Abstract
Increased reactive oxygen species (ROS) levels produced by hyperglycemia and angiotensin-II (AT-II) are considered among the pathogenic factors in the malignant transformation of diabetic renal cells. We aimed to investigate the potential role of AT-II in the increased cancer risk seen in diabetes; measuring oxidative damage to renal DNA and protective antioxidant defenses, including adiponectin (Adp) and plasma antioxidant capacity by the Ferric Reducing Ability of Plasma (FRAP) method. In the kidney of streptozotocin (STZ)-induced (55 mg/kg) diabetic rats either treated or not treated for 3 weeks with losartan, an AT-II type 1 receptor antagonist (20 mg/kg/day); we measured 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) levels, as an index of oxidative DNA damage, circulating Adp and FRAP. Diabetic rats showed significantly higher 8-oxodGuo levels in renal DNA (8.48 ± 0.98 × 10−6 dG, mean ± SEM n = 11) than normoglycemic ones (1.18 ± 0.04 × 10−6 dG, mean ± SEM, n=7) and lower plasma Adp and FRAP levels in comparison to normoglycemics. The treatment of diabetic rats with losartan significantly (P < 0.01) reduced 8-oxodGuo levels (5.4 ± 0.58 × 10−6 dG, mean ± SEM n=9) in renal DNA and conserved FRAP values. Moreover, an inverse correlation was found between 8-oxodGuo in kidney DNA and circulating Adp levels in normoglycemic and diabetic rats. Losartan treatment preserves FRAP levels, reduces DNA oxidative injury and thus the carcinogenesis risk. Furthermore, our results indicate that Adp plasma levels are a further marker of oxidative injury to the kidney and confirm that it is an important part of the plasma antioxidant defense.
Keywords: STZ-induced diabetes, oxidative DNA damage, 8-oxodGuo, malondialdehyde (MDA) losartan, adiponectin (Adp), FRAP
Introduction
Insulin resistance and diabetes are risk factors for cancer1–3 including kidney and urinary tract cancer. The underlying mechanism(s) responsible for such an association remains unclear.4,5 Abnormal cellular metabolism, caused by insulin resistance and/or hyperglycemia, has been recognized as a possible ethiogenic condition predisposing susceptible cells to malignant transformation by triggering a pro-oxidant status and a permanent pro-inflammatory condition.6 To further exploit the relation between cancer and metabolism and to find predictive and valuable biomarkers of malignancy, the attention of this research is focused on monitoring endogenous factors recognized as protective against insulin resistance, including adiponectin (Adp).7 In fact, Adp opposes a variety of pro-neoplastic events such as inflammation, insulin resistance, cell growth and proliferation. Accordingly, Adp plasma levels are reduced in diabetes and in end-stage renal disease associated with increased risk of several cancers8,9 including renal cell carcinoma.10
Increased levels of reactive oxygen species (ROS) play a pivotal role in the development of diabetic complications; including nephropathy, a well-known risk factor for renal malignancy.11 Among the sources of ROS in diabetes are hyperglycemia and activation of the renin-angiotensin system, which is responsible for increased tissue angiotensin-II (AT-II) levels, a potent hypertrophic and pro-oxidant factor strongly involved in diabetes nephrophaty.12 At present, it is unknown whether the deleterious effects of AT-II in the diabetic kidney include ROS-induced oxidative DNA damage, a process which by favoring the accumulation of mutations13 plays a role in the carcinogenesis process.14,15
We previously published that, diabetic rats sacrificed 2 weeks after streptozotocin (STZ) injection showed clinical signs of diabetic nephropathy associated with increased lipid and protein oxidation, and that all these injuries were reduced in animals treated with losartan,16 an AT-II type 1 receptor (AT1) antagonist.17
In order to evaluate the potential of AT-II and diabetes to cause ROS and DNA damage and increase cancer risk, in the model of early diabetes described above, we measured oxidative damage to kidney DNA, plasma antioxidant capacity and we also analyzed circulating Adp levels.
Materials and methods
Ethics statement
The experimental procedures were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) standard guidelines for the care of animals, and were approved by the Ethics Committee for Animal Experiments of the University of Florence.
Experimental design and induction of diabetes
Wistar rats aged 12–14 weeks (Charles River, Calco, Italy) were randomly divided into four groups (see below for description) and allowed free access to standard dried chow diet and water whose consumption was daily monitored. Two groups of rats were administered losartan by putting it in their drinking water (20 mg/kg/day) until the end of the experiment (3 weeks later). After 1 week, two groups, including one of the two receiving losartan, were injected in the tail vein with citrate buffer pH 4.5 (normoglycemic, N, and normoglycemic, losartan-treated, NLos). The other two groups underwent a single injection of STZ (55 mg/kg in citrate buffer pH 4.5). We checked the plasma glycemia of rats 48 h after STZ injection, and only those rats whose glycemia was higher than 14 mM were considered diabetic and included in our schedule of treatment (diabetic, D, and diabetic, losartan treated, DLos). The losartan concentration was adjusted according to body weight and water consumption to maintain a dosage of 20 mg/kg/day, and the treatment was interrupted 24 h before sacrifice. The body weight and water consumption of each animal were measured three times a week. Twenty-four hours before sacrifice, the animals were fasted overnight. Two weeks after the STZ injection, the rats were weighed, sacrificed, their blood collected in heparinized tubes while their abdominal fat and kidneys were collected and weighed.
Experimental groups: N: normoglycemic rats; NLos: normoglycemic rats treated with losartan for 3 weeks; D: diabetic rats; DLos: diabetic rats treated with losartan for 3 weeks after the induction of hyperglycemia
8-oxodGuo assay
The kidney tissues were homogenized in 50 mM phosphate buffer solution (PBS) containing 0.1 M dithiothreitol and then centrifuged at 4 ℃ for 20 min at 2000 × g. These pellets were used for 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo). The kidney pellets were re-suspended and the DNA was isolated using the method recommended by ESCOOD group.18 The purified DNA (about 50 µg) was hydrolyzed with P1 nuclease (10 IU) and alkaline phosphatase (7 IU). The hydrolyzed mixture was filtered using Micropure-EZ enzyme remover (Amicon, MA, USA) and 50 µl was injected into an HPLC apparatus. The nucleosides were separated by a C18 reverse-phase column (Supelco, 5 µm, I.D. 0.46 cm×25 cm). The 8-oxodGuo and 2dG in the DNA were detected using an ESA Coulochem II electrochemical detector in line with a UV detector as previously described.19
MDA determination as index of lipid oxidation
MDA levels were assayed as already reported16 in kidneys for normoglycemics (N), STZ-induced diabetic (D) not treated and treated with losartan (20 mg/kg/day for 3 weeks).
Determination of Adp plasma levels
We used an ELISA kit from AdipoGen Inc (Vinci Biochem, Italy) (limit of detection: 50 pg/ml) to determine the amount of Adp in rat plasma following the manufacturer's instructions. At our concentrations, CV (coefficient of variation) was 7.4% (within) and 8.4% (between) as we reported previously.20
Ferric reducing ability of plasma (FRAP) assay
The FRAP assay was performed in the plasma according to the method of Benzie and Strain.21 Briefly, FRAP reagent (900 µl) containing 10mM 2,4,6-tripyridyl-S-triazine in 40 mM HCl, 300 mM acetate buffer (pH 3.6) and 20 mM FeCl3 was added to 30 µl of plasma. The change of absorbance (at 593 nm) between the final reading and the blank was calculated for each sample and related to the absorbance of ferric standard solutions.
Statistical analysis
Data were presented as means ± SEM and were analyzed using ANOVA two way analysis and Pearson correlation. A value of P <0.05 was considered statistically significant. For statistical analysis, Graph Pad Prism 5 software (San Diego, CA, USA) was used.
Results
Metabolic parameters
Two weeks after STZ injection, rats from the D and DLos groups showed hyperglycemia, a significant increase in water and food consumption, kidney hypertrophy, and reduction in body weight and in abdominal fat in comparison to normoglycemic rats. Losartan treatment did not affect any of these parameters both in normoglycemic and diabetic rats (Table 1).
Table 1.
Metabolic characteristics of normoglycemic (N), normoglycemic losartan treated (NLos), diabetic (D) and diabetic losartan treated (DLos) rats
| Group | Plasma glycemia (mM) | Body weight (g) | Daily water consumption (ml) | Daily food intake (g) | Kidney weight/ Body weight | Adipose mass/ body weight (%) |
|---|---|---|---|---|---|---|
| N (n = 7) | 7.0 ± 0.01 | 262.1 ± 10.1 | 29.9 ± 2.2 | 17.9 ± 0.8 | 0.70 ± 0.02 | 1.60 ± 0.02 |
| NLos (n = 7) | 9.0 ± 0.03 | 254.7 ± 9.1 | 29.3 ± 1.6 | 19.4 ± 0.5 | 0.79 ± 0.01 | 1.52 ± 0.01 |
| D (n = 11) | 20.0 ± 0.18** | 133.8 ± 6.8** | 128.6 ± 0.9** | 21.7 ± 5.4** | 0.96 ± 0.06** | 0.45 ± 0.09** |
| DLos (n = 9) | 18.7 ± 0.25## | 127.5 ± 10.2## | 130.1 ± 8.6## | 27.3 ± 1.4## | 1.05 ± 0.04## | 0.47 ± 0.07## |
Values are expressed as the mean ± SEM of the number (n) of rats.
**P < 0.001 vs. N.
P < 0.001 vs. NLos.
Oxidative DNA damage
We evaluated oxidative DNA damage in the kidneys of STZ-induced diabetic rats by measuring 8-oxodGuo levels. Two weeks after STZ injection, the amount of 8-oxodGuo in the kidney DNA of the D group reached the value of 8.48 ± 0.98 × 10−6 dG, about six-fold higher than that found in normoglycemics (N) (Figure 1). In the kidneys of diabetic rats treated with losartan (20 mg/kg/day for 3 weeks), 8-oxodGuo levels were 5.4 ± 0.58 × 10 −6 dG, a value only three-fold higher than those measured in normoglycemic rats. Losartan treatment did not modify 8-oxodGuo levels in the kidney DNA of normoglycemic rats (NLos).
Figure 1.
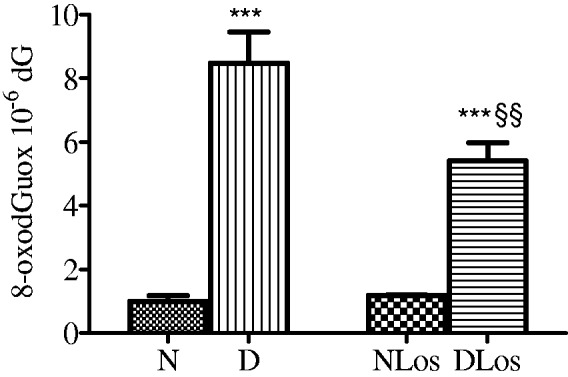
Levels of 8-oxodGuo in kidney DNA of normoglycemic (N; n = 7), diabetic (D; n = 11), normoglycemic losartan treated (NLos, n = 7), and diabetic losartan treated (DLos, n = 9) rats. Values are means ± SEM. ***P < 0.001 D versus N and NLos versus DLos ; §§P < 0.01 DLos versus D
Oxidative damage to lipids
Confirming that already reported, at the present experimental settings, oxidation damage to kidney lipids, measured as MDA levels was found 80% higher (P < 0.05) in diabetic (D) than in normoglycemic tissues (N) (2.4 ± 0.2 μM). Losartan treatment completely prevented diabetes-induced MDA increase making lipid oxidation in diabetic kidney not different from that measured in normoglycemics (2.7 ± 0.5 μM).
Adp plasma levels
As shown in Figure 2, Adp levels were significantly (P < 0.001) lower in diabetic (D) than normoglycemic rats (N). Losartan treatment did not modify circulating Adp levels in diabetic rats (DLos).
Figure 2.
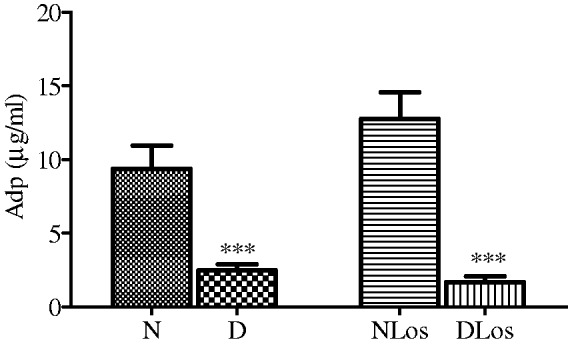
Adp levels in the plasma of normoglycemic (N; n = 6), diabetic (D; n = 9), normoglycemic losartan treated (NLos; n = 6) and diabetic losartan treated (DLos; n = 6) rats. Values are means ± SEM. ***P < 0.001 D versus N and DLos versus. NLos
Correlation between Adp plasma levels and 8-oxodGuo in renal DNA
An inverse correlation was found between oxidative DNA damage (8-oxodGuo) and Adp levels in renal tissue from normoglycemic (N) and diabetic rats (D) (Figure 3). The same correlation persisted in normoglycemic and diabetic rats treated with losartan (r = 0.89; n = 12, P < 0.001; data not shown).
Figure 3.
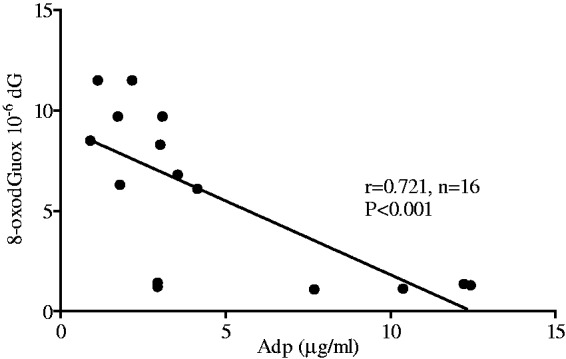
Correlation between 8-oxodGuo levels in kidney DNA and circulating Adp levels in normoglycemic (N; n = 6) and diabetic rats (D; n = 9)
Plasma antioxidant status
Total plasma antioxidant status was estimated by the FRAP assay. As Figure 4(a) shows, FRAP levels were reduced in diabetic (D) vs. normoglycemic (N) rats. In animals treated with losartan (DLos), this reduction was not evident thus indicating the drug exerted a protective effect against STZ-induced reduction of antioxidant defenses. Moreover, as reported in Figure 4(b), FRAP was well correlated with Adp in normoglycemic (N) as well as in diabetic rats (D). The correlation also existed in normoglycemic (NLos) and diabetic rats treated with losartan (DLos) (r = 0.76, n = 12 P < 0.01, data not shown).
Figure 4.
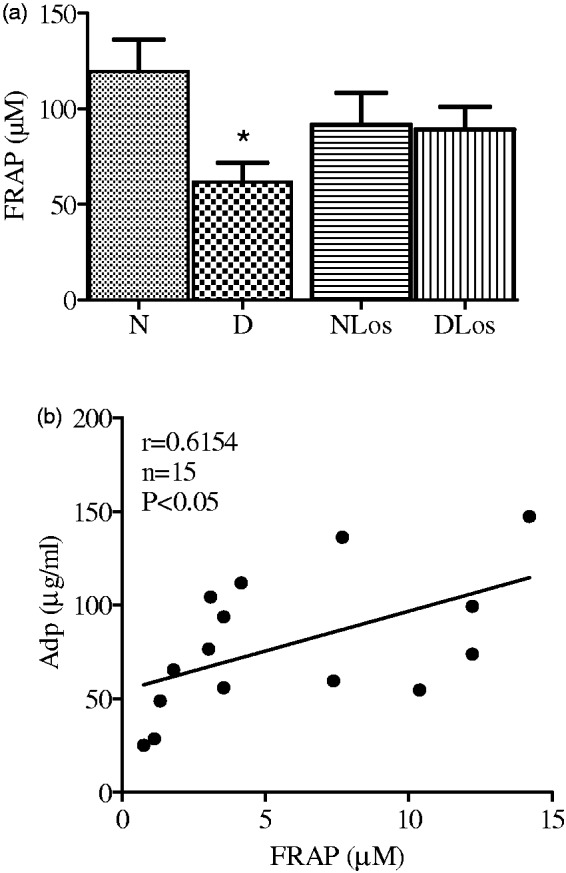
FRAP values in normoglycemic (N; n = 7), diabetic (D; n = 11), normoglycemic losartan treated (NLos; n = 7), and diabetic losartan treated (DLos; n = 9) rats. Values are means ± SEM. *P < 0.05 D versus N (a). Correlation between FRAP and Adp levels in the plasma of normoglycemic (N; n = 7) and diabetic rats (D; n = 9) (b)
Discussion
Experimental diabetes summarizes a condition of extreme metabolic stress triggered by hyperglycemia, increased AT-II levels, and rapid weight reduction, contributing to systemic oxidation damage. In this context, we report here for the first time that 8-oxodGuo levels are found to be higher in the kidney of two-week diabetic rats than in renal tissue of normoglycemic rats. The presence of 8-oxodGuo in DNA is a marker of oxidation damage and it is highly mutagenic, causing G:C>T:A transversions, frequently found in the mutated genes of tumor tissues.15,22 Moreover, our diabetic animals show a lower total plasma antioxidant status and Adp levels than normoglycemics. Interestingly, circulating Adp correlates with 8-oxodGuo levels in the kidney, corroborating the idea that the protective role of this cytokine is extended to controlling the oxidative damage of DNA, an event predisposing to carcinogenesis.22 Moreover, oxidative lipid injury, measured as MDA levels, increased in kidney from diabetic rats in comparison to normoglycemics, confirming previously reported data by our lab.16
In these conditions, treatment with losartan reduces oxidative damage to lipids and to DNA and conserves the plasma antioxidant capacity. Interestingly, these effects are independent of the amelioration of hyperglycemia or on rat body weight gain. This indicates that all of the protective effects observed in diabetic animals treated with losartan can be ascribed to the pharmacodynamic features of the drug including antagonism at the AT-II type 1 receptor. Losartan does not oppose the decrease of Adp plasma levels in accordance with a lack of effect of the drug on adipose mass, the major source of Adp.23
From these results, we endorse the concept that systemic oxidative stress is associated with dysregulation of Adp secretion as already described in humans.24 Adp levels are strongly reduced in diabetic rats but, even if low, are still enough to play a role as an antioxidant limiting STZ-induced oxidative DNA damage. Furthermore, we add the evidence that Adp and 8-oxodGuo were found to be inversely correlated in the kidneys of N rats, irrespective of the treatment received. This result indicates that Adp plasma levels can be considered as an additional marker of renal DNA damage (basal or STZ-induced), thus potentially extending the physiopathological significance of measuring Adp plasma levels. To get further inside the relation between oxidant/antioxidant status, other than Adp, we measured FRAP levels. Our results show that FRAP levels decreased significantly in D vs. N rats. In losartan treated diabetic rats, when oxidant status is reduced, FRAP levels were conserved. However, our results also show a relationship between FRAP and Adp irrespective of the glycemic status, indicating that Adp might be considered part of the plasma antioxidant capacity.
In conclusion, our data indicate that diabetes results in the disruption of the fine balance between oxidant and antioxidant status leading to increased oxidative DNA damage and consequently an increased risk of carcinogenesis.25
Even if the translation of results from bench to patients is often dangerous, our data sustains the addition of losartan to the therapy of diabetic patients at high risk of the malignant transformation of renal cells.
ACKNOWLEDGEMENTS
This work was supported by grants from the University of Florence (Fondo ex-60%).
Author contributions
ML and LR designed the research and wrote the paper. EB, FT and CDS performed the experiments. All authors read and approved the final manuscript.
References
- 1.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin. Diabetologia 2012; 55: 948–58. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes care 2010; 33: 1674–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endoc Relat Cancer 2009; 16: 1103–23. [DOI] [PubMed] [Google Scholar]
- 4.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29: 254–58. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Cancer J Clin 2010; 60: 207–21. [DOI] [PubMed] [Google Scholar]
- 6.De Berardinis RJ. Is cancer a disease of abnormal cellular metabolism?. Genet Med 2008; 10: 767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 2007; 86: s858–66. [DOI] [PubMed] [Google Scholar]
- 8.Saxena A, Baliga MS, Ponemone V, Kaur K, Larsen B, Fletcher E, Greene J, Fayad R. Mucus and adiponectin deficiency: role in chronic inflammation-induced colon cancer. Int J Colorectal Dis 2013; 28: 1267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao LM, Weinstein SJ, Pollak M, Li Z, Virtamo J, Albanes D, Chow WH, Purdue MP. Prediagnostic circulating adipokine concentrations and risk of renal cell carcinoma in male smokers. Carcinogenesis 2013; 34: 109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spyridopoulos TN, Petridou ET, Skalkidou A, Dessypris N, Chrousos GP, Mantzoros CS. Low adiponectin levels are associated with renal cell carcinoma: a case-control study. Obesity and Cancer Oncology Group. Int J Cancer 2007; 120: 1573–78. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 2011; 54: 1013–18. [DOI] [PubMed] [Google Scholar]
- 12.Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep 2012; 14: 360–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–69. [DOI] [PubMed] [Google Scholar]
- 14.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–60. [DOI] [PubMed] [Google Scholar]
- 15.Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med (Berl) 1996; 74: 297–312. [DOI] [PubMed] [Google Scholar]
- 16.Manni ME, Bigagli E, Lodovici M, Zazzeri M, Raimondi L. The protective effect of losartan in the nephropathy of the diabetic rat includes the control of monoamine oxidase type A activity. Pharm Res 2012; 65: 465–71. [DOI] [PubMed] [Google Scholar]
- 17.Burnier M. Angiotensin II type 1 receptor blockers. Circulation 2001; 103: 904–12. [DOI] [PubMed] [Google Scholar]
- 18.Gedik CM, Collins A. ESCODD (European Standards Committee on Oxidative DNA Damage). Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J 2005; 19: 82–4. [DOI] [PubMed] [Google Scholar]
- 19.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxy-guanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med 2000; 28: 13–7. [DOI] [PubMed] [Google Scholar]
- 20.Baldasseroni S1, Mannucci E, Orso F, Di Serio C, Pratesi A, Bartoli N, Marella GA, Colombi C, Foschini A, Valoti P, Mossello E, Fumagalli S, Marchionni N, Tarantini F. Adiponectin in outpatients with coronary artery disease: independent predictors and relationship with heart failure. Nutr Metab Cardiovasc Dis 2012; 22: 292–99. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239: 270–76. [DOI] [PubMed] [Google Scholar]
- 22.Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem 2006; 387: 373–79. [DOI] [PubMed] [Google Scholar]
- 23.Moschen AR, Wieser V, Tilg H. Adiponectin: key player in the adipose tissue-liver crosstalk. Curr Med Chem 2012; 19: 5467–73. [DOI] [PubMed] [Google Scholar]
- 24.Yanagawa Y1, Morimura T, Tsunekawa K, Seki K, Ogiwara T, Kotajima N, Machida T, Matsumoto S, Adachi T, Murakami M. Oxidative stress associated with rapid weight reduction decreases circulating adiponectin concentrations. Endocr J 2010; 57: 339–45. [DOI] [PubMed] [Google Scholar]
- 25.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009; 27: 120–39. [DOI] [PubMed] [Google Scholar]


