Abstract
Inflammation and its subsequent endothelial dysfunction have been reported to play a pivotal role in the initiation and progression of chronic vascular diseases. Inhibiting the attachment of monocytes to endothelium is a potential therapeutic strategy for vascular diseases treatment. α-Melanocyte stimulating hormone is generated from a precursor hormone called proopiomelanocortin by post-translational processing. However, whether α-melanocyte stimulating hormone plays a role in regulating endothelial inflammation is still unknown. In this study, the effects of α-melanocyte stimulating hormone on endothelial inflammation in human umbilical vein endothelial cell lines were investigated. And the result indicated that α-melanocyte stimulating hormone inhibits the expression of endothelial adhesion molecules, including vascular adhesion molecule-1 and E-selectin, thereby attenuating the adhesion of THP-1 cells to the surface of endothelial cells. Mechanistically, α-melanocyte stimulating hormone was found to inhibit NF-κB transcriptional activity. Finally, we found that the effect of α-melanocyte stimulating hormone on endothelial inflammation is dependent on its receptor melanocortin receptor 1.
Keywords: α-Melanocyte stimulating hormone, endothelial inflammation, VCAM-1, NF-κB
Introduction
Atherosclerosis, one of the most common vascular diseases, involves a very complex pathological process with accumulation of modified lipids, inflamed endothelial cells (ECs), and leukocytes in the arteries.1 Inflammation and its subsequent endothelial dysfunction have been reported to play a pivotal role in the initiation and progression of atherosclerosis.2 Increased circulating cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) are also associated with such cardiovascular events. TNF-α stimulates the expression of monocyte chemotactic protein-1 and adhesion molecules, including vascular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin. These chemokines and adhesion molecules play key roles in the firm adhesion of monocytes to activated ECs.3,4 Inhibiting the attachment of monocytes to endothelium is a potential therapeutic strategy for atherosclerosis treatment.
α-Melanocyte stimulating hormone (α-MSH) is generated from a precursor hormone called proopiomelanocortin by post-translational processing.5 Multiple lines of evidence have shown that α-MSH has potent anti-inflammatory effects when administered systemically or locally.6 α-MSH and other melanocortins family members such as β-MSH, c-MSH, and adrenocorticotropic hormone bind to melanocortin receptors (MC-Rs). MC-Rs belong to the superfamily of G-protein-coupled receptors with seven transmembrane domains and bind the melanocortin peptides with differential affinity.7 Studies have shown that the anti-inflammatory effects of α-MSH in vitro are mediated mainly via engagement of melanocortin receptor 1 (MC-1 R).8 Importantly, α-MSH has been reported to suppress the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as chemokines such as IL-8 and interferon c (IFN-c) upon treatment with α-MSH.9 However, whether α-MSH plays a role in regulating endothelial inflammation is still unknown. In this study, the effects of α-MSH on endothelial inflammation in human umbilical vein endothelial cell (HUVEC) lines were investigated. It was found that α-MSH inhibits the expression of endothelial adhesion molecules and attenuates the adhesion of THP-1 cells to the surface of ECs.
Materials and methods
Cell culture
HUVECs from Lonza, USA were used in this study. Cells were maintained in EBM-2 media with supplemental growth factors according to the manufacturer’s instructions in an incubator with 5% CO2 at 37℃. Human monocytic leukemia cell line THP-1 cells were purchased from the ATCC, USA. Cells were maintained in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, antibiotic–antimycotic, and L-glutamine (Life Technologies).
RNA isolation and real-time polymerase chain reaction
Total RNA from cultured cells was isolated using Qiazol (Qiagen, USA) following the manufacturer’s instructions. RNA (2 µg) was used as templates for reverse transcription polymerase chain reaction (PCR) to synthesize cDNA. Then real-time PCR was carried out by a StepOne Plus Real-time PCR System using SYBR Green expression assays (Applied Biosystems) in a 20-µl reaction volume. Gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase using the ΔΔCt method.
Adhesion assay
HUVECs were cultured with 10 µg/mL TNF-α in the presence or absence of α-MSH for 6 h. After labelling with 0.2 mg/L calcein red AM for 30 min at 37℃, THP-1 cells were seeded onto confluent HUVECs and incubated for 2 h at 37℃. Then co-cultured cells were washed with 1× phosphate-buffered saline (PBS) containing 1% bovine serum albumin. All imaging was performed using a Leica video imaging system. Digital images were captured over three regions in each well at 200× magnification. Four wells were used in each group. One of the three regions was randomly chosen from each group for statistical analysis. Attached cells in each field were counted and normalized to untreated group.
