Abstract
Although carboplatin is one of the standard chemotherapeutic agents for non-small cell lung cancer (NSCLC), it has limited therapeutic efficacy due to activation of a survival signaling pathway and the induction of multidrug resistance. Curcumin, a natural compound isolated from the plant Curcuma longa, is known to sensitize tumors to different chemotherapeutic agents. The aim of this study is to evaluate whether curcumin can chemosensitize lung cancer cells to carboplatin and to analyze the signaling pathway underlying this synergism. We investigated the synergistic effect of both agents on cell proliferation, apoptosis, invasion, migration, and expression of related signaling proteins using the human NSCLC cell line, A549. A549 cell was treated with different concentrations of curcumin and carboplatin alone and in combination. Combined treatment with curcumin and carboplatin inhibited tumor cell growth, migration, and invasion compared with either drug alone. Matrix metalloproteinase (MMP)-2 and MMP-9 were more efficiently downregulated by co-treatment than by each treatment alone. mRNA and protein expression of caspase-3 and caspase-9 and proapoptotic genes was increased in cells treated with a combination of curcumin and carboplatin, whereas expression of the antiapoptotic Bcl-2 gene was suppressed. Co-treatment of both agents substantially suppressed NF-κB activation and increased expression of p53. Phosphorylation of Akt, a protein upstream of NF-κB, was reduced, resulting in inhibition of the degradation of inhibitor of κB(IκBα), whereas the activity of extracellular signal-regulated kinase (ERK1/2) was enhanced. Our study demonstrated that the synergistic antitumor activity of curcumin combined with carboplatin is mediated by multiple mechanisms involving suppression of NF-κB via inhibition of the Akt/IKKα pathway and enhanced ERK1/2 activity. Based on this mechanism, curcumin has potential as a chemosensitizer for carboplatin in the treatment of patients with NSCLC.
Keywords: Curcumin, carboplatin, synergism, lung neoplasm, proliferation
Introduction
Lung cancer is the most common cause of cancer-related mortality in the United States, with 226,160 new cases and 160,340 deaths in 2012.1 Over the past 10 years, the development of individualized molecular targeted therapies has improved the success rates of the treatment for non-small cell lung cancer (NSCLC).2 In spite of these advances, the five-year combined survival rate is still approximately 16% in most countries.3,4
Prevalence of target oncogene mutations is low: only 10–20% in whites and 30–40% in Asians for epidermal growth factor receptor mutation and 2–7% for anaplastic lymphoma receptor tyrosine kinase rearrangement.3,4 For these reasons, platinum-based chemotherapy is still the standard therapy for advanced NSCLC lacking a targetable mutation. Carboplatin, an analog of cisplatin, is widely used for the treatment of several cancers including lung, head, neck, and ovarian cancers.5,6 Although carboplatin and cisplatin have similar mechanisms of action, carboplatin is considerably less toxic than cisplatin.7 In clinical practice, platinum-based chemotherapeutic agents offer a one-year survival rate of only 40–50% for patients with advanced NSCLC.8 This result can be explained by the activation of survival signaling pathways and the induction of multidrug resistance by chemotherapy.9 Given this reality, any agent that downregulates the survival signaling pathway can function as a chemosensitizer and improve the efficacy of cancer treatment.
Curcumin, a yellow natural compound extracted from turmeric, has been used as a dietary ingredient for centuries. Extensive research on curcumin performed in South East Asian countries in the last decade has revealed that curcumin has anti-inflammatory, anti-infective, and antitumorigenesis effects.10,11 Of these, the anticancer effects involve modulation of survival signaling pathways and multiple related molecules.12 Several studies have demonstrated that curcumin can sensitize lung cancer cells to various chemotherapeutic agents including gemcitabine, cisplatin, docetaxel, and vinorelbine.13–16 Although carboplatin is a commonly used chemotherapeutic agent for NSCLC, few studies have been performed to determine the synergistic effect of curcumin and carboplatin in NSCLC.
In this study, we used lung cancer cell lines to study the synergistic effect of a combination of curcumin and carboplatin on cell viability and genes related to apoptosis and elucidate the signaling pathway underlying this synergism.
Material and methods
Cell culture
The human alveolar adenocarcinoma cell line A549 was obtained from the American Type Culture Collection (Rockville, MD, USA). The cell line was maintained in continuous exponential growth by passaging two to three times a week in RPMI 1640 medium (WelGENE, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin solution (10,000 units/mL penicillin and 10 mg/mL streptomycin). Cells were cultured at 37℃ in a 5% CO2 humidified incubator and were split regularly before they attained approximately 80% confluence.
Drugs and reagents
Carboplatin (Sigma, St. Louis, MO, USA) was dissolved in distilled water and curcumin (from Curcuma longa [Turmeric]; Sigma) was dissolved in dimethyl sulfoxide (DMSO) to give a 50 mM stock solution. The concentration of DMSO in the experimental solutions was less than 0.1%. Antibodies specific for cleaved caspase-3 (Asp175), cleaved caspase-9 (Asp315), phosphorylated p53 (Ser15), phosphorylated IκBα (Ser32), ERK1/2, phosphorylated ERK1/2 (Thr202/Tyr204), Akt (C67E7), and phosphorylated Akt (Thr308) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against NF-κB (C-20), IκBα (C-21), p53 (FL-393), p21 (H-164), Bax (B-9), Bcl-2 (N-19), Lamin B (M-20), β-actin (I-19), and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell viability assay
Cells were seeded at a density of 1 × 104 cells/well in 96-well culture plates. After 24 h the medium was replaced with serum-free medium and the cells were treated with control solvent (0.1% DMSO) or different doses of carboplatin (5–100 µM), curcumin (5–50 µM), or carboplatin (10 µM, 25 µM) plus curcumin (10 µM, 25 µM) for 24 h. After 24 h, MTT (3-[4,5-dimethythiazol-2-yl]-2,5 - diphenyltetrazolium bromide) solution was added to each well to a final concentration of 0.5 µg/mL and incubated for 1 h at 37℃. The insoluble purple formazan product was dissolved in DMSO and absorbance was measured at 540 nm with an ELISA reader. Cell viability was calculated according to the following equation
All experiments were repeated at least three times.
Analysis of cytotoxic synergy between carboplatin and curcumin
Cells were cultured for 24 h and then the culture medium was replaced with fresh medium containing carboplatin and curcumin separately or in combination at ratios of 1:1, 1:2, and 1:5. After 24 h cell viability was determined by the MTT assay as described earlier. The synergistic effects of carboplatin and curcumin were quantitatively analyzed by calculation of the combination index (CI) using the Compusyn software program, which utilizes the Chou–Talalay equation method. CI analysis was used to calculate the interaction between treatment agents. The CI value was calculated using the following equation
where CA,x and CB,x are the concentrations of agent A and agent B used in combination that inhibit cell growth by x% and ICx,A and ICx,B are concentrations required for x% inhibition by agent A and agent B alone. Values of CI < 1, CI = 1, and CI > 1 indicate synergism, an additive effect, and antagonism, respectively. A more detailed description of degrees of synergism and antagonism is provided by Chou and Hayball.17
Observation of cell morphology by in situ Wright staining
Cells were seeded into 12-well culture plates at a density of 8 × 104 cells/well. After incubation for 24 h, the medium was replaced with serum-free medium and the cells were treated with agents as described earlier and cultured for a further 24 h. After treatment, the cells were washed twice with phosphate buffered saline (PBS) and then fixed and subjected to Giemsa Wright staining according to the manufacturer’s instructions (Fisher Scientific, Kalamazoo, MI, USA). After staining, the cells were dried and morphologic changes were observed and photographed using a phase-contrast microscope.
Scratch wound-healing motility assay
Cells were seeded in six-well plates and cultured to subconfluence. Cell monolayers were scratched with a sterile 200 µl pipette tip, creating a cell-free area or “wound” approximately 1 mm in width. Plates were washed twice with sterile PBS to remove loose cell debris and the medium was replaced with fresh medium containing 1% FBS and carboplatin and/or curcumin. A defined area of the wound was photographed under phase-contrast microscopy before treatment. After 24 h the same area was re-photographed and the remaining wound area was determined by image analysis. The wound closure rate was determined using the initial and final wound areas during the wounding experiments with the percentage of wound closure calculated as ([initial − final]/initial) × 100. All experiments were repeated three times.
Invasion assay
The invasive ability of cancer cells with or without the indicated treatment was examined by a transwell migration assay using membranes (8 µm pore size, 6.5 mm diameter; Corning Costar, Cambridge, MA, USA) coated with matrigel (2.5 mg/mL). Cells were trypsinized, washed, and resuspended at 1 × 105 cells/mL in serum-free media. The upper wells of the chambers were filled with cells in serum-free medium (5 × 104 cells/well) with or without curcumin (10 µM) and/or carboplatin (50 or 100 µM), and the lower wells contained medium with 10% FBS. After incubation for 24 h, the non-invading cells on the upper surface of the inserts were removed with a cotton swab and invading cells on the lower surface were fixed and stained with crystal violet Cell Stain Solution (Cell Biolabs, San Diego, CA, USA). Evaluation of cell invasion was performed by light microscopy, and the cells in four randomly selected fields were counted. The experiments were performed three times in triplicate.
Matrix metalloproteinase activity assay
The activity of MMP-2 and MMP-9 in the conditioned medium was measured by gelatin zymography. Briefly, samples (cell culture supernatants) were prepared with standard sodium dodecyl sulfate (SDS)-gel loading buffer before loading. The prepared samples were loaded onto 10% SDS polyacrylamide gels (0.75 mm thick) containing 0.1% gelatin. After electrophoresis, the gels were washed twice with distilled water containing 2.5% Triton X-100 (Sigma) for 20 min and then incubated in zymography reaction buffer (100 mM Tris–HCl, pH 7.5, 100 mM CaCl2, 500 mM NaCl, 0.2 mM ZnCl2, 0.02% NaN3, 2.5% TX-100) for 16 h at 37℃. The gels were stained with Coomassie brilliant blue R-250 for 1 h followed by destaining with methanol–acetic acid–water for 30 min. MMP-2 and MMP-9 activity appeared as clear bands against the blue background of the stained gel. Relative activity levels of MMP-2 and MMP-9 were semi-quantified using a Gel-Pro Analyzer.
Semi-quantitative reverse transcription (RT)-PCR analysis
Total RNA was isolated from A549 cells using TRIzol reagent™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. Total RNA (2 µg) was transcribed to cDNA with the oligo-dT primer and Moloney murine leukemia virus RTase (Invitrogen). PCR was carried out in a 20 µL reaction mixture containing 2.5 U Taq DNA polymerase buffer, 2 µL of each primer, and 2 µL cDNA. The sequences of the primers used in this study are summarized in Table 1. PCR was performed under the following conditions: initial denaturation at 95℃ for 5 min, followed by 25–30 cycles of 94℃ for 30 s, annealing at the specific temperature for each primer for 30 s, and 72℃ for 30 s, with a final extension step at 72℃ for 10 min. PCR products were resolved by electrophoresis on 2% agarose gels and visualized with ethidium bromide.
Table 1.
Sequences of PCR primers used in semi-quantitative reverse transcription (RT)-PCR analysis
| Gene | Sequence | Length (bp) | Annealing Temp. (℃) |
|---|---|---|---|
| caspase-3 | F: 5′-CAGTGGAGGCCGACTTCTTG-3′ | 399 | 57 |
| R: 5′-TGGCACAAAGCGACTGGAT-3′ | |||
| caspase-9 | F: 5′-TGTCCTACTCTACTTTCCCAGGTT-3′ | 198 | 57 |
| R: 5′-GTGAGCCCACTGCTCAAAGAT-3′ | |||
| p53 | F: 5′-TCCTTACTGCCTCTTGCTTCTC-3′ | 266 | 60 |
| R: 5′-TAACTGCACCCTTGGTCTCC-3′ | |||
| p21 | F: 5′-GGAAGACCATGTGGACCTGT-3′ | 197 | 60 |
| R: 5′-GGCGTTTGGAGTGGTAGAAA-3′ | |||
| NF-κB | F: 5′-GTGAATCTCCTGCCCTTTCA-3′ | 340 | 60 |
| R: 5′-CACCAATGATGTGTGATTGTCTC-3′ | |||
| MMP-9 | F: 5′-CTGTCCGTGAGGGTGTTGAG-3′ | 249 | 55 |
| R: 5′-CTCCCAGCCTATGCTTACCC-3′ | |||
| β-acitin | F:5′-ACAGGAAGTCCCTTGCCATC-3′ | 248 | 55 |
| R:5′-AGGGAGACCAAAAGCCTTCA-3′ |
Western blot analysis
Cells were harvested by trypsinization (trypsin 0.5%, 1 mM ethylenediamine tetraacetic acid [EDTA]), washed with PBS, lysed using a radioimmunoprecipitation assay cell lysis buffer (150 mM NaCl, 1% triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM Tris–HCl, pH 7.5, and 2 mM EDTA containing a mixture of protease inhibitors and phosphatase inhibitors (GenDEPOT, Barker, TX, USA), and centrifuged at 13,000 r/min for 30 min at 4℃. The protein concentration in the supernatants was determined using a BCA Protein Assay Kit (Sigma). Equal amounts of extracted protein (40 µg/sample) were separated by SDS polyacrylamide gel electrophoresis using a 10% gel with a Tris-glycine-SDS buffer and transferred to 0.45 -µm polyvinylidene difluoride membranes. The membranes were blocked with 5% (w/v) non-fat dried milk (Difco/Becton Dickinson, Atlanta, GA, USA) in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) and incubated for 2 h with the indicated antibody at a dilution of 1:200 at room temperature. The signal was visualized using enhanced Western Blotting Luminol Reagent (Santa Cruz Biotechnology) and recorded on an X-ray film.
Statistical analysis
The data were subjected to one-way ANOVA followed by Dunnett’s multiple range testing using Graph-Pad Prism version 5.00 for Windows (Graphpad Prism Software, La Jolla, CA, USA). All data are expressed as means ± SD and a value of P < 0.05 was accepted as statistically significant.
Results
Curcumin and carboplatin have a synergistic antiproliferative effect
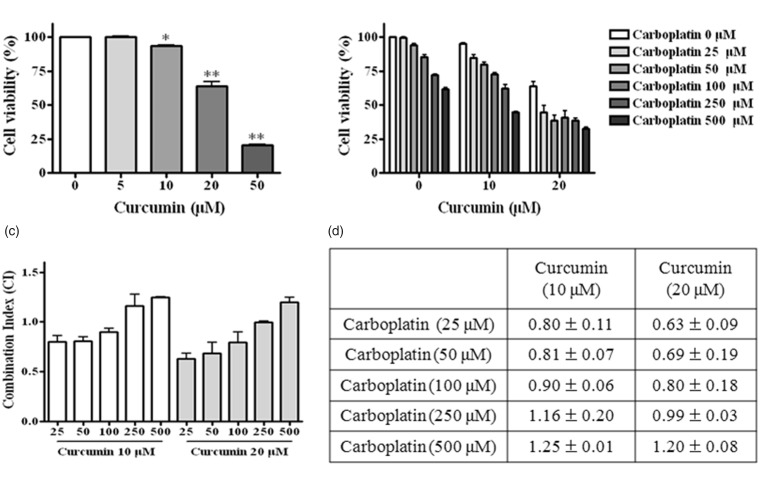
To investigate the synergism between these two agents, we treated A549 cells with different concentrations of curcumin (5, 10, 20, and 50 µM) and carboplatin (25, 50, 100, 250, and 500 µM) alone and in combination at different ratios for 24 h (Figure 1(a) and (b)). The MTT assay showed that curcumin and carboplatin alone and in combination reduced cell viability in a dose-dependent manner. DMSO showed no significant effects on cell morphology and viability (data not shown).
Figure 1.
Synergistic effect of curcumin and carboplatin on cell growth inhibition. A549 cells were treated with different concentrations of curcumin and/or carboplatin for 24 h and cell death was measured by the MTT assay as described in “Material and methods” section. Cell viability (a, b) and combination index (c, d) were determined. Combining curcumin with carboplatin resulted in enhanced cytotoxicity in A549 cells. Values represent mean ± SD from three independent experiments. *, P < 0.05; **, P < 0.01 compared with control
The results from the MTT assay were analyzed using Compusyn software to determine the CI values of the two agents in A549 cells for different treatment ratios. A CI of 1 indicates an additive effect, <1 indicates a synergistic effect, and >1 suggests an antagonistic effect of the combination. As shown in Figure 1(c) and (d), when curcumin and carboplatin were administered in ratios of 1:10, 1:5, and 1:2.5, the CI values ranged from 0.6 to 0.9. Although the CI value of a combination of 20 µM curcumin and carboplatin was lower than that of a combination of 10 µM curcumin and carboplatin, cell viability was less than 50% for the combination of 20 µM curcumin and carboplatin. For this reason, we selected 10 µM curcumin and 50 or 100 µM carboplatin (CI value <1) alone or in combination for use in subsequent experiments.
Morphological changes
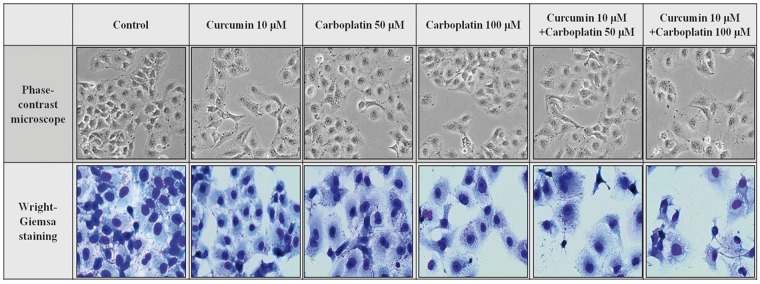
As shown in Figure 2, Wright–Giemsa staining of cells showed changes in cell morphology, cell shrinkage, rounding and partial detachment, loss of cell–cell contacts, and membrane blebbing in treated cells compared to control cells. These morphologic changes were distinctly increased after treatment with the combination of curcumin and carboplatin.
Figure 2.
Cellular morphologic changes. A549 cells were seeded in 12-well culture plates. After 24 h the cells were treated with the indicated concentration of drugs for a further 24 h. Cells were photographed under a phase contrast inverted microscope and then fixed and subjected to Wright–Giemsa staining. (A color version of this figure is available in the online journal.)
Inhibition of cell migration and invasion
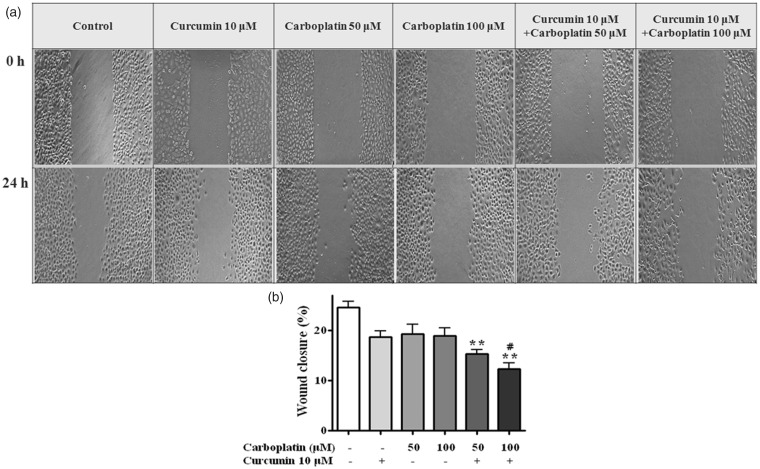
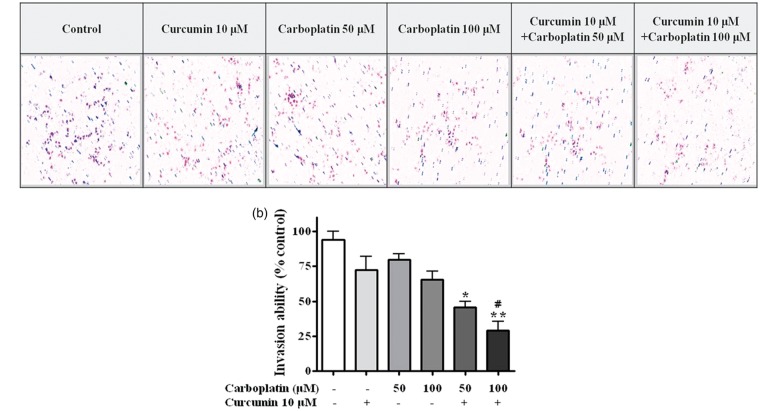
Treatment with 10 µM curcumin and 50 or 100 µM carboplatin in combination significantly reduced cell migration (P < 0.01) compared to untreated cells (Figure 3). Moreover, the migration inhibitory effect of a combination of 10 µM curcumin and 100 µM carboplatin was significantly higher than that of 100 µM carboplatin alone (P < 0.05). Combination treatment also significantly inhibited cell invasion in the matrigel invasion assay (P < 0.05) (Figure 4).
Figure 3.
Wound healing assay. After the cells had formed a confluent monolayer, scratches in the monolayer were made using a 200 µL tip and closure of the scratch was examined under a microscope. (a) Representative photographs show the same area at 0 h and after 24 h of treatment. (b) Cells migrating into the wound area were counted based on the line of the wound at 0 h. *, P < 0.05; **, P < 0.01 compared with control; #, P < 0.05 compared with 100 μM carboplatin
Figure 4.
Matrigel cell invasion assay. After incubation for 24 h with the indicated concentration of curcumin or carboplatin, cells that migrated to the lower chamber were fixed, stained, and counted using light microscopy (magnification, ×200) as described in “Material and methods” section. (a) Random fields were scanned (four fields per filter of the well) for the presence of cells on the lower side of the membrane. (b) Cells that migrated through the membrane were quantified. Carboplatin and curcumin synergistically suppressed the migration of A549 cells. *, P < 0.05; **, P < 0.01 compared with control. (A color version of this figure is available in the online journal.)
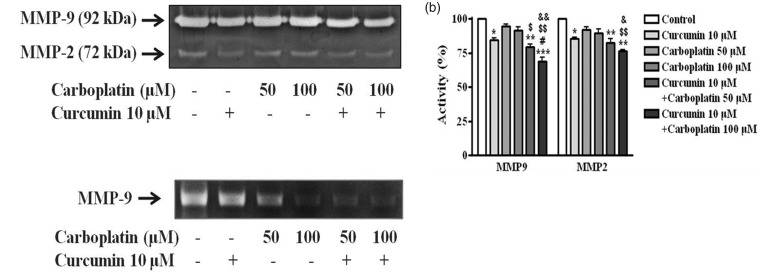
Downregulation of MMP-2 and MMP-9 activity
To examine whether the anti-invasive activities of curcumin or carboplatin correlated with the inhibition of MMP-2 and MMP-9 activities, we examined the activity levels of gelatinolytic MMPs using a gelatin zymography assay (Figure 5(a)). Cells were treated with curcumin, carboplatin, or both agents for 24 h and the culture supernatants were used for the assay. As shown in Figure 5(a), curcumin decreased activity levels of the pro-forms of zymogen MMP-2 and MMP-9 by 84.3 and 86.6%, respectively. However, carboplatin did not have an obvious effect on the pro-forms. Carboplatin with 10 µM curcumin treatment induced downregulation of MMP-2/-9 activity in a dose-dependent manner. The treatment of 100 µM carboplatin with curcumin inhibited the activity of MMP-2 and MMP-9 by 68.9 and 76.3%, respectively (Figure 5(b)). To further examine whether the inhibition of pro-form MMP-9 activity by curcumin and carboplatin resulted from a decrease in MMP-9 mRNA expression, the levels of MMP-9 mRNA in A549 cells were determined by RT-PCR. As shown in Figure 5(c), curcumin alone slightly inhibited MMP-9 mRNA expression in A549 cells, whereas carboplatin alone inhibited expression in a dose-dependent manner. In particular, the combination of curcumin and carboplatin decreased MMP-9 mRNA expression by approximately 40% compared with control cells. These results demonstrated that a combination of curcumin and carboplatin has a synergistic antimetastatic effect in A549 cells through inhibition of MMP-2 and MMP-9.
Figure 5.
Matrix metalloproteinase (MMP)-2/-9 in A549 cells. (a) Cells were incubated with curcumin or carboplatin as indicated for 24 h. MMP-2/-9 activities were examined using gelatin zymography. (b) Quantification of MMP-2/-9 activity by curcumin and/or carboplatin was compared with the control. Data are expressed as the mean ± SD. *, p < 0.05; ** p < 0.01; ***, p < 0.001 versus control group. #, p < 0.05 versus treatment group with 10 μM curcumin. $, p < 0.05; $$, p < 0.01 versus treatment group with 50 μM carboplatin; &, p < 0.05, &&, p < 0.01 versus treatment group with 100 μM carboplatin. (c) MMP-2/-9 mRNA levels were measured by RT-PCR analysis
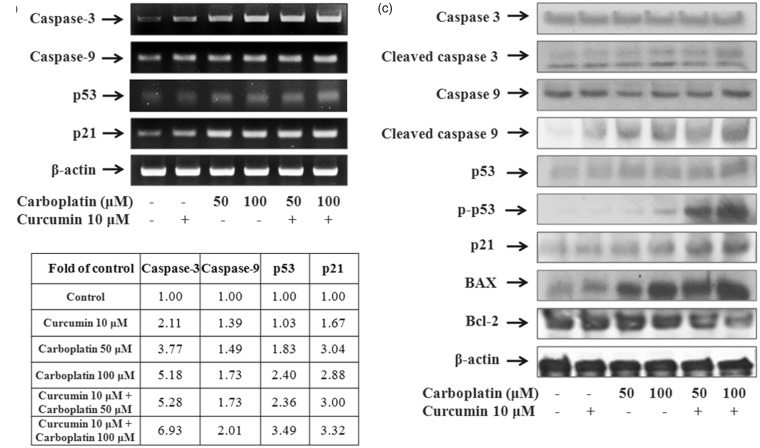
Expression of apoptotic genes
The mRNA expression of caspase-3, caspase-9, p53, and p21 was significantly upregulated by the combination of curcumin and carboplatin (Figure 6(a) and (b)).
Figure 6.
Effect of curcumin and carboplatin on the expression of genes involved in apoptosis and cell survival. A549 cells were treated for 24 h with curcumin and carboplatin alone or in combination, and the expression of caspase-3, caspase-9, p53, and p21 was examined by RT-PCR analysis (a) and quantitated using the Image J program (b). (c) Protein levels of apoptosis-related genes were examined using western blot analysis
The effect of curcumin and carboplatin on the expression of apoptosis-related proteins caspase-3, caspase-9, p53, p21, Bax, and Bcl-2 was also examined. As shown in Figure 6(c), the combination of curcumin and carboplatin caused obvious cleavage of both caspase-3 and caspase-9, suggesting that the antiproliferative effect of the combination treatment is mediated through the induction of apoptosis. In addition, the expression of the proapoptotic genes p53, p21, and Bax was upregulated by the combination of curcumin and carboplatin, whereas expression of the antiapoptotic gene Bcl-2 was significantly decreased by 81.8 and 52.6% by treatment with 10 µM curcumin and either 50 or 100 µM carboplatin, respectively.
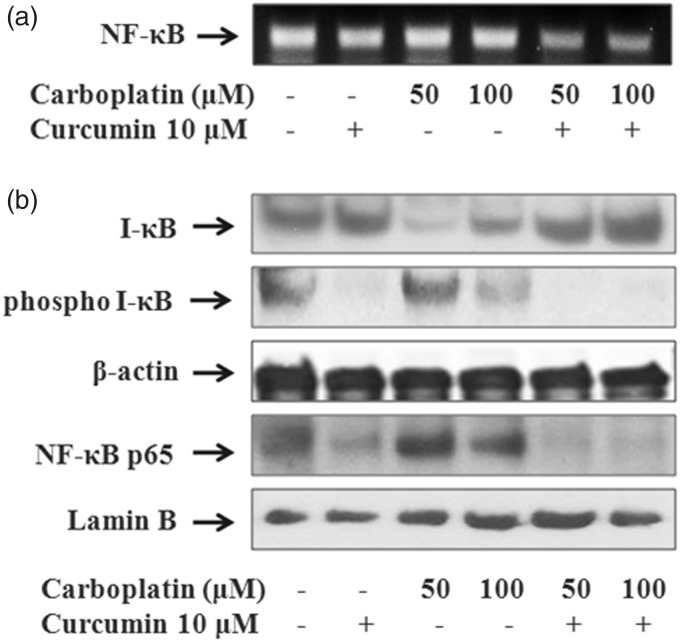
Suppression of NF-κB activation
As shown in Figure 7, the combination of carboplatin with curcumin greatly suppressed mRNA and nuclear protein levels of NF-κB compared with the single drug treatment. The treatment of curcumin with or without carboplatin induced the activity of I-κB, an inhibitor of NF-κB proteins, through inhibiting phosphorylation of I-κB. We examined the NF-κB p65 activity in nuclear extract of A549 cells using western blotting, because NF-κB activation requires nuclear translocation of the p65 subunit of NF-κB. Curcumin treatment effectively blocked p65 nuclear translocation (Figure 7(b)). These results indicate that curcumin may sensitize A549 cells to the effects of carboplatin by inhibiting NF-κB activity and thus regulating the expression of apoptosis-related genes.
Figure 7.
Effect of curcumin and carboplatin on NF-κB activity. A549 cells were treated with curcumin and carboplatin as indicated, and the expression of NF-κB was examined using RT-PCR (a). Curcumin treatment with carboplatin induces I-κB activity and inhibits phosphorylation of I-κB and nuclear translocation of NF-κB p65. Cytosolic and nuclear lysates were analyzed by western blot analysis. β-actin and lamin B were used as a loading control for cytoplasmic and nuclear proteins, respectively (b)
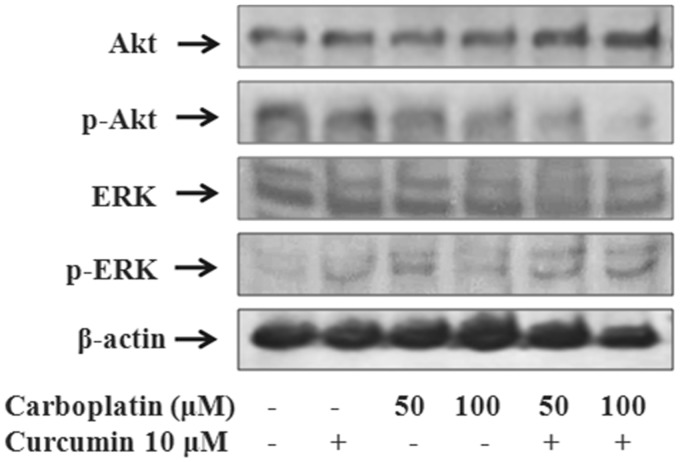
Regulation of Akt and ERK1/2
Treatment of A549 cells with 10 µM curcumin in combination with 50 or 100 µM carboplatin for 24 h affected phosphorylation of Akt and ERK1/2 to a greater extent than treatment with each agent alone. As shown in Figure 8, the combination of curcumin and 100 µM carboplatin downregulated Akt phosphorylation by 34.9% and increased ERK1/2 phosphorylation by 181.6%. These results indicate that the synergistic antineoplastic effect of the combination of curcumin and carboplatin on A549 cells is mediated by inactivation of Akt and activation of ERK1/2.
Figure 8.
Effect of curcumin and carboplatin on the phosphorylation of Akt and MAPK. A549 cells were treated with curcumin and carboplatin as indicated and the expression of Akt and MAPK-activated genes was examined using western blot analysis
Discussion
This study aimed to evaluate the effect of curcumin combined with carboplatin on cell viability and apoptosis and underlying mechanism in human lung cancer cells. Curcumin is known to sensitize cancer cells to various chemotherapeutic agents, but protect normal organs from chemotherapy-induced toxicity.18
In this study, curcumin or carboplatin alone decreased the cell viability of A549 cells in a dose-dependent manner, whereas a combination of curcumin (10 or 20 µM) and carboplatin (20–100 µM) had a greater inhibitory effect on cell viability. In addition to cell proliferation and apoptosis, the combination of curcumin and carboplatin had a synergistic therapeutic effect on cancer cell invasion and migration. It is possible that these effects involve downregulation of MMP-2 and MMP-9 by curcumin.19 MMP-2 and MMP-9 are responsible for extracellular matrix degradation and overexpression of these genes is closely related to tumor metastasis.20 Our experiment demonstrated that combination of curcumin and carboplatin inhibited MMP-2 and MMP-9 expression in the A549 cell line.
One of the major signaling pathways responsible for chemoresistance is the NF-κB pathway. The heterodimeric transcription factor NF-κB is a significant player in cancer development and progression.21 NF-κB transcriptionally regulates the expression of various proteins that participate in prosurvival signaling pathways, such as Bcl-2, cyclin D, and vascular endothelial growth factor.22,23 NF-κB is activated by nuclear translocation following its release from a complex with nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα). Phosphorylation of IκBα is one of the protein kinase B (Akt)-dependent signaling pathways responsible for the activation of NF-κB.24 Chemotherapy-induced activation of NF-κB is thought to play a critical role in the acquisition of chemoresistance.25 Several reports have shown that downregulation of NF-κB intensifies therapeutic efficacy25–28 and that suppression of NF-κB activity is associated with the induction of apoptosis in cancer chemotherapy.29
Curcumin has been shown to inhibit NF-κB pathway.18,30 Sakamoto et al.31 reported that platinum-based anticancer drugs such as carboplatin and oxaliplatin augment NF-κB activation, whereas curcumin decreases nuclear expression of NF-κB and potentiates the sensitivity of colorectal cancer cells to chemotherapeutic agents. We found that curcumin, either as a single agent or combined with carboplatin, suppressed NF-κB activation. This inhibition of NF-κB is through sequestration in the cytoplasm by IκBα. Curcumin combined with carboplatin also reduced expression of antiapoptotic protein Bcl-2, one of several downstream targets of NF-κB.32 Bcl-2 family proteins are critical regulators of the induction of apoptosis through interactions at the mitochondrial membrane that influence the release of cytochrome c,33 which in turn activates the intracellular caspases responsible for cleavage of cellular proteins during apoptosis. Chanvorachote et al.13 reported that curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Initiation of mitochondrial apoptosis is mediated by the Bcl-2 family and the tumor suppressor protein p53, and the subsequent increase in the activity of caspase-3 and caspase-9. Mitochondrial cytochrome c activates caspase-9 as an initial caspase, which subsequently induces activation of the effector caspase-3.34 Caspase activation is a hallmark of apoptosis and is known to be regulated by NF-κB.35 Curcumin-induced NF-κB inhibition exerts antitumor potency through activation of the p53–p21 protein complex.36
We further observed that a combination of curcumin and carboplatin inhibited phosphorylation of Akt and enhanced phosphorylation of ERK1/2 in the lung cancer cell line. Curcumin has been shown to inhibit phosphorylation of Akt/mTOR and its downstream targets in many cancer cell lines37,38 and Akt phosphorylation plays a critical role in regulation of the NF-κB pathway.35 Therefore, inhibition of Akt phosphorylation by curcumin might downregulate NF-κB. Activation of ERK1/2, one of the mitogen-activated protein kinases, is associated with inhibition of apoptosis in response to diverse external stimuli.39 On the other hand, activation of ERK1/2 in response to certain DNA damage stimuli, including etoposide, adriamycin, and platinum compounds, promotes cell apoptosis by activating proapoptotic signaling molecules and G2-M arrest.38 A report by Ono et al.40 support involvement of ERK1/2 activation in curcumin-induced apoptosis in gall bladder carcinoma cells. Therefore, the enhanced phosphorylation of ERK1/2 by curcumin combined with carboplatin in lung cancer cells is an interesting finding of this study.
In summary, we report that curcumin combined with carboplatin showed synergistic effect on cell invasion and metastasis through activation of cell apoptosis in human lung cancer cells. These beneficial effects were mediated by NF-κB through inhibition of the Akt/IKKα pathway and activation of ERK1/2 with subsequent activation of caspases and p53. Our results provide evidence for the potential as chemosensitizer of curcumin in the treatment of lung cancer, especially in patients frail to platinum-based chemotherapy. Further investigation for the activity of curcumin in animals and humans is required to confirm its potential as a chemosensitizer.
Acknowledgements
The authors sincerely thank Dr Donghwa Kim at University of South Florida, for his comments on the manuscript. We declare no competing financial interests.
Authors’ contributions
JHK and HSK managed the overall experiment and wrote the manuscript. HYL and IKK conducted the experiments and analyzed the data. JHH, CDY, and HHK participated in the interpretation of the studies and manuscript editing. HSM and SHL supervised the project. All authors read and approved the final manuscript. JHK and HSK contributed equally to this work.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19: 1893–907. [DOI] [PubMed] [Google Scholar]
- 2.Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer: an evolving story. Annu Rev Med 2008; 59: 429–42. [DOI] [PubMed] [Google Scholar]
- 3.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol 2008; 3: 819–31. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013; 31: 1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pivot X, Cals L, Cupissol D, Guardiola E, Tchiknavorian X, Guerrier P, Merad L, Wendling JL, Barnouin L, Savary J, Thyss A, Schneider M. Phase II trial of a paclitaxel-carboplatin combination in recurrent squamous cell carcinoma of head and neck. Oncology 2001; 60: 66–71. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara K, Sakuragi N, Suzuki S, Yoshida N, Maehata K, Nishiya M, Koshida T, Sawai H, Aotani E, Kohno I. First-line intraperitoneal carboplatin-based chemotherapy for 165 patients with epithelial ovarian carcinoma: results of long term follow up. Gynecol Oncol 2003; 90: 637–43. [DOI] [PubMed] [Google Scholar]
- 7.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level (review). Oncol Rep 2003; 10: 1663–82. [PubMed] [Google Scholar]
- 8.Cosaert J, Quoix E. Platinum drugs in the treatment of non-small-cell lung cancer. Br J Cancer 2002; 87: 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merk J, Rolff J, Dorn C, Leschber G, Fichtner I. Chemoresistance in non-small-cell lung cancer: can multidrug resistance markers predict the response of xenograft lung cancer models to chemotherapy? Eur J Cardiothorac Surg 2011; 40: 29–33. [DOI] [PubMed] [Google Scholar]
- 10.Limtrakul P. Curcumin as chemosensitizer. Adv Exp Med Biol 2007; 595: 269–300. [DOI] [PubMed] [Google Scholar]
- 11.Chen TY, Chen DY, Wen HW, Ou JL, Chiou SS, Chen JM, Wong ML, Hsu WL. Inhibition of enveloped viruses infectivity by curcumin. PLoS One 2013; 8: e62482–e62482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye MX, Li Y, Yin H, Zhang J. Curcumin: updated molecular mechanisms and intervention targets in human lung cancer. Int J Mol Sci 2012; 13: 3959–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanvorachote P, Pongrakhananon V, Wannachaiyasit S, Luanpitpong S, Rojanasakul Y, Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Invest 2009; 27: 624–35. [DOI] [PubMed] [Google Scholar]
- 14.Rocks N, Bekaert S, Coia I, Paulissen G, Gueders M, Evrard B, Van Heugen JC, Chiap P, Foidart JM, Noel A, Cataldo D. Curcumin-cyclodextrin complexes potentiate gemcitabine effects in an orthotopic mouse model of lung cancer. Br J Cancer 2012; 107: 1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen S, Sharma H, Singh N. Curcumin enhances vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun 2005; 331: 1245–52. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Guo R, Xu Y, Zheng Y, Hou Z, Dai X, Zhang Z, Zheng D, Xu H. Synergistic antitumor efficiency of docetaxel and curcumin against lung cancer. Acta Biochim Biophys Sin 2012; 44: 147–53. [DOI] [PubMed] [Google Scholar]
- 17.Chou TC, Martin N. Compusyn for drug combinations and for general dose-effect Analysis. PC Software for quantization of synergism and antagonism and determination of IC50, ED50 and LD50. ComboSyn, Inc. Paramus, NJ, 2005.
- 18.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 2010; 62: 919–30. [DOI] [PubMed] [Google Scholar]
- 19.Lin HJ, Su CC, Lu HF, Yang JS, Hsu SC, Ip SW, Wu JJ, Li YC, Ho CC, Wu CC, Chung JG. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol Rep 2010; 23: 665–70. [PubMed] [Google Scholar]
- 20.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med 2008; 359: 2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujioka S, Son K, Onda S, Schmidt C, Scrabas GM, Okamoto T, Fujita T, Chiao PJ, Yanaga K. Desensitization of NFkappaB for overcoming chemoresistance of pancreatic cancer cells to TNF-alpha or paclitaxel. Anticancer Res 2012; 32: 4813–21. [PubMed] [Google Scholar]
- 22.Tergaonkar V, Bottero V, Ikawa M, Li Q, Verma IM. IkB kinase-independent IkBa degradation pathway: functional NF-kB activity and implications for cancer therapy. Mol Cell Biol 2003; 23: 8070–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 1999; 19: 5923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kB through utilization of the IkB kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 2001; 276: 18934–40. [DOI] [PubMed] [Google Scholar]
- 25.Jones DR, Broad RM, Madrid LV, Baldwin AS, Jr, Mayo MW. Inhibition of NF-kappaB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis. Ann Thorac Surg 2000; 70: 930–37. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SG, Park J, Park JY, Park CH, Lee KH, Cho JW, Hwang JI, Seong JY. Anti-cancer activity of a novel small molecule compound that simultaneously activates p53 and inhibits NF-kappaB signaling. PLoS One 2012; 7: e44259–e44259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kausar H, Jeyabalan J, Aqil F, Chabba D, Sidana J, Singh IP, Gupta RC. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett 2012; 325: 54–62. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Zheng X, Wang Q, Yan J, Li D, Zhou Y, Lin Y, Zhang L, Wang X. Concurrent blockade of NF-kappaB and Akt pathways potentiates cisplatin's antitumor activity in vivo. Anticancer Drugs 2012; 23: 1039–46. [DOI] [PubMed] [Google Scholar]
- 29.Wang YT, Liu HS, Su CL. Curcumin-enhanced chemosensitivity of FDA-approved platinum (II)-based anti-cancer drugs involves downregulation of nuclear endonuclease G and NF-kB as well as induction of apoptosis and G2/M arrest. Int J Food Sci Nutr 2014; 65: 368–74. [DOI] [PubMed] [Google Scholar]
- 30.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, Liotta D, Fu H. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol 2008; 74: 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto K, Maeda S. Targeting NF-kappaB for colorectal cancer. Expert Opin Ther Targets 2010; 14: 593–601. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka S, Sclabas GM, Schmidt C, Niu J, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Inhibition of constitutive NF-kB activity by IkBa M suppresses tumorigenesis. Oncogene 2003; 22: 1365–70. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett 2000; 466: 6–10. [DOI] [PubMed] [Google Scholar]
- 34.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors 2013; 39: 27–36. [DOI] [PubMed] [Google Scholar]
- 35.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 2011; 12: 695–708. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Dai Q, Lu N, Wei L, Ha J, Rong J, Mu R, You Q, Li Z, Guo Q. LYG-202 inhibits the proliferation of human colorectal carcinoma HCT-116 cells through induction of G1/S cell cycle arrest and apoptosis via p53 and p21(WAF1/Cip1) expression. Biochem Cell Biol 2011; 89: 287–98. [DOI] [PubMed] [Google Scholar]
- 37.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the mammalian target of rapamycin-raptor complex. Cancer Res 2009; 69: 1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther 2008; 7: 2609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006; 58: 621–31. [DOI] [PubMed] [Google Scholar]
- 40.Ono M, Higuchi T, Takeshima M, Chen C, Nakano S. Antiproliferative and apoptosis-inducing activity of curcumin against human gallbladder adenocarcinoma cells. Anticancer Res 2013; 33: 1861–66. [PubMed] [Google Scholar]