Abstract
The composite of poly-lactic-co-glycolic acid (PLGA) and calcium phosphate cements (CPC) are currently widely used in bone tissue engineering. However, the properties and biocompatibility of the alendronate-loaded PLGA/CPC (APC) porous scaffolds have not been characterized. APC scaffolds were prepared by a solid/oil/water emulsion solvent evaporation method. The morphology, porosity, and mechanical strength of the scaffolds were characterized. Bone marrow mesenchymal stem cells (BMSCs) from rabbit were cultured, expanded and seeded on the scaffolds, and the cell morphology, adhesion, proliferation, cell cycle and osteogenic differentiation of BMSCs were determined. The results showed that the APC scaffolds had a porosity of 67.43 ± 4.2% and pore size of 213 ± 95 µm. The compressive strength for APC was 5.79 ± 1.21 MPa, which was close to human cancellous bone. The scanning electron microscopy, cell counting kit-8 assay, flow cytometry and ALP activity revealed that the APC scaffolds had osteogenic potential on the BMSCs in vitro and exhibited excellent biocompatibility with engineered bone tissue. APC scaffolds exhibited excellent biocompatibility and osteogenesis potential and can potentially be used for bone tissue engineering.
Keywords: Bone tissue engineering, alendronate, mesenchymal stem cells, biocompatibility
Introduction
Ideal materials for bone tissue engineering should have good biocompatibility, easy molding, and suitable pore sizes, which would ensure the supply of nutrients and ingrowth of new bone tissue.1–3 Single biological material is usually impossible to achieve all the functions. Therefore, composition of different materials becomes the focus of research. Calcium phosphate bone cement (CPC) is a bone repair material with good biocompatibility and bone conduction, and is considered as an ideal matrix material for repairing bone defect.4–7 Poly-lactic-co-glycolic acid (PLGA) has been widely applied in bone tissue engineering owing to its excellent biocompatible and biodegradable properties and has been approved by FDA for its clinical uses. PLGA can also be used for sustained release of carrier medical materials, drugs, and cytokines.8 Alendronate is a third generation of nitrogen-containing bisphosphonates and can strongly inhibit bone resorption, prevent bone loss, increase bone mass, and reduce the incidence of fracture.9,10 CPC porous scaffold containing alendronate-loaded PLGA microparticles provides 3D space for cells to adhere and proliferate, and the loaded alendronate promotes osteoblast differentiation. In this study, we used alendronate-loaded PLGA microspheres and CPC for bone tissue engineering, which not only increased the mechanical properties and absorbability of CPC, but also improved the osteogenic activity via slow release of alendronate. Our results also showed that bone marrow mesenchymal stem cells (BMSCs) had excellent adhesion, proliferation, and compatibility with the engineered bone tissues.
Materials and methods
Materials
PLGA 50:50, inherent viscosity 1.13 dL/g, and mw 50,000 was purchased from Lakeshore Biomaterials (Birmingham, AL, USA). Alendronate, polyvinyl alcohol (PVA; mw 30,000–70,000), percoll, PBS, cell counting kit-8 (CCK-8), and culture plates was purchased from Sigma (St Louis MO, USA). DMEM/F12 media, fetal calf serum (FCS) were purchased from HyClone (Logan, Utah, USA). Penicillin/streptomycin/amphotericin B was obtained from Sangon Biotech (Shanghai, China).
Preparation of microsphere and scaffold
The alendronate-loaded microspheres were prepared using solid/oil/water (s/o/w) emulsion solvent evaporation method as reported previously with slight modifications.11,12 PLGA with a lactic to glycolic acid ratio of 50:50 and a weight–average molecular weight of 20,000 Da was used for the microsphere preparation. Briefly, PLGA was dissolved into dichloromethane and the organic solution was mixed by vortexing for 30 s with 300 µl of 1% alendronate in water. The mixture of water–oil emulsion was added into a 0.3% w/v aqueous PVA solution with stirring at 800–1000 rpm for 5 h to evaporate the solvent. The separated microspheres were washed thrice and resuspended in water followed by lyophilization on ATR FD 3.0 system (ATR Inc., St. Louis, MO, USA) and stored until further use at 4℃. PLGA microspheres and CPC powder were mixed at a ratio of 3:1 at room temperature. The mixture of PLGA and CPC were injected as a paste into a mold with diameter of 6 mm and length of 10 mm, resulting in alendronate-loaded PLGA/CPC (APC) scaffolds. Each piece of scaffold containing 2 mg of alendronate was lyophilized and stored at −20℃ until further use. CPC without PLGA scaffolds (referred as CPC scaffolds) or CPC with PLGA but without alendronate scaffolds (referred as CPC/PLGA scaffolds) were also prepared as controls.
Characterization of microsphere and scaffold
The microspheres and scaffolds were sputter-coated with gold and observed by scanning electron microscopy (SEM, JSM-6390, JEOL, Tokyo, Japan) at 10 kV. The encapsulation efficiency of APC microspheres was determined by suspending 50 mg microspheres into 2 mL PBS buffer (pH = 7.2) and incubating at 37℃ for 2 h during which the supernatant was periodically analyzed as described previously.13 Alendronate release from PLGA microspheres was measured in PBS at 37℃ with 5% CO2 and 95% humidity, using a UV-spectrophotometer and the amount of alendronate released was calculated using a standard calibration curve. The compressive strength of the scaffolds was determined at a loading speed of 1 mm/min using an RGD-5 Test Instrument (Shenzhen, Guangdong, China). Five scaffolds (diameter of 6 mm and length of 10 mm) were tested for each group. The porosity of the scaffolds in distilled water was assessed by the Archimedes method using the following formula: Porosity = (m2−m1)/(m2−m3)× 100%, where m1 is the dry weight of scaffold, m2 is the saturated wet weight of scaffold, and m3 is the wet weight of scaffold suspended in water.
Biocompatibility in vitro
Isolation and culturing of rabbit BMSCs in vitro:
All experimental animals were approved by the Shandong University Laboratory Animal Care (Jinan, Shandong, China) and our study was carried out in strict accordance with the Guidelines on the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research and the Guidelines of Animal Care. Bone marrow fluid was collected in the centrifuge tube under aseptic conditions from the tibia and femur condyle of four anaesthetized New Zealand rabbits (0.80 kg, one month old, male). Bone marrow fluid–PBS mixture (10 mL) was allowed to stand for 12 min, and then supernatant was slowly transferred to a 15 mL centrifuge tube containing an equal amount of Percoll. After 30 min of centrifugation (2000 rpm) in no braking mode, mononuclear cells were collected from the interphase (cloud-like cell layer), and mixed with 10 mL of culture medium consisting of 89% DMEM/F12, 10% FCS, and 1% penicillin/streptomycin/amphotericin B, 10 mmol/L β-glycerophosphate, 50 µmol/L ascorbic acid and 0.1 µmol/L dexamethasone. Cells were cultured at the condition of 37℃, 5% CO2, and 95% humidity. The culture medium was changed every two days, and the cells were subcultured by trypsin digestion at 90% confluence. We choose well growing cells at the third generation for the following experiments.
Cell seeding:
The CPC scaffold, PLGA/CPC scaffold, and APC scaffold were placed in the culture plate respectively, and cell suspensions were seeded on the scaffolds surface (2 × 105 cells/scaffold). The culture plate with scaffold material and cells were cultured at 37℃ with 5% CO2 and 95% humidity. The culture medium was changed every day.
Examination of cell morphology:
To observe cell adhesion on the scaffold surface, the cell-containing scaffolds were rinsed with PBS gently before the specimens were fixed with 2.5% glutaraldehyde in PBS for 1–2 h, followed by washing with PBS, and subsequent dehydration sequentially in 30, 50, 70, 80, 90, and 100% ethanol at 37℃ for 10–15 min. Subsequently, the samples were sputter-coated with gold and examined by scanning electron microscopy (SEM, JSM-6390, JEOL, Tokyo, Japan) at 10 kV.
Examination of cell adhesion and proliferation:
The CPC scaffold, PLGA/CPC scaffold, and APC scaffold were placed in 96-well plates. Control wells were left blank without scaffold. BMSCs at a density of 5 × 103/cm2 were seeded on well containing scaffolds (n = 3 × 15) and the blank control wells (n = 15). The cells in the 96-well plates with or without scaffold were cultured at 37℃ with 5% CO2 and 95% humidity. After 4, 8, and 12 h, CCK-8 was used to determine cell adhesion. Briefly, 10 µl of CCK-8 solution was added to each well, and the 96-well plate was continuously incubated at 37℃ for 1 h. Subsequently, the absorbance was read at 450 nm with an automated microplate reader (BioTek, Highland Park, IL, USA). All procedures were repeated three times and cell adhesion for four groups was analyzed.
To determine the cell proliferation, scaffold was placed in the 96-well plates as described above. BMSCs at a density of 3 × 104/cm2 were seeded on the scaffold wells (n = 3 × 20) and the blank control wells (n = 20). The culture plates were cultured at 37℃ with 5% CO2 and 95% humidity. The culture medium was changed every day. On days 1, 3, 5, and 7 after cell seeding, cell proliferation was determined using CCK-8 assay as described above. All procedures were repeated three times and cell proliferation for four groups was analyzed.
Cell cycle analysis:
Scaffold was placed in the 96-well plates as described above. BMSCs at a density of 1 × 109/L were seeded on the wells with or without scaffold. After incubation for one week, cells were collected for cell-cycle analysis by flow cytometry.
Alkaline phosphatase (ALP) activity:
BMSCs were seeded on the wells containing CPC scaffold, PLGA/CPC scaffold, APC scaffold and blank wells at a density of 3 × 105 cells/well in 96-well plates. After 7 and 14 days of culture, alkaline phosphatase (ALP) activity was quantified by determining the specific conversion of p-nitrophenyl phosphate into p-nitrophenol. The enzyme reaction was carried out at 37℃ water bath for 60 min and then terminated by adding distilled water containing 2 M NaOH and 0.2 mM EDTA. The absorbance of p-nitrophenol was measured at 410 nm using a spectrophotometer. The absorbance was directly converted to ALP activity level based on a standard curve, which was obtained with pNP values ranging from 0 to 600 µmol/mL.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD) and the statistical analysis was determined using single factor analysis of variance (ANOVA) with Bonferroni’s post hoc tests for comparison. All statistical analyses were performed with SPSS software (version 17.0, Chicago, IL, USA) and differences were considered significant at p < 0.05.
Results
Characterization of microsphere and scaffold
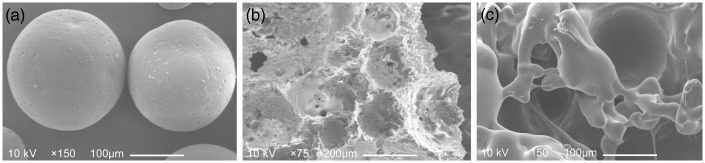
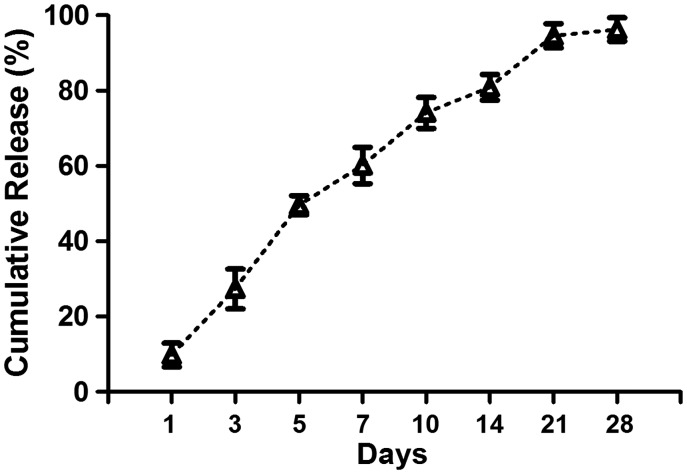
Representative SEM micrographs of alendronate-loaded PLGA microspheres and APC scaffold were shown in Figure 1(a) and (b). Characterization of microspheres and scaffolds are shown in Table 1 and Table 2, respectively. Alendronate-loaded PLGA with uniform size of microspheres were successfully prepared by s/o/w emulsion solvent evaporation method and exhibited good sphericity. Alendronate-loaded PLGA microspheres had a mean diameter of 210 ± 90 (mean ± SD) µm. It is worth noting that microspheres showed narrow size distributions with a diameter of 210 µm, which was promising for good reproducibility and repeatable release behavior. The encapsulation efficiency for PLGA microsphere had a high value of 75.12% for alendronate. Our in vitro release data shown in Figure 2 exhibited that alendronate-loaded PLGA microspheres expressed a common exponential tendency with an initial burst; accordingly, 60% of total release had been achieved during first seven days. Up to day 28, the total alendronate release was almost completed. The diameter of 213 ± 95 µm pore can be seen in the APC scaffold with high porosity. PLGA microspheres were uniformly embedded in scaffold. The porosity of the APC scaffolds was 67.43 ± 4.2%, and the compressive strength was 5.79 ± 1.21 Mpa, which were lower than those of CPC scaffolds, but was close to the cancellous bone that can fully meet the clinical requirement. The addition of alendronate had no impact on the features of microsphere and scaffold.
Figure 1.
Scanning electron micrographs of alendronate-loaded PLGA microspheres (a), APC scaffold without cell (b) and BMSCs seeded on APC scaffold (c). (a) Alendronate-loaded PLGA microparticles; (b) APC scaffold which had been soaked in PBS for 15 days for the dissolving of microspheres; (c) Cell morphology on the APC scaffold after five days of seeding.
Table 1.
Characterization of microsphere.
| Diameter(µm) | Encapsulation (%) | |
|---|---|---|
| No drug-loaded PLGA microparticles | 215 ± 92 | 0 |
| Alendronate-loaded PLGA microparticles | 210 ± 90 | 75.12 ± 6.03 |
Table 2.
Characterization of scaffold.
| Pore (µm) | Porosity (%) | Compressive strength (Mpa) | |
|---|---|---|---|
| CPC scaffold without PLGA | 3 ± 1.9 | 4.43 ± 2.2 | 8.80 ± 1.51 |
| No drug-loaded PLGA/CPC scaffold | 208 ± 86 | 66.21 ± 3.0 | 5.86 ± 1.07 |
| Alendronate-loaded PLGA/CPC (APC) scaffold | 213 ± 95 | 67.43 ± 4.2 | 5.79 ± 1.21 |
Figure 2.
Drug release profile of alendronate-loaded PLGA microspheres.
Data represents mean ± 2SD.
Cell morphology
SEM showed that BMSCs can attach and spread over the APC scaffold surface (Figure 1(c)). After five days of seeding, cells were well distributed and firmly attached on the surface of the scaffold.
Cell adhesion and proliferation
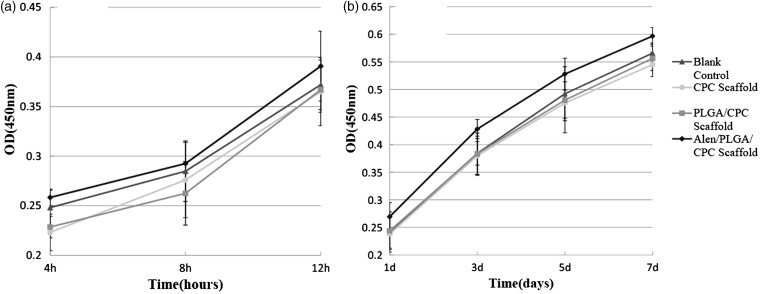
The effect of scaffold on cell adhesion and proliferation was tested using CCK-8 assay, which measures the metabolic activity and the number of cells. The results showed that BMSCs on the APC scaffold had significantly higher cell adhesion and proliferation than those on CPC or PLGA/CPC scaffolds after one day of incubation (Figure 3(a) and (b)). These results suggest that APC scaffold promotes BMSCs adhesion and proliferation.
Figure 3.
Adhesion (a) and proliferation (b) of cells on APC scaffolds and control (blank control, CPC scaffolds, and PLGA-CPC scaffolds).
Data represents mean ± 2SD.
Cell-cycle analysis
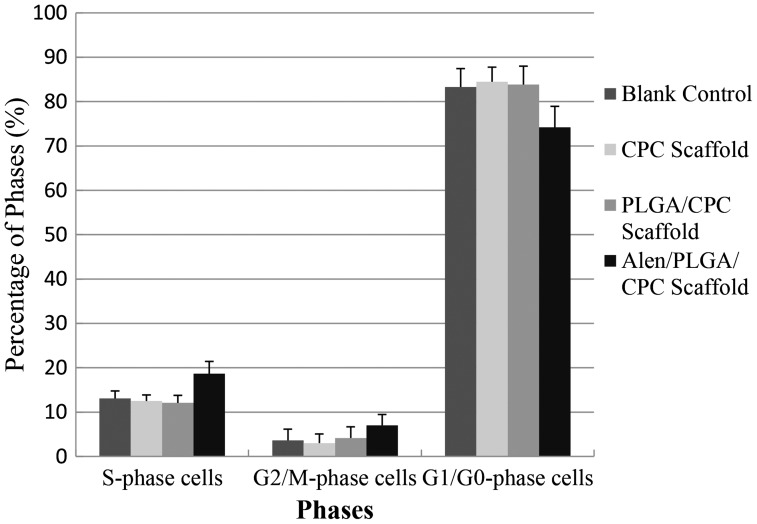
BMSCs seeded on APC scaffold exhibited a higher percentage of S and G2/M phases than those seeded on CPC or PLGA/CPC scaffolds or blank wells (Figure 4). In addition, the results also showed that most cells seeded on the CPC scaffold did not enter the cell cycle and lag in the G0/G1 phase. These results indicated that cells on APC scaffold were promoted to enter the proliferative phase.
Figure 4.
Cell cycle analysis results of BMSCs on APC scaffolds and control (blank control, CPC scaffolds and PLGA-CPC scaffolds).
Data represents mean ± 2SD.
ALP activity
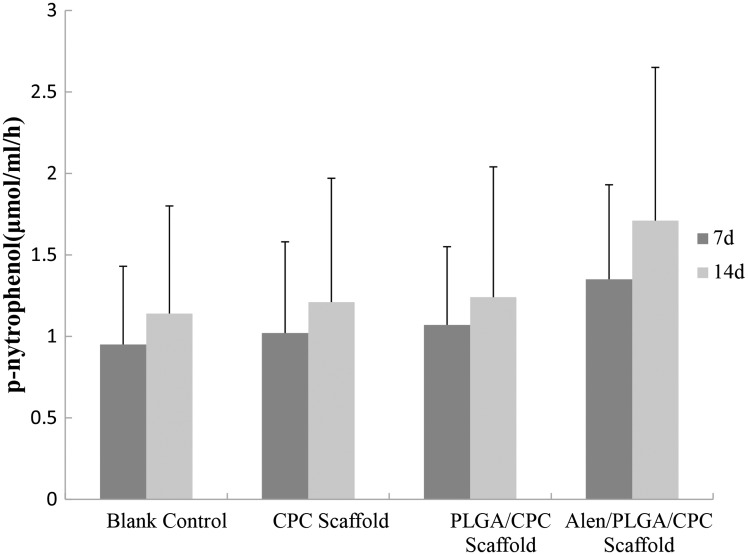
ALP is a well-known enzyme considered as an osteogenic marker. The results showed that BMSCs seeded on APC scaffold had higher ALP activity than those seeded on CPC or PLGA/CPC scaffolds or blank wells after one and two weeks of culture (Figure 5). This is probably due to the high proliferation rate and high osteogenic potential of the BMSCs seeded on the APC scaffold.
Figure 5.
The ALP activity of BMSCs seeded on APC scaffolds and control (blank control, CPC scaffolds, and PLGA-CPC scaffolds).
Data represents mean ± 2SD.
Discussion
The treatment of bone defects can present complex and difficult management dilemmas for the orthopedic surgeon. Currently, autologous bone graft is the clinical gold standard for the treatment of bone defects. However, limited source of autologous bone and complications restricted its clinical applications. Allogenic bone transplantation has potential risk of immune rejection and disease transmission. Composite bone substitute materials, also referred as tissue engineered bones with the biodegradable and controlled-release drug delivery system is being extensively investigated for treatment of bone defect. Ideal bone tissue engineering bone materials require a good material properties and biocompatibility to promote new bone ingrowth and nutrient supply.
As a type of bone restorative material, CPC has the following advantages: wide variety of sources, no additional wound at the donor site; good biocompatibility;14 solid–liquid mixture producing a paste with injectable and temporary plastic;15,16 self-curing at room temperature and does not generate heat;16 high osteoconductivity and biodegradable activity; close to the mineral composition and properties of bone tissue;17 and no obvious toxic and side effect and no immunogenicity and carcinogenicity.18,19 However, this kind of cement solidified product is hydroxyapatite. Although it contains hydroxyapatite porous structure, the pore size only has several microns or less. Thus, it cannot allow vascular permeation and cell ingrowth. Furthermore, such bone cement is very stable in the body, and the absorption and degradation usually occur only on the surface and the absorption rate is very slow.20,21 Therefore, CPC, particularly its degradation speed, needs to be improved. One of the reasons for the slow degradation of CPC is due to its lack of pore structure. After the formation of microspheres, porous scaffold is conducive to cell and bone ingrowth. Thus, improvement on CPC’s poor mechanical properties and slow absorption is critical for its clinical applications.22–24 PLGA is the polylactic acid (polylactic acid, PLA) and polyglycolic acid (polyglycolic acid, PGA) co-polymer which was used to improve material property of CPC in this study. PLA and PGA are α-polyester-based polymer material with high plasticity, good mechanical strength and biocompatibility, and excellent degradability in vivo. The degradation rate can be adjusted by the molecular weight and the ratio of PLA and PGA.25 Degradation products of PLA and PGA are eventually decomposed into non-toxic harmless products, e.g. CO2 and H2O through the ester bond, and excreted by the kidneys or other pathways. Studies have shown that drug encapsulation rate of PLGA for simvastatin reaches more than 90%, and drug could be sustainably released over three weeks.26 Our in vitro study showed that alendronate encapsulation rate of PLGA was about 75%, and the profiles of alendronate could release over 28 days.
Alendronate is a third generation nitrogen-containing bisphosphonate that can efficiently inhibit bone resorption, prevent bone loss, increase bone mass and decrease the incidence of fractures.9 Bisphosphonate drugs can damage the cytoskeleton of osteoclasts, affect the ruffled border and sealing zone in the process of osteoclastic bone resorption, directly inhibit osteoclast formation and promote apoptosis of osteoclasts and indirectly inhibit osteoclast differentiation and bone resorption through osteoblasts.10,27 Studies have shown that oral administration of bisphosphonates is complicated by its poor bioavailability (generally < 5%)28 and only 50% of the absorbed drug can be selectively taken up by the skeleton, while the rest is excreted in urine.29 Intravenous administration can improve the utilization of drugs, but may cause complications, e.g. osteonecrosis of the jaw, renal complications, acute syndrome, and gastrointestinal dysfunction.30,31 Local sustained-release of the drug can effectively overcome the defects of the two routes of administration. In addition, it reduces the dosage of drugs and effectively improves its utilization by the bone.32 Our present results also suggest that cell adhesion, proliferation as well as osteogenic differentiation of the BMSCs on APC scaffolds was higher than those on the pure CPC scaffold or PLGA/CPC scaffold, suggesting APC scaffold facilitates cell osteogenic differentiation via the use of alendronate.
Aperture, porosity, and connectivity of the pores are important indicators of porous materials. Although the appropriate pore size of artificial porous materials remains controversial, the consensus view is that the pore size should meet the required spaces for osteons and bone cells to grow in the materials. In 1870s, Klawitter et al.33 considered that suitable bone tissue ingrowth aperture is at least 100 µm, and suitable aperture of carrier is 100–500 µm. Mistry et al.34 believed that a suitable aperture for bone tissue engineering scaffold is 200–400 µm, a pore size that provides a suitable space for cell adhesion, growth, and functioning. New bone tissue can grow into the internal space from the surface of the scaffold, thus forming a network structure. When the porosity is between 30 and 50%, the pores are connected with each other and the scaffold could meet the clinical requirements of the initial strength.35,36 Only with suitable aperture, porosity, and pore connectivity, can the scaffold achieve the purpose of repairing the bone defect. In this study, we found that the distribution of pore size for APC scaffold is around 213 µm and the pores are connected via small channel, which resembles to the structures of the cancellous bones. Porous structure of the composite tissue engineering bone is not only conducive to cell adhesion and ingrowth, blood vessels and nerve ingrowth, but also in favor of the infiltration of nutrients and the excretion of metabolites. In this study, the APC scaffolds were successfully prepared by a s/o/w emulsion solvent evaporation method and exhibited suitable porous structure and compressive strength. In addition, we showed that cells seeded on pure CPC scaffold had a lower proliferation rate than those seeded on PLGA/CPC or APC scaffold, suggesting porous structure have a good capacity of supporting cell growth.
In conclusion, the results obtained in this study demonstrated that APC scaffold has a pore size of 213 ± 95 µm, a porosity of 67.43 ± 4.2%, and a compressive strength of 5.79 ± 1.21 Mpa, which support rapid cell adhesion and proliferation, and osteogenic differentiation. Furthermore, APC scaffold has an excellent biocompatibility with engineered bone tissues in vitro.
ACKNOWLEDGEMENTS
This work was financially supported by the Shandong Provincial Natural Science Foundation, China (No. ZR2011HQ058) and the Independent Innovation Foundation of Shandong University, IIFSDU (No. 2012ZD027). We would like to state that none of the authors has any potential financial conflict of interest related to this manuscript.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. YHL, ZDW, and JML managed the design, preparation of microsphere and scaffold, and evaluated its characterization. WW, CWD, and HXZ managed the biocompatibility of scaffold in vitro. YHL and ZDW managed data collection and analyzed the results. YHL and JML managed the manuscript preparation.
References
- 1.Zhu X, Eibl O, Scheideler L, Geis-Gerstorfer J. Characterization of nano hydroxyapatite/collagen surfaces and cellular behaviors. J Biomed Mater Res A 2006; 79: 114–27. [DOI] [PubMed] [Google Scholar]
- 2.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011; 32: 9622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalwani G, Henslee AM, Farshid B, Lin L, Kasper FK, Qin YX, Mikos AG, Sitharaman B. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 2013; 14: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantz BR, Ison IC, Fulmer MT, Poser RD, Smith ST, VanWagoner M, Ross J, Goldstein SA, Jupiter JB, Rosenthal DI. Skeletal repair by in situ formation of the mineral phase of bone. Science 1995; 267: 1796–8. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Aslani A, Sambito MA, Kalkhoran NM, Slamovich EB, Webster TJ. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A 2008; 84: 265–72. [DOI] [PubMed] [Google Scholar]
- 6.Vorndran E, Geffers M, Ewald A, Lemm M, Nies B, Gbureck U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater 2013; 9: 9558–67. [DOI] [PubMed] [Google Scholar]
- 7.Bohner M, Gbureck U, Barralet JE. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials 2005; 26: 6423–9. [DOI] [PubMed] [Google Scholar]
- 8.Ruhe PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly (dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release 2005; 106: 162–71. [DOI] [PubMed] [Google Scholar]
- 9.Bone HG1, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350: 1189–99. [DOI] [PubMed] [Google Scholar]
- 10.Russell R, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008; 19: 733–59. [DOI] [PubMed] [Google Scholar]
- 11.Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology 2011; 22: 035101–035101. [DOI] [PubMed] [Google Scholar]
- 12.Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 2012; 33: 7164–73. [DOI] [PubMed] [Google Scholar]
- 13.Kuljanin J, Janković I, Nedeljković J, Prstojević D, Marinković V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe (III) ions. J Pharm Biomed Anal 2002; 28: 1215–20. [DOI] [PubMed] [Google Scholar]
- 14.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements: Competitive drug carriers for the musculoskeletal system? Biomaterials 2006; 27: 2171–7. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda M, Masada K, Takeuchi E. Treatment of enchondroma of the hand with injectable calcium phosphate bone cement. J Hand Surg Am 2006; 31: 98–102. [DOI] [PubMed] [Google Scholar]
- 16.Yu L, Li Y, Zhao K, Tang Y, Cheng Z, Chen J, Zang Y, Wu J, Kong L, Liu S, Lei W, Wu Z. A Novel Injectable Calcium Phosphate Cement-Bioactive Glass Composite for Bone Regeneration. PloS One 2013; 8: e62570–e62570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 2002; 395: 44–44. [DOI] [PubMed] [Google Scholar]
- 18.Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials 2000; 21: 1283–1283. [DOI] [PubMed] [Google Scholar]
- 19.Julien M, Khairoun I, LeGeros RZ, Delplace S, Pilet P, Weiss P, Daculsi G, Bouler JM, Guicheux J. Physico-chemical–mechanical and in vitro biological properties of calcium phosphate cements with doped amorphous calcium phosphates. Biomaterials 2007; 28: 956–65. [DOI] [PubMed] [Google Scholar]
- 20.Hong CY, Lin SK, Kok SH, Wong MY, Hong YC. Histologic reactions to a newly developed calcium phosphate cement implanted in the periapical and periodontal tissues. J Formos Med Assoc 1990; 89: 297–297. [PubMed] [Google Scholar]
- 21.Bohner M, Gbureck U, Barralet JE. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials 2005; 26: 6423–9. [DOI] [PubMed] [Google Scholar]
- 22.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A 2005; 74: 533–533. [DOI] [PubMed] [Google Scholar]
- 23.Link DP, van den Dolder J, van den Beucken JJ, Cuijpers VM, Wolke JG, Mikos AG, Jansen JA. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A 2008; 87: 760–760. [DOI] [PubMed] [Google Scholar]
- 24.van de Watering FC, van den Beucken JJ, Walboomers XF, Jansen JA. Calcium phosphate/poly (d, l-lactic-co-glycolic acid) composite bone substitute materials: evaluation of temporal degradation and bone ingrowth in a rat critical-sized cranial defect. Clin Oral Implants Res 2012; 23: 151–9. [DOI] [PubMed] [Google Scholar]
- 25.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 2012; 30: 546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nath SD, Son S, Sadiasa A, Min YK, Lee BT. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int J Pharm 2013; 443: 87–94. [DOI] [PubMed] [Google Scholar]
- 27.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A 1999; 96: 133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conte PF, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004; 9: 28–37. [DOI] [PubMed] [Google Scholar]
- 29.Cremers SC, Pillai GC, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44: 551–70. [DOI] [PubMed] [Google Scholar]
- 30.Body JJ, Diel I, Bell R. Profiling the safety and tolerability of bisphosphonates. Semin Oncol 2004; 31: 73–8. [DOI] [PubMed] [Google Scholar]
- 31.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol 2006; 7: 508–14. [DOI] [PubMed] [Google Scholar]
- 32.von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 2005; 26: 6941–9. [DOI] [PubMed] [Google Scholar]
- 33.Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J Biomed Mater Res 1971; 5: 161–229. [Google Scholar]
- 34.Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol 2005; 94: 1–22. [DOI] [PubMed] [Google Scholar]
- 35.Goshima J, Goldberg VM, Caplan AI. The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin Orthop Relat Res 1991; 262: 298–311. [PubMed] [Google Scholar]
- 36.Nade S, Armstrong L, McCartney E, Baggaley B. Osteogenesis after bone and bone marrow transplantation: the ability of ceramic materials to sustain osteogenesis from transplanted bone marrow cells: preliminary studies. Clin Orthop Relat Res 1983; 181: 255–63. [PubMed] [Google Scholar]