Immunocytochemistry
HUVECs were fixed with 4% paraformaldehyde for 10 min at RT, followed by permeabilization with 0.1% Triton X-100 for 15 min on ice. Then cells were blocked with 5% normal goat serum in PBS for 1 h at RT. After incubating with primary antibodies for 2 h at RT, cells were incubated with Alexa-594-conjugated secondary antibodies for 1 h at RT. After washing with PBS for three times, cells were mounted with VECTASHIELD® Mounting Media containing DAPI (4′, 6-diamidino-2-phenylindole) (Vector labs, USA). Signals were recorded using a deconvolution fluorescence microscope system (BZ-8000, Keyence, Osaka, Japan).
Western blot analysis
HUVECs were lysed in cell lysis buffer (Cell signaling, USA) supplemented with the complete protease inhibitor and phosphatase inhibitor cocktail (Roche, USA).
Protein concentration was determined by a BCA Protein Assay. The extracted protein was then subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and electrotransferred to Immobilon-P membrane (Millipore, USA).10 After being blocked for 2 h in TBS containing 5% non-fat dry milk and 0.5% Tween-20, membranes were sequentially incubated with primary antibodies for 3 h and horseradish peroxidase conjugated secondary antibodies for 2 h at RT. Blots were developed with Immunocruz (Santa Cruz Biotechnology).
Statistical analysis
All data are presented as means ± SEM resulting from at least three independent experiments. Statistical analysis was performed using one-way variance analysis (ANOVA) followed by Dunnett’s post hoc test. P < 0.05 was considered as a significant difference.
Results
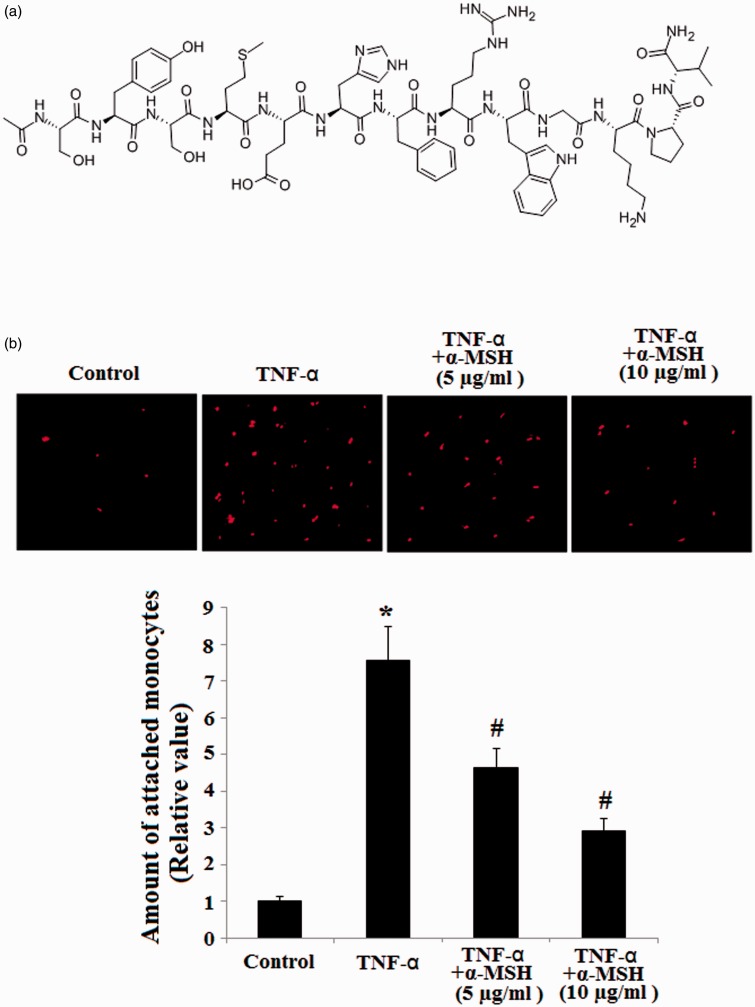
The molecular structure of α-MSH is shown in Figure 1(a). One of the objectives of this study was to investigate the contributory roles of α-MSH in inhibiting endothelial inflammation. To address this, we examined the effects of α-MSH on TNF-α-induced monocytic attachment of THP-1 to ECs. HUVECs were stimulated with TNF-α for 6 h in the presence or absence of 5 µg/mL or 10 µg/mL α-MSH, and then HUVEC monolayers were incubated with fluorescent dye-labeled human monocytic cells (THP-1). As shown in Figure 1(b), cell attachment induced by TNF-α was markedly attenuated in the pretreatment of cells with α-MSH in a dose dependent manner.
Figure 1.
α-MSH ameliorates the endothelial adhesion of THP-1 induced by TNF-α in HUVECs. (a) Molecular structure of α-MSH; (b) representative fluorescence microscopic images of endothelial adhesion of THP-1 and quantitative data for corresponding images (*, P < 0.001 vs. non-treated control, n = 4; #, P < 0.001 vs. TNF-α-treated group). (A color version of this figure is available in the online journal.)
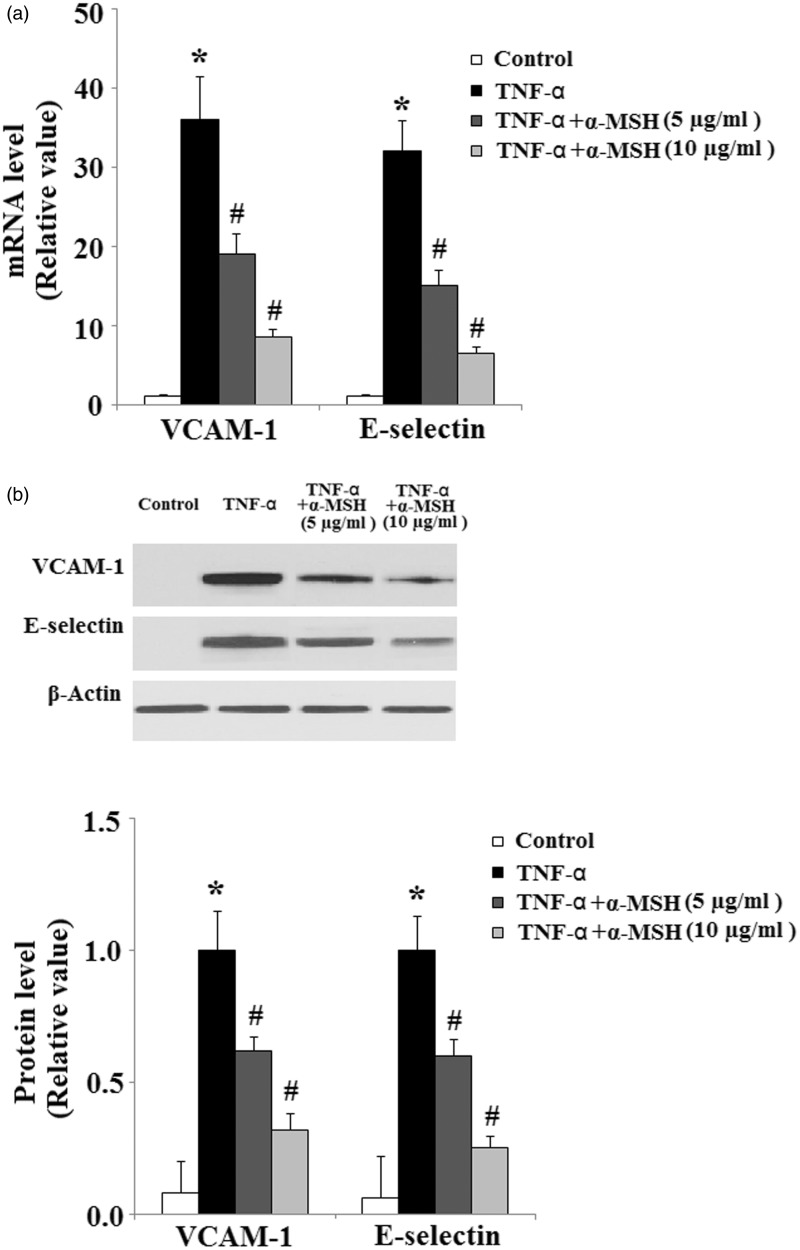
One of the earliest events in endothelial inflammation induced by the pro-inflammatory cytokine TNF-α is the expression of adhesion molecules on the cell surface. We assessed the expression of VCAM-1, E-selectin, and ICAM-1 at mRNA levels. As shown in Figure 2(a), treatment of HUVECs with TNF-α strongly induced VCAM-1 and E-selectin at mRNA levels. In contrast, pretreatment with α-MSH mitigated the induction of VCAM-1 and E-selectin at mRNA levels. Importantly, Western blot results indicated that α-MSH ameliorated the induction of VCAM-1 and E-selectin at protein levels (Figure 2(b)).
Figure 2.
Effects of α-MSH on TNF-α-mediated induction of inflammatory adhesion molecules. (a) α-MSH inhibits the expression of VCAM-1 and E-selectin at mRNA levels (*, P < 0.01 vs. non-treated control, n = 4; #, P < 0.001 vs. TNF-α-treated group); (b) α-MSH inhibits the expression of VCAM-1 and E-selectin at protein levels (*, P < 0.001 vs. non-treated control, n = 4; #, P < 0.001 vs. TNF-α-treated group)
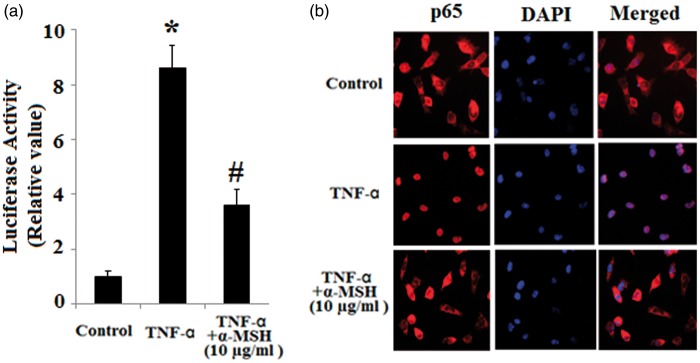
NF-κB has been reported to play a pivotal role in the activation of both the VCAM-1 and E-selectin promoters in response to inflammatory stimuli.11 Then we examined the inhibitory effect of α-MSH on NF-κB activation. First, we assessed the effects of α-MSH on NF-κB transcriptional activity by performing luciferase reporter assays. As shown in Figure 3(a), TNF-α drastically induced NF-κB luciferase activity in HUVECs transfected with NF-κB-Luc reporter plasmid, which were markedly suppressed by α-MSH. In addition, immunostaining results revealed that TNF-α-induced p65 nuclear translocation was blocked by pretreatment with α-MSH, thereby confirming the inhibition effect of α-MSH in NF-κB activation (Figure 3(b)).
Figure 3.
Inhibitory effects of α-MSH on NF-κB activation in endothelial cells. (a) NF-κB luciferase reporter assays (*, P < 0.001 vs. non-treated control, n = 4; #, P < 0.001 vs. TNF-α-treated group); (b) Effects of α-MSH on p65 nuclear translocation. The cells were immunostaining with anti-p65 antibodies. DAPI staining was used as control of nuclear expression. (A color version of this figure is available in the online journal.)
MC-1 R plays a pivotal role in regulating the anti-inflammatory effects of α-MSH.
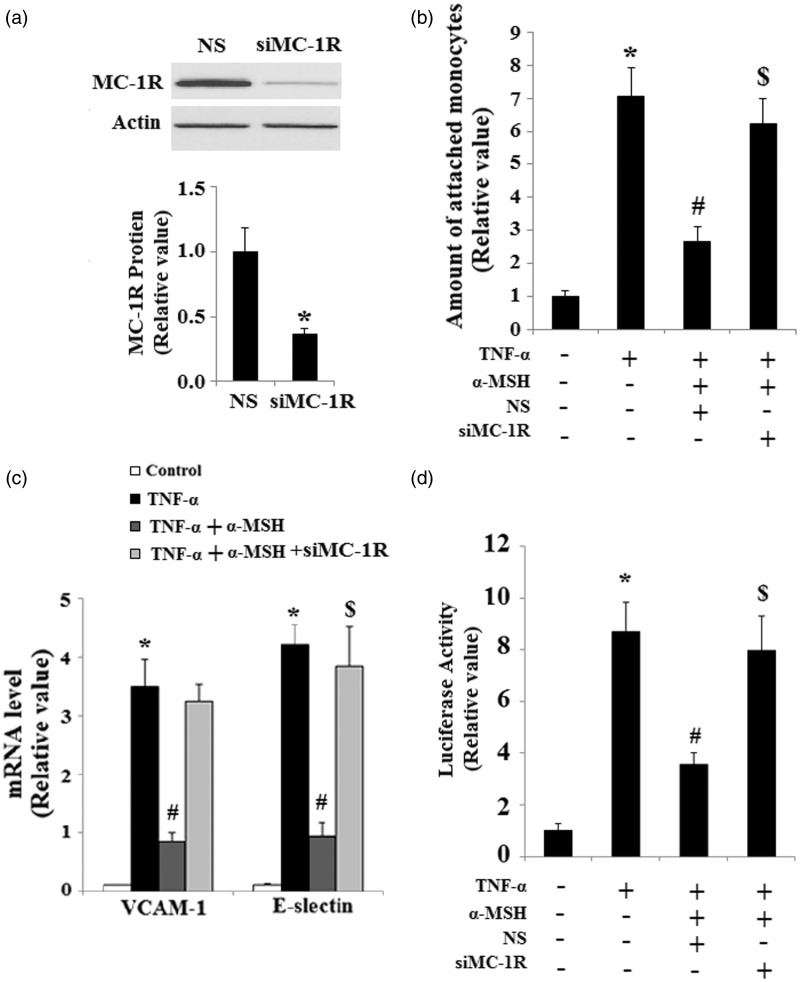
In order to determine whether or not the effect of α-MSH on endothelial inflammation is dependent on MC-1 R, we inhibited the expression of MC-1 R using MC-1 R siRNA. Successful suppression of MC-1 R was confirmed by Western blot analysis as shown in Figure 4(a) . The inhibitory effect of α-MSH on the monocytic attachment to ECs is abolished when HUVECs are transfected with MC-1 R siRNA (Figure 4(b)). Most importantly, real-time PCR result revealed that the inhibitory effect of α-MSH on the induction of VCAM-1 and E-selectin is abolished by silencing the expression of MC-1 R (Figure 4(c)). Notably, luciferase reporter assays revealed that the inhibitory effect of α-MSH on the activation of NF-κB is abolished by transfection with MC-1 R siRNA (Figure 4(d)).
Figure 4.
The effects of α-MSH on inhibiting the endothelial adhesion of THP-1 are dependent on MC-1 R. (a) The expression of MC-1 R was knocked down using small RNA interferences, and Western blot analysis confirmed the successful knockdown of MC-1 R (P < 0.001 vs. non-specific [NS] group); (b) MC-1 R silencing completely abolishes the inhibitory effects of α-MSH on endothelial adhesion of THP-1 (*, #, $, P < 0.001 vs. the previous column group); (c) Real-time PCR and quantification analysis reveals that MC-1 R silencing abolishes the effects of α-MSH on inhibiting the expression of VCAM-1 and E-selectin (*, #, $, P < 0.001 vs. the previous column group); (d) NF-κB luciferase reporter assays (*, #, $, P < 0.001 vs. the previous column group)
Discussion
Recruitment of immune cells to the surface of the endothelium plays a critical role in the pathological development of vascular inflammatory diseases.12 This process is mediated by the induction of endothelial adhesion molecules such as VCAM-1 and E-selectin. In this study, we found that monocyte adhesion to ECs caused by TNF-α is significantly attenuated by pretreatment with α-MSH. Importantly, the induction of VCAM-1 and E-selectin is prevented by pretreatment with α-MSH. Mechanistically, pretreatment with α-MSH decreases the transcriptional activity of NF-κB. In addition, we found that the protective effects of α-MSH against endothelial inflammation are dependent on MC-1 R. These findings suggest that α-MSH is a negative regulator of vascular inflammation.
The anti-inflammatory effects of α-MSH in vitro have been found in a variety of animal models. For example, α-MSH has shown anti-inflammatory potential using animal models of autoimmune uveitis as well as corneal injury.13 In addition, α-MSH has been reported to suppress the oxidative stress induced by ultraviolet radiation in skin keratinocytes and melanocytes via the MC1R-mediated cAMP-PKA pathway.14 It is also notable that α-MSH exerts anti-oxidative and anti-apoptotic effects in retinal vascular ECs by inhibiting the transcription factor FoxO4.15
An important molecular mechanism underlying the anti-inflammatory effects of α-MSH, such as modulation of pro-inflammatory cytokine and adhesion molecule expression, appears to be the suppression of NF-κB activation.16,17 Under normal conditions, NF-κB exists in the cytoplasm as a heterodimer of the p50 and p65 subunits. Activation of NF-κB is controlled by a family of inhibitors referred to as IκBα, which can retain the entire complex in cytoplasm. Inflammatory cytokines are able to induce phosphorylation and subsequent degradation of IκB, thereby leading to the liberation of NF-κB heterodimers, which can then translocate to the nucleus, bind specific DNA sequences, and affect target gene expression. In this study, we found that pretreatment with α-MSH prevents the nuclear translocation of NF-κB, which is consistent with previous studies.
In addition to inhibiting the generation of pro-inflammatory mediators such as TNF-α and IL-1β, α-MSH was also found to induce IL-10, a cytokine with potent immunosuppressive activities, strongly suggesting that this cytokine plays a key role in mediating the molecular anti-inflammatory mechanisms of α-MSH.18,19 MC-1 R is an important member of the five subtypes. Our data display that the inhibitory effects of α-MSH on inflammation are dependent on MC-1 R. Taken together, our findings suggest that MC-1 R may help protect against endothelial dysfunction in vascular diseases.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No. 81370318, to Y.Z.) and Natural Science Foundation of Jilin Province (No. 201115058, to Y.Z.).
Author contributions
YZ, YY, and GF designed the study; YY, WZ, LM, HY, and NL carried out the experiment; YY, GF, and YZ analyzed the data; YZ prepared the manuscript.
References
- 1.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 2008; 54: 24–38. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, Anderson JL, 3rd, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511. [DOI] [PubMed] [Google Scholar]
- 3.Mayer K, Merfels M, Muhly-Reinholz M, Gokorsch S, Rosseau S, Lohmeyer J, Schwarzer N, Krüll M, Suttorp N, Grimminger F, Seeger W. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol 2002; 283: H811–8. [DOI] [PubMed] [Google Scholar]
- 4.Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, Visioli F, Distante A, De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol 2003; 23: 622–9. [DOI] [PubMed] [Google Scholar]
- 5.Böhm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol 2006; 126: 1966–75. [DOI] [PubMed] [Google Scholar]
- 6.Luger TA, Brzoska T. Alpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis 2007; 66(Suppl 3): iii52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzzetti R, McLoughlin L, Lavender PM, Clark AJ, Rees LH. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignancies. J Clin Invest 1989; 83: 733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam CW, Getting SJ. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy 2004; 3: 311–5. [DOI] [PubMed] [Google Scholar]
- 9.Luger TA, Scholzen T, Grabbe S. The role of a-melanocyte stimulating hormone in cutaneous biology. J Invest Dermatol Symp Proc 1997; 2: 87–93. [DOI] [PubMed] [Google Scholar]
- 10.Zhou F, Zhang L, Gong K. LEF-1 activates the transcription of E2F1. Biochem Biophys Res Commun 2008; 365: 149–53. [DOI] [PubMed] [Google Scholar]
- 11.Collins T, Cybulsky MI. NF-kB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 2001; 107: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation in atherosclerosis. Nature 2002; 420: 868–74. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH). Ann N Y Acad Sci 2000; 917: 239–47. [DOI] [PubMed] [Google Scholar]
- 14.Henri P, Beaumel S, Guezennec A, Poumès C, Stoebner PE, Stasia MJ, Guesnet J, Martinez J, Meunier L. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J Cell Physiol 2011; 227: 2578–85. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Dong L, Liu X, Jiang Y, Zhang L, Zhang X, Li X, Zhang Y. α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS One 2014; 9: e93433–e93433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manna SK, Aggarwal BB. α-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kB activation induced by various inflammatory agents. J Immunol 1998; 161: 2873–80. [PubMed] [Google Scholar]
- 17.Brzoska T, Kalden DH, Scholzen T, Luger TA. Molecular basis of α-MSH/IL-1 antagonism. Ann N Y Acad Sci 1999; 885: 230–8. [DOI] [PubMed] [Google Scholar]
- 18.Rheins LA, Cotleur AL, Kleier RS, Hoppenjans WB, Saunder DN, Nordlund JJ. Alpha-melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice. J Invest Dermatol 1989; 93: 511–7. [DOI] [PubMed] [Google Scholar]
- 19.Grabbe S, Bhardwaj RS, Steinert M, Simon MM, Schwarz T, Luger TA. Alpha-melanocyte stimulating hormone induces hapten-specific tolerance in mice. J Immunol 1996; 156: 473–8. [PubMed] [Google Scholar]