Abstract
In this study, we examined whether local deferoxamine (DFO) administration can promote angiogenesis and bone repair in steroid-induced osteonecrosis of the femoral head (ONFH). Steroid-induced ONFH was induced in 65 mature male New Zealand white rabbits by methylprednisolone in combination with lipopolysaccharide. Six weeks later, the rabbits received no treatment (model group, N = 15), bilateral core decompression (CD group, N = 20) or CD in combination with local DFO administration (DFO group, N = 20). Six weeks after the surgery, vascularization in the femoral head was evaluated by ink artery infusion angiography and immunohistochemical staining for von Willebrand Factor (vWF). Bone repair was assessed by histologic analysis and micro-computed tomography (micro-CT). Immunohistochemical staining was performed to analyze the expression of vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1α (HIF-1α), bone morphogenetic protein-2 (BMP-2), and osteocalcin (OCN). Ink artery infusion angiography and microvessel analysis by immuohistochemical staining for vWF showed more blood vessels in the DFO group than other groups. The expression of HIF-1α, VEGF, BMP-2, and OCN, indicated by immunohistochemical staining, was higher in the DFO group compared with other groups. Micro-CT scanning results indicated that the DFO group had larger volume of newly formed bone than the CD group. This work indicated that local DFO administration improved angiogenesis and bone repair of early stage ONFH in rabbit model, and it may offer an efficient, economic, and simple therapy for early stage ONFH.
Keywords: Osteonecrosis, deferoxamine, hypoxia-inducible factor-1α, angiogenesis, bone repair
Introduction
Steroid-induced osteonecrosis of the femoral head (ONFH) is one of the most serious complications in patients who have long-term glucocorticoid use or heavy use in a short time as treatment for diseases including glomerulonephritis, inflammatory bowel disease, and immunosuppression after renal transplants.1 It is a chronic progressive disease and if not treated properly and promptly, 85% of symptomatic patients at stages I and II will have to undergo hip arthroplasty in 2 y because of the articular cartilage collapse.2 Besides, corticosteroid-induced ONFH tends to occur in those aged 30–50 y and the joint prosthesis is not durable enough in younger patients with corticosteroid-induced ONFH.3,4 Thus, numerous studies have been performed to find a novel method to avoid the collapse and arthroplasty.
So far, many surgical therapies including core decompression (CD), vascularized bone grafting, stem cells transplantation, and rotational osteotomy are used to treat early-stage ONFH. CD was the most commonly used method to treat ONFH in the past, but the efficacy is controversial.5 Although the vascularized fibular graft has acquired satisfactory success rate in early stage osteonecrosis, it also has serious complications.6 Stem cells transplantation is a potentially effective method by providing more osteogenic progenitor cells and cell factors that induce angiogenesis and bone formation. However, its clinical application is limited due to the high death rate of stem cells after transplantation and limited sources.
Hypoxia-inducible factor-1 (HIF-1) plays a crucial role in regulating the adaptive response of cells to hypoxia. So far, more than 100 target genes of HIFs including vascular endothelial growth factor (VEGF) have been found, and they are particularly relevant to angiogenesis, erythrocytopoiesis, and cytoprotection. Recent studies showed that HIF-1α and its target gene VEGF play important roles in angiogenic–osteogenic coupling during bone development and regeneration.7 Deferoxamine (DFO), an iron chelator, is usually used to treat disorders of excess iron. Also, DFO is a HIF prolyl hydroxylase inhibitor (PHI) and inhibits degradation of HIF-1α both in vitro and in vivo under normoxia, subsequently increasing the expression of HIFs target genes.8–10 Recently, it has been demonstrated that in bone fracture and bone defect model, local DFO injection can promote angiogenesis and bone formation.11,12 However, the effect of DFO on vascularization and bone repair in ONFH has not been studied.
So, the present study is to study whether local DFO administration promotes vascularization and bone repair of steroid-induced ONFH in a rabbit model.
Materials and methods
All the animals used were from the Experimental Animal Center of Xi’an Jiaotong University, China. The experimental protocols were approved by the Animal Ethical Committee of the Xi'an Jiaotong University and adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Establishment of animal ONFH model and treatments
Sixty-five mature male New Zealand (age: 28 weeks) were housed at the Experimental Animal Center of Xi’an Jiaotong University and received standard laboratory diets. Osteonecrosis was induced by methods according to the previously published protocols.13 Briefly, the rabbits received an intravenous injection of 10 µg/kg body weight of lipopolysaccharide (Sigma, St. Louis, MO, USA). Twenty-four hours later, the rabbits received three intramuscular injections of methylprednisolone (Pfizer, New York, USA) at 20 mg/kg body weight at a time interval of 24 h. It was reported that six weeks after the last injection of methylprednisolone, ONFH gradually developed. During the establishment of ONFH model, five rabbits died of infection. Six weeks after the last injection of methylprednisolone, five rabