise training slows mammary tumour growth in adolescent rats. Eur J Cancer Prev 2003; 12: 281–7. [DOI] [PubMed] [Google Scholar]
- 15.Thompson HJ, Westerlind KC, Snedden JR, Briggs S, Singh M. Inhibition of mammary carcinogenesis by treadmill exercise. J Natl Cancer Inst 1995; 87: 453–6. [DOI] [PubMed] [Google Scholar]
- 16.Thompson HJ, Westerlind KC, Snedden JR, Briggs S, Singh M. Exercise intensity dependent inhibition of 1-methyl 1-nitrosourea induced mammary cancer in female F344 rats. Carcinogenesis 1995; 16: 1783–6. [DOI] [PubMed] [Google Scholar]
- 17.S’aez C, Barriga C, Garcia JJ, Rodriguez AB, Ortega E. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effect of the hormone melatonin. Mol Cell Biochem 2007; 294: 19–24. [DOI] [PubMed] [Google Scholar]
- 18.Whittal-Strange KS, Charan S, Parkhouse WS. Exercise during puberty and NMU-induced mammary tumorigenesis in rats. Breast Cancer Res Treat 1998; 47: 1–8. [DOI] [PubMed] [Google Scholar]
- 19.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol 2011; 50: 167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podhorska-Okołów M, Krajewska B, Carraro U, Zabel M. Apoptosis in mouse skeletal muscles after physical exercise. Folia Histochem Cytobiol 1999; 37: 127–8. [PubMed] [Google Scholar]
- 21.Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia 2000; 5: 187–200. [DOI] [PubMed] [Google Scholar]
- 22.Kobierzycki C, Wojnar A, Dziegiel P. Expression of SATB1 protein in the ductal breast carcinoma tissue microarrays – preliminary study. Folia Histochem Cytobiol 2013; 51: 333–8. [DOI] [PubMed] [Google Scholar]
- 23.Thune I, Furberg AS. Physical activity and cancer risk: doserespons and cancer, all sites and site-specific. Med Sci Sports Exerc 2001; 33: 530–50. [DOI] [PubMed] [Google Scholar]
- 24.McTiernan A, Kooperberg Ch, White E, Wilcox S, Coates R, Adams-Campbell L, Woods N, Ockene JA. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA 2003; 290: 1331–6. [DOI] [PubMed] [Google Scholar]
- 25.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med 2008; 42: 636–47. [DOI] [PubMed] [Google Scholar]
- 26.Thompson HJ, Wolfe P, McTiernan A, Jiang W, Zhu Z. Wheel running induced changes in plasma biomarkers and the carcinogenic response in the 1-methyl 1-nitorosourea induced rat model for breast cancer. Cancer Prev Res 2010; 3: 1484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat 1997; 46: 135–41. [DOI] [PubMed] [Google Scholar]
- 28.Thompson HJ, Ronan AM, Ritacco KA, Tagliaferro AR, Meeker LD. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res 1988; 48: 2720–3. [PubMed] [Google Scholar]
- 29.Thompson HJ, Ronan AM, Ritacco KA, Tagliaferro AR. Effect of type and amount of dietary fat on the enhancement of rat mammary tumorigenesis by exercise. Cancer Res 1989; 49: 1904–8. [PubMed] [Google Scholar]
- 30.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc 1999; 31: 57–66. [DOI] [PubMed] [Google Scholar]
- 31.Bacurau RFP, Belmonte MA, Seelaender MCL, Costa Rosa LF. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem Funct 2000; 18: 249–28. [DOI] [PubMed] [Google Scholar]
- 32.De Lima C, Alves L, Iagher F, Machado AF, Kryczyk M, Yamazaki RK, Brito GA, Nunes EA, Naliwaiko K, Fernandes LC. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur J Appl Physiol 2008; 104: 957–64. [DOI] [PubMed] [Google Scholar]
- 33.Costa Rosa LF. Exercise as a time-conditioning effector in chronic disease: a complementary treatment strategy. Evid Based Complement Alternat Med 2004; 1: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gago-Dominguez M, Castelao JE, Pike MC, Sevanian A, Haile R. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 2829–39. [DOI] [PubMed] [Google Scholar]
- 35.De Lima C, Alves L, Iagher F, Franzoi Machado A, Kryczyk M, Key Yamazaki R, Pereira Brito GA, Nunes E, Naliwaiko K, Cláudio Fernandes L. Tumor growth reduction in Walker 256 tumor-bearing rats performing anaerobic exercise: participation of Bcl-2, Bax, apoptosis, and peroxidation. Appl Physiol Nutr Metab 2011; 36: 533–8. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez MJ, Schemmel RA, Dugan L, Gray JI, Welsch CW. Dietary fish oil inhibits human breast carcinoma growth: a functional of increased lipid peroxidation. Lipids 1993; 28: 827–32. [DOI] [PubMed] [Google Scholar]
- 37.Palozza P, Calviello G, Maggiano N, Lanza P, Ranelletti FO, Bartoli GM. Beta-carotene antagonizes the effects of eicosapentaenoic acid on cell growth and lipid peroxidation in WiDr adenocarcinoma cells. Free Radic Biol Med 2000; 28: 228–34. [DOI] [PubMed] [Google Scholar]
- 38.El-Aziz MA, Hassan HA, Mohamed MH, Meki AR, Abdel-Ghaffar SK, Hussein MR. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma: an animal model. Int J Exp Pathol 2005; 86: 383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaafar H, Mohamad Idris F, Mohd Nafi SN. The association between phenotype and size of breast tumors induced by 1-methyl-1-nitrosourea (MNU) injection in rats. Med Sci Monit 2009; 15: 129–34. [PubMed] [Google Scholar]
- 40.Faustino-Rocha A, Silva A, Teixeira-Guedes CI, Lopes C. Ultrasonographic, thermographic and histologic evaluation of MNU-induced mammary tumors in female Sprague–Dawley rats. Biomed Pharmacother 2013; 68: 771–6. [DOI] [PubMed] [Google Scholar]
- 41.Steiner JL, Davis JM, McClellan JL, Enos RT, Murphy EA. Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Int J Oncol 2013; 42: 1466–72. [DOI] [PubMed] [Google Scholar]
- 42.Podhorska-Okolow M, Sandri M, Zampieri S. Apoptosis of myofibres and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropathol Appl Neurobiol 1998; 24: 518–31. [DOI] [PubMed] [Google Scholar]
- 43.Phaneuf S, Leeuwenburgh C. Apoptosis and exercise. Med Sci Sports Exerc 2000; 33: 393–6. [DOI] [PubMed] [Google Scholar]
- 44.Podhorska-Okolow M, Dziegiel P, Murawska-Cialowicz E, Saczko J, Kulbacka J, Gomulkiewicz A, Rossini K, Jethon Z, Carraro U, Zabel MM. Effect of adaptive exercise on apoptosis in cells of rat renal tubuli. Eur J Appl Physiol 2007; 99: 217–26. [DOI] [PubMed] [Google Scholar]
- 45.Staunton MJ, Gaffney EF. Apoptosis. Basic concepts and potential significance in human cancer. Arch Pathol Lab Med 1998; 122: 310–9. [PubMed] [Google Scholar]
- 46.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev 2001; 10: 287–301. [PubMed] [Google Scholar]
- 47.Teixeira GR, Fávaro WJ, Pinheiro PF, Chuffa LG, Amorim JP, Mendes LO, Fioruci BA, Oba E, Martins OA, Martinez M, Martinez FE. Physical exercise on the rat ventral prostate: steroid hormone receptors, apoptosis and cell proliferation. Scand J Med Sci Sports 2012; 22: 86–92. [DOI] [PubMed] [Google Scholar]
- 48.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer. Epub ahead of print 2014. DOI: 10.1002/cncr.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]