Abstract
Vaspin, a novel adipocyte factor secreted from visceral adipose tissues, is associated with obesity and insulin resistance and can regulate glucose and lipid metabolism, increase insulin sensitivity, and suppress inflammation; however, the underlying mechanisms remain unknown. Proliferation and maladaptive differentiation are important pathological mechanisms underlying obesity. This study aimed to evaluate the effects of vaspin on the proliferation and differentiation of preadipocyte 3T3-L1 cells and to explore the likely mechanisms responsible for 3T3-L1 differentiation. Vaspin was added to cultured 3T3-L1 cells, and the differentiation of adipocytes was evaluated using Oil Red O staining. The AKT signaling pathway and specific differentiation factors related to the differentiation of preadipocyte 3T3-L1 cells, peroxisome proliferator-activated γ and the CCAAT/enhancer-binding protein (C/EBP) family, were evaluated using reverse transcription polymerase chain reaction (RT-PCR) and western blot analyses during the early phase of differentiation. Additionally, adiponectin mRNA, interleukin-6 mRNA (IL-6 mRNA), and glucose transporter-4 (GLUT4) protein levels were measured in the differentiated adipocytes. The results indicated that vaspin promotes the intracellular accumulation of lipids and increases differentiation-related factors, including peroxisome proliferator-activated receptor γ, C/EBPα, and free fatty acid-binding protein 4 (FABP4), in a dose-dependent manner. Additionally, vaspin (200 ng/mL) increased the mRNA and protein levels of C/EBPβ, peroxisome proliferator-activated γ, C/EBPα, and FABP4. Moreover, compared with the control, significantly smaller eight-day differentiated adipocytes were observed, and these cells exhibited decreased IL-6 mRNA and increased GLUT4 mRNA levels; these results also indicated the potential of vaspin to promote the insulin-mediated AKT signaling pathway during the early phase of differentiation. In conclusion, vaspin is able to promote the differentiation of 3T3-L1 preadipocytes and may increase their sensitivity to insulin and suppress obesity.
Keywords: Obesity, vaspin, preadipocyte, adipogenesis
Introduction
Obesity is considered as a global human health problem.1 Obesity significantly increases the risk for developing type 2 diabetes, hypertension, coronary heart disease, stroke, and several types of cancers.2–4 Adipose tissue is not only a large energy storage organ but also an important endocrine organ.5 Fat cells produce and secrete a variety of adipokines, which affect insulin sensitivity, glucose and lipid levels, and satiety and appetite.6
Adipose tissue dysfunction plays a key role in the development of obesity and obesity-related diseases.7,8 An imbalance between energy intake and consumption can trigger obesity.9 An increased number of fat cells is primarily caused by the proliferation of preadipocytes and their continuous differentiation into mature adipocytes.10 The mechanisms underlying the complications of obesity are related to changes in the proliferation, differentiation, and function of fat cells.11 The differentiation of fibroblast-like preadipocytes into mature adipocytes involves morphological and biochemical changes. During differentiation, the action of adipogenic genes, including CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor-γ (PPAR-γ), induces adipogenesis.12
Vaspin, which belongs to the family of serine protease inhibitors, is a visceral adipose tissue-specific serine protease inhibitor. Hida et al.13 initially identified the novel adipocyte factor vaspin in the visceral adipose tissue of obese rats with type 2 diabetes (OLETF rats). Administering vaspin to obese mice or rats improved glucose tolerance and insulin sensitivity by inhibiting the expression of proinflammatory adipocytokines, including leptin, resistin, and tumor necrosis factor-α, in mesenteric and subdermal white adipose tissues.13 Vaspin administration reduces food intake and has sustained blood glucose-lowering effects in mice.14 The expression of vaspin in visceral adipose tissue fat and subcutaneous fat tissue was positively correlated with BMI and body fat content, as well as with an increase in body fat and the deterioration of the metabolic disorder. The level of vaspin mRNA was positively correlated with body fat content and plasma insulin level; however, vaspin mRNA was negatively correlated with insulin sensitivity.15 A decreased serum vaspin level and improved insulin sensitivity were observed in obese patients undergoing gastric bypass surgery, indicating that the increased level of vaspin in adipose tissue was closely related to obesity, insulin resistance, and type 2 diabetes.16 However, little is known concerning the potential of obesity for inducing the expression of vaspin, the effect of vaspin on the metabolism of adipocytes and the molecular mechanisms underlying obesity.
The present study aimed to evaluate the effects of vaspin on lipid accumulation and at various times during the differentiation process of adipocytes and to explore the molecular mechanisms underlying the role of vaspin in the proliferation and differentiation of preadipocytes, providing new evidence for the prevention and treatment of obesity and its related diseases.
Materials and methods
Cell culture
Mouse 3T3-L1 preadipocytes (Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences; School of Basic Medicine, Peking Union Medical College, Beijing, China) were grown in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose containing 10% fetal bovine serum (FBS) at 37℃ in a humidified atmosphere with 5% CO2. The cells were subcultured after reaching 80% confluence. To induce adipogenesis, 3T3-L1 cells were cultured until confluent; two days after reaching confluence (day 0), the culture medium was exchanged with a differentiation/induction medium containing 10 µg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone and 10% FBS in DMEM for two days. The cell culture medium was subsequently replaced with DMEM containing 10 µg/mL insulin and 10% FBS for an additional two days. The cells were fed DMEM containing 10% FBS every other day for the next 5–8 days; on day 8, >90% of the cells exhibited an adipocyte phenotype. Recombinant human vaspin (PeproTech, USA) was added to the culture medium at various concentrations during the adipogenic period of eight days. Vaspin cytotoxicity was evaluated using a 3 -(4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for cell viability.
Oil Red O staining
The cells were fixed with 10% formaldehyde/phosphate-buffered saline (PBS) and stained with Oil Red O solution, 6 and 8 days after differentiation induction (0.5% Oil Red O-isopropyl alcohol/H2O (3:2, v/v)). After staining, Oil Red O was extracted from the cells using isopropyl alcohol, and the absorbance was measured at 490 nm. The lipid droplet sizes were analyzed using Image Pro Plus 6.0. At least 10 microscopic fields were examined per well to determine the adipocyte size.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells, and the RNA concentrations were measured using a spectrophotometer. A total of 1 µg of mRNA was reverse transcribed into cDNA and then amplified using the SYBR-Green I reagent (Takara, Tokyo, Japan) in a fluorescence thermocycler. Mouse Gapdh was used as an internal control. The following primer sequences were used: PPAR-γ, 5′-CAC TCG CAT TCC TTT GAC-3′ (forward) and 5′-ACT TTG ATC GCA CTT TGG-3′ (reverse); CCAAT/enhancer-binding protein α (C/EBPα), 5′-GGT GGA CAA GAA CAG CAA-3′ (forward) and 5′-CGG TCA TTG TCA CTG TC-3′ (reverse); CCAAT/enhancer-binding protein β (C/EBPβ), 5′-CAA GAA GAC GGT GGA CAA-3′ (forward) and 5′-CTG CTC CAC CTT CTT GAC-3′ (reverse); free fatty acid-binding protein 4 (FABP4), 5′-GTG ATG CCT TTG TGG GAA C-3′ (forward) and 5′-ATC CCC ATT TAC GCT GAT G-3′ (reverse); and Gapdh, 5′-GCT GAG TAT GTC GTG GAG T-3′ (forward) and 5′-GTT CAC ACC CAT CAC AAA C-3′ (reverse).
Western blot analysis
Total protein was extracted and subsequently quantified using a BCA protein quantification kit (Beyotime, Shanghai, China). A total of 30 µg of protein from each sample was separated using SDS-PAGE (10–15%) and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% non-fat milk in TBS-T for 2 h at room temperature. After incubation with the primary antibodies at 4℃ overnight, the membranes were thoroughly washed with TBS-T before incubation with the secondary anti-rabbit/mouse horseradish peroxidase-conjugated antibody for 2 h at room temperature. After washing the membranes again with TBS-T, the bands were visualized with enhanced chemiluminescence reagents (Millipore). Primary antibodies against mouse PPAR-γ (Cell Signaling Technology, Danvers, MA, USA), FABP4, phospho-AKT (p-AKT), AKT, and β-actin (Epitomics, CA, USA) were used.
Statistics
All of the analyses were performed using SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). All values were presented as the mean ± SEM. Statistical comparisons between the two groups were performed using a two sample t-test. The differences among multiple groups were assessed using two-way ANOVA followed by Dunnett’s test. Probabilities of P < 0.05 were considered to be statistically significant.
Results
Effect of vaspin on the proliferation of 3T3-L1 preadipocytes
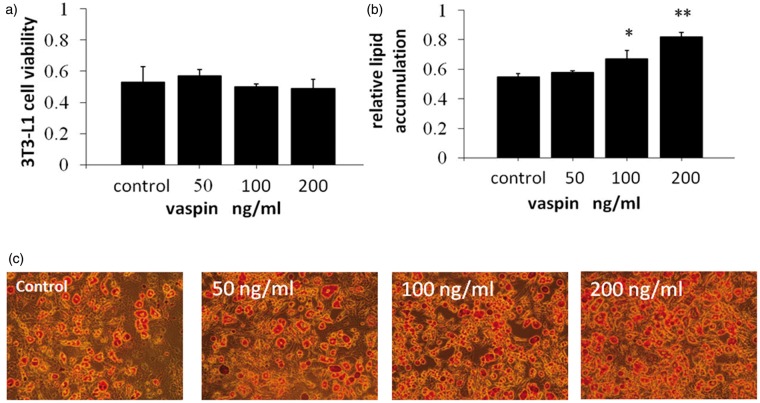
3T3-L1 preadipocytes were incubated with various concentrations of vaspin (0, 50, 100, or 200 ng/mL) for 48 h; the proliferative activity was assessed using the MTT assay. The findings revealed that vaspin exhibited no significant effect on the proliferation of 3T3-L1 preadipocytes (Figure 1a).
Figure 1.
Vaspin treatment enhanced lipid accumulation during adipocyte differentiation in 3T3-L1 cells. (a) 3T3L1 preadipocytes were treated with various concentrations of vaspin during differentiation, and cell viability was assessed using an MTT assay. (b) Oil Red O was extracted from the cells using isopropyl alcohol, and the absorbance was measured at 490 nm. (c) The cells were incubated with or without vaspin for six days. The cells were fixed with formalin and then stained with Oil Red O (200 × magnification). *P < 0.05 compared with control. (A color version of this figure is available in the online journal.)
Effect of vaspin on lipid accumulation on day 6 of 3T3-L1 preadipocyte differentiation
The proliferation of 3T3-L1 preadipocytes was evaluated through the colorimetric analysis of lipid droplets in isopropanol-stained cells. Vaspin induced lipid accumulation in a dose-dependent manner. The levels of lipid accumulation in the 50, 100, and 200 ng/mL groups were increased by 1.0-fold, 1.20-fold (P < 0.05) and 1.49-fold (P < 0.01), respectively, compared with that of the control group (Figure 1b and c). This result indicates that vaspin can promote lipid accumulation and adipocyte differentiation.
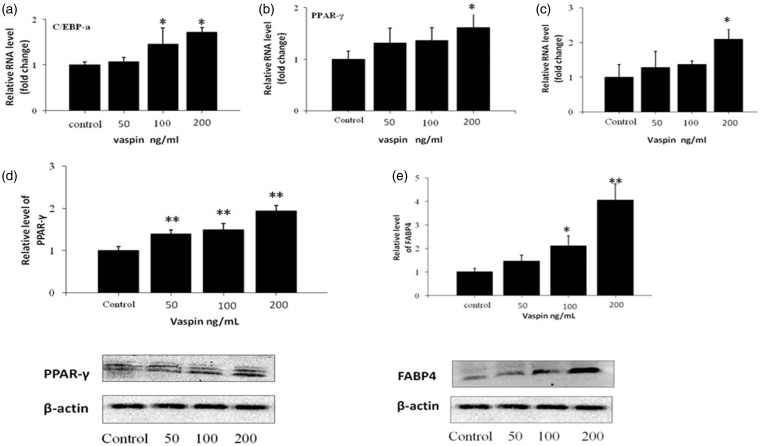
Effects of vaspin on specific transcription factors during adipocyte differentiation
The expression of PPAR-γ, C/EBP, and FABP4 increased in a dose-dependent manner with as the concentration of vaspin increased (50, 100, or 200 ng/mL). However, no significant difference was observed between the 50 ng/mL and 100 ng/mL groups. At a concentration of 200 ng/mL, the mRNA levels of PPAR-γ, C/EBPα, and FABP4 increased by 1.61-fold (P < 0.05), 1.72-fold (P < 0.01), and 2.08-fold (P < 0.05), respectively, compared with those of the control group (Figure 2a to c). Similarly, western blot analysis revealed that the expression levels of PPAR-γ and FABP4 were increased by vaspin in a dose-dependent manner. PPAR-γ was increased by 1.38-fold, 1.48-fold, and 1.96-fold (50, 100, or 200 ng/mL, respectively), respectively. FABP4 was increased by 1.45-fold, 2.10-fold (P < 0.05), and 4.05-fold (P < 0.01) (50, 100, or 200 ng/mL), respectively (Figure 2d and e).
Figure 2.
Effects of various concentrations of vaspin on the expression of adipocyte-specific markers. The cells were incubated with or without vaspin for six days. (a, b, and c) The mRNA levels of the C/EBP and PPAR-γ transcription factors and the expression of the adipogenic marker gene FABP4 were analyzed using RT-PCR analysis on day 6 of differentiation. (d and e) The protein expression levels of PPAR-γ and FABP4 were assessed using western blot analysis on day 6 of differentiation. *P < 0.05 compared with the control group; **P < 0.01 compared with the control group
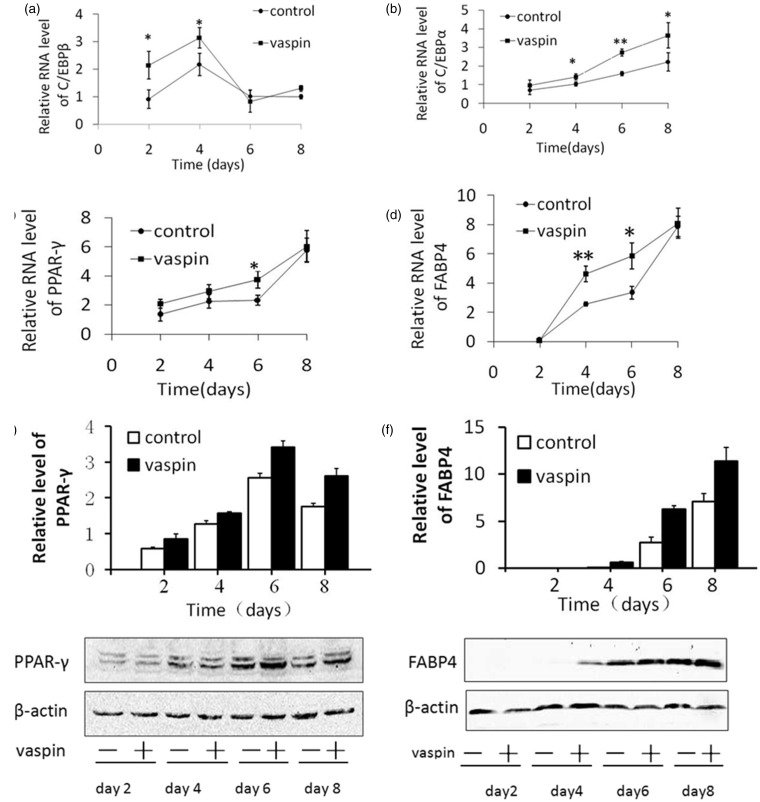
To evaluate the effects of vaspin on the differentiation of 3T3-L1 preadipocytes at various time points, we added 200 ng/mL vaspin and subsequently collected these cells on days 2, 4, 6, and 8. Additionally, the differentiation medium and vaspin were replaced simultaneously during differentiation. We observed that vaspin increased the C/EBPβ mRNA levels by 2.35-fold (P < 0.05) and 1.44-fold (P < 0.05) on days 2 and 4, respectively (Figure 3a). However, C/EBPα mRNA was increased by 1.35-fold (P < 0.05), 1.71-fold (P < 0.01), and 1.63-fold (P < 0.05) on days 4, 6, and 8, respectively (Figure 3b). PPAY-γ mRNA was increased on days 2, 4, 6, and 8, with a 1.6-fold increase (P < 0.05) on day 6 (Figure 3c). FABP4 mRNA was increased by 1.79-fold (P < 0.01) and 1.75-fold (P < 0.05) on days 4 and 6, respectively, compared with that of the control group (Figure 3d). Additionally, western blot analysis showed that vaspin significantly increased the expression of PPAR-γ on days 2, 4, 6, and 8, with the most obvious increase appearing on day 6. Increased expression of FABP4 was not detected on day 2; however, significant increases were observed on days 4, 6, and 8 compared with the control group.
Figure 3.
Effects of vaspin on the expression of adipocyte-specific markers throughout the differentiation period. Vaspin (200 ng/mL) was added to the differentiation medium, and the cells were harvested on culture days 2, 4, 6, and 8. (a, b, c, and d, respectively) The mRNA levels of C/EBPβ, C/EBPα, PPAR-γ and FABP4 were analyzed using RT-PCR analysis. (e and f) The protein levels of PPAR-γ and FABP4 were assessed using western blot analysis. *P < 0.05 compared with the control group; **P < 0.01 compared with the control group
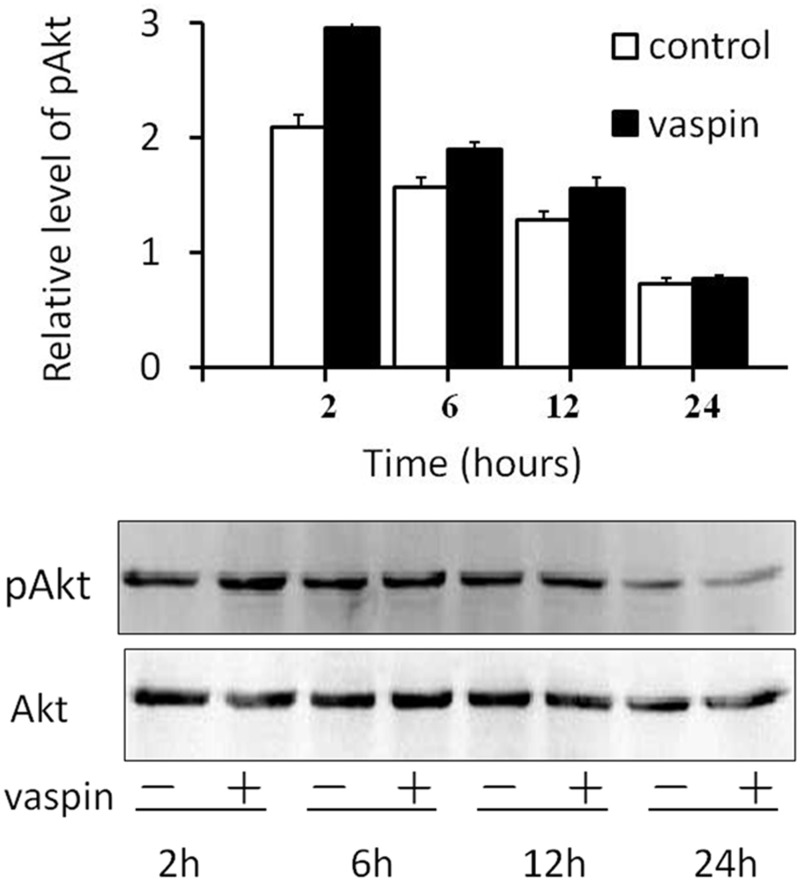
Effects of vaspin on the AKT signaling pathway during adipocyte differentiation
3T3-L1 preadipocytes were cultured in the presence of 200 ng/mL vaspin and then harvested at 2, 6, 12, and 24 h after the administration of vaspin. Western blot analysis revealed that the expression of p-AKT increased 2, 6, and 12 h after the administration of vaspin compared to that of the control group; the most obvious increase occurred at 2 h (P < 0.01). Additionally, this effect gradually weakened as the culture aged, and no increase was detected on day 24 (Figure 4).
Figure 4.
Effects of vaspin on the AKT signaling pathway during the first 24 h of differentiation. Vaspin (200 ng/mL) was added to the differentiation medium, and the cells were harvested after 2, 6, 12, or 24 h of culture. Western blot analyses were used to assess the protein levels of p-AKT and AKT, **P < 0.01 compared with the control group
Changes in mature adipocytes in the presence of vaspin for eight days
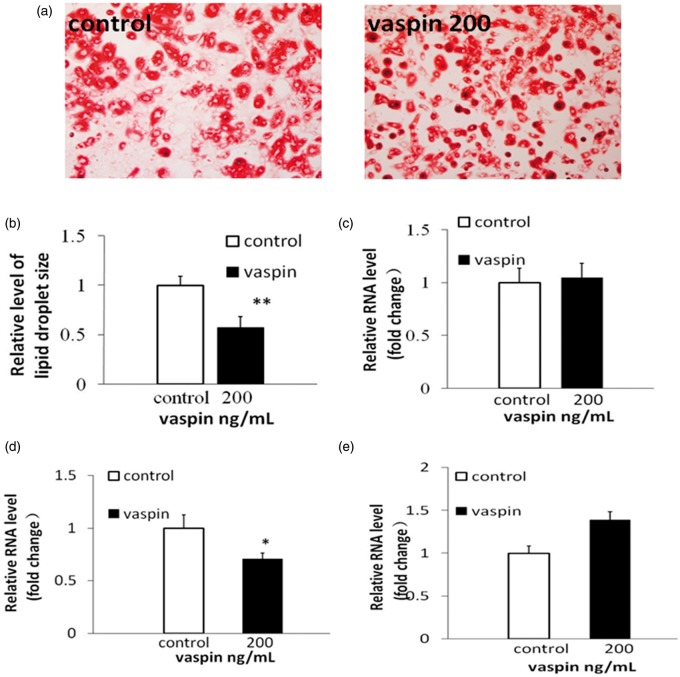
Vaspin, 200 ng/mL, was added to the cells, and the vaspin and differentiation medium was simultaneously replaced. In response, approximately 80–90% of the 3T3-L1 preadipocyte cells differentiated into adipocytes. Smaller lipid droplets were observed in the adipocytes incubated with 200 ng/mL vaspin in relation to the control group (Figure 5a and b). The mRNA levels of adiponectin, interleukin-6 (IL-6) and glucose transporter 4 (GLUT4) were examined in the adipocytes differentiated for eight days in the presence of 200 ng/mL vaspin. No significant changes were observed for adiponectin (Figure 5c). However, IL-6 mRNA was significantly reduced (Figure 5d), and the level of GLUT4 mRNA was significantly increased (Figure 5e) compared with those of the control group.
Figure 5.
3T3-L1 preadipocytes were treated with 200 ng/mL vaspin during differentiation. On day 8 of differentiation, (a) the cells were stained with Oil Red O. (b) The sizes of the lipid droplets were analyzed using Image Pro Plus 6.0. (c, d, and e) The mRNA levels of adiponectin, IL-6 and GLUT4 were analyzed using RT-PCR analysis. *P < 0.05 compared with the control group; **P < 0.01 compared with the control group. (A color version of this figure is available in the online journal.)
Discussion
Vaspin, which is a new adipocyte factor, is associated with obesity, insulin resistance, and inflammation.15 However, little is known regarding the effects of vaspin on adipocytes, glucolipid metabolism, insulin sensitivity, and inflammation. According to the literature, the expression of vaspin was increased in obese rat and human adipose tissue, and elevated vaspin expression has been associated with obesity and insulin resistance.17,18 Previous findings have demonstrated that the circulating level of vaspin may reflect the body fat content19 because the regulation of adipocyte differentiation and insulin resistance is associated with obesity.20 The present study aimed to explore the effects of vaspin on the differentiation of 3T3-L1 preadipocytes.
The differentiation of 3T3-L1 preadipocytes is a complex process that requires the modulation of several transcription factors, including PPAR-γ and the family of CCAAT/enhancer-binding proteins: C/EBPβ, C/EBPδ, and C/EBPα. Adipocyte differentiation is driven by the activation of fat-related genes due to changes in transcription. Members of the CCAAT/enhancer-binding protein family (C/EBPα, C/EBPβ, and C/EBPδ) play important roles in adipogenesis.21 C/EBPβ, which is expressed early in the adipocyte differentiation program, initiates mitotic clonal expansion.22 In our study, C/EBPβ expression was significantly increased compared to the control group during the early phase of differentiation.
In response to an adipogenic induction, C/EBPβ and C/EBPδ are first activated to promote PPAR-γ and C/EBPα expression.21 The transcription factor PPAR-γ is a master regulator of adipocyte differentiation, and its activation is necessary and sufficient for this process.12 The activation of C/EBPα and PPAR-γ leads to terminal differentiation through their subsequent transactivations of adipocyte-specific genes, such as FABP4, lipoprotein lipase, and fatty acid synthase.12,23
In this study, vaspin promoted the expression of the transcription factors C/EBPα and PPAR-γ in a dose-dependent manner, increased FABP4 and promoted the formation of fat. The expression of C/EBPα and PPAR-γ was gradually increased with prolonged differentiation compared with that of the control group. These findings indicated that vaspin promoted the differentiation of preadipocytes and that C/EBPβ, C/EBPα, and PPAR-γ were involved in the differentiation of preadipocytes.
The phosphatidylinositol 3 kinase (PI3K/AKT) signaling pathway involves the proliferation, differentiation, apoptosis and regulation of glucose transporters, and other cellular functions. AKT plays a key role in the insulin signaling pathway; the activation of AKT has been confirmed as an important target for controlling obesity and diabetes. The PI3K pathway that is activated by the phosphorylation of AKT is an important component of this system.24,25 In the absence of insulin, activated AKT can cause 3T3-L1 cells to differentiate.24 The cascade reaction of the PI3K/AKT pathway plays an important role in the differentiation of fat and activates PPAR-γ and C/EBPα during the differentiation of 3T3-L1 preadipocytes.25 The PI3K inhibitor LY294002 can inhibit the differentiation of 3T3-L1 preadipocytes and the expression of C/EBPα and PPAR-γ.26
Moreover, AKT activates its downstream substrates via phosphorylation and subsequently modulates C/EBPβ and C/EBPα.27,28 Studies have reported that vaspin can prevent the free fatty acid -induced apoptosis of vascular endothelial cells by up-regulating the PI3K/AKT pathway and inhibiting the high glucose-induced phosphorylation of vascular smooth muscle cells (VSMCs) by AKT, indicating that vaspin may be involved in the proliferation and activation of VSMCs through the PI3K/AKT pathway.29 In this study, we determined the effect of vaspin on the PI3K/AKT signaling pathway during the early differentiation of 3T3-L1 cells and noted that the phosphorylation of AKT in the presence of 200 ng/mL vaspin was more obvious than that in the control group. This effect was most obvious 2 h after the administration of vaspin and lasted for 24 h. The findings suggest that the phosphorylation of AKT may be involved in the differentiation of 3T3-L1 preadipocytes.
Adipocyte dysfunction often manifests as inflammation, adipokine dysregulation, and insulin resistance and is considered to be a contributory factor to the development of diabetes.30 The ability of small subcutaneous adipocytes to promote lipometabolism and improve insulin sensitivity has been reported. The PPAR-γ agonist thiazolidinediones can stimulate the differentiation of preadipocytes, increasing the number of small adipocytes by up-regulating the expression of GLUT4 and adiponectin to improve insulin sensitivity and prevent excess lipidosis.31–33 The size of adipocytes is an important determinant of adipokine secretion. Leptin, IL-6, IL-8, and adiponectin expression all exhibit a significant linear correlation with adipocyte size.34 In the present study, we found that mature adipocytes incubated with vaspin for eight days were smaller than those of the control group. The adiponectin mRNA level was not affected; however, the IL-6 mRNA level was significantly reduced, and the GLUT4 mRNA level was significantly increased. These findings indicate that the administration of vaspin can alter the function of mature adipocytes and reduce the expression of proinflammatory factors, in addition to improving lipid metabolism and increasing insulin sensitivity.
In conclusion, we observed that vaspin was able to promote the expression of FABP4 by increasing the expression of transcription factors, such as PPAR-γ, C/EBPβ, and C/EBPα. These effects can promote the differentiation of 3T3-L1 preadipocytes. The PI3K/AKT signaling pathway may be involved in this process. Additionally, the administration of vaspin led to significantly smaller adipocytes, reduced expression of IL-6 and increased expression of GLUT4, suggesting that vaspin could potentially be used to increase insulin sensitivity and inhibit obesity.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81270236 and 81400258) and Shaanxi Province Scientific and Technological Project (2012k16-06-01).
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; CFS conducted the experiments, PL and GLL wrote the manuscript.
References
- 1.Hossain P, Kawar B, Nahas ME. Obesity and diabetes in the developing world – a growing challenge. N Engl J Med 2007; 356: 213–5. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875–80. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005; 96: 939–49. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009; 54: 1847–56. [DOI] [PubMed] [Google Scholar]
- 6.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–56. [DOI] [PubMed] [Google Scholar]
- 7.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 2008; 582: 117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern N, Osher E, Greenman Y. Hypoadiponectinemia as a marker of adipocyte dysfunction – part II: the functional significance of low adiponectin secretion. J Cardiometab Syndr 2007; 2: 288–94. [DOI] [PubMed] [Google Scholar]
- 9.Dahlman I, Arner P. Obesity and polymorphisms in genes regulating human adipose tissue. Int J Obes (Lond) 2007; 31: 1629–41. [DOI] [PubMed] [Google Scholar]
- 10.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell 1996; 87: 377–89. [DOI] [PubMed] [Google Scholar]
- 11.Achike FI, To N-HP, Wang H, Kwan C-Y. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol 2011; 38: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA 2005; 102: 10610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011; 54: 1819–23. [DOI] [PubMed] [Google Scholar]
- 15.Heiker JT. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J Pept Sci 2014; 20: 299–306. [DOI] [PubMed] [Google Scholar]
- 16.Handisurya A, Riedl M, Vila G, Maier C, Clodi M, Prikoszovich T, Ludvik B, Prager G, Luger A, Kautzky-Willer A. Serum vaspin concentrations in relation to insulin sensitivity following RYGB-induced weight loss. Obes Surg 2010; 20: 198–203. [DOI] [PubMed] [Google Scholar]
- 17.Klöting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun 2006; 339: 430–6. [DOI] [PubMed] [Google Scholar]
- 18.Shaker OG, Sadik NAH. Vaspin gene in rat adipose tissue: relation to obesity-induced insulin resistance. Mol Cell Biochem 2013; 373: 229–39. [DOI] [PubMed] [Google Scholar]
- 19.Youn BS, Klöting N, Kratzsch J, Lee N, Park JW, Song ES, Ruschke K, Oberbach A, Fasshauer M, Stumvoll M, Blüher M. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008; 57: 372–7. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. Mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPAR gamma) deficiency and PPAR gamma agonist improve insulin resistance. J Biol Chem 2001; 276: 41245–54. [DOI] [PubMed] [Google Scholar]
- 21.Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol 2012; 9: 689–702. [DOI] [PubMed] [Google Scholar]
- 22.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 2003; 100: 44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78: 783–809. [DOI] [PubMed] [Google Scholar]
- 24.Xu JF, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem 2004; 279: 35914–22. [DOI] [PubMed] [Google Scholar]
- 25.Green CJ, Göransson O, Kular GS, Leslie NR, Gray A, Alessi DR, Sakamoto K, Hundal HS. Use of AKT inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/AKT in the insulin-dependent regulation of glucose and system A amino acid uptake. J Biol Chem 2008; 283: 27653–67. [DOI] [PubMed] [Google Scholar]
- 26.Gao J-Z, Zheng R-D, Wang C-B, Ying Y-Q, Luo X-P. Effects of PI3K inhibitor LY294002 on the differentiation of mouse preadipocytes and the expression of C/EBPalpha and PPARgamma. Zhongguo dang dai er ke za zhi = Chin J Contemporary Pediatr 2011; 13: 823–6 [Article in Chinese]. [PubMed] [Google Scholar]
- 27.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3 beta in cellular signaling. Prog Neurobiol 2001; 65: 391–426. [DOI] [PubMed] [Google Scholar]
- 28.Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBP alpha kinase. Mol Cell Biol 1999; 19: 8433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Peng W, Zhuang J, Lu Y, Jian W, Wei Y, Li W, Xu Y. Vaspin attenuates high glucose-induced vascular smooth muscle cells proliferation and chemokinesis by inhibiting the MAPK, PI3K/AKT, and NF-kappa B signaling pathways. Atherosclerosis 2013; 228: 61–8. [DOI] [PubMed] [Google Scholar]
- 30.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012; 308: 1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang JG, Park CY, Ihm SH, Yoo HJ, Park H, Rhee EJ, Won JC, Lee WY, Oh KW, Park SW, Kim SW. Mechanisms of adipose tissue redistribution with rosiglitazone treatment in various adipose depots. Metabolism 2010; 59: 46–53. [DOI] [PubMed] [Google Scholar]
- 32.Dubuisson O, Dhurandhar EJ, Krishnapuram R, Kirk-Ballard H, Gupta AK, Hegde V, Floyd E, Gimble JM, Dhurandhar NV. PPAR gamma-independent increase in glucose uptake and adiponectin abundance in fat cells. Endocrinology 2011; 152: 3648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang Y-C, Cho H-J. Ascofuranone stimulates expression of adiponectin and peroxisome proliferator activated receptor through the modulation of mitogen activated protein kinase family members in 3T3-L1, murine pre-adipocyte cell line. Biochem Biophys Res Commun 2012; 422: 423–8. [DOI] [PubMed] [Google Scholar]
- 34.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007; 92: 1023–33. [DOI] [PubMed] [Google Scholar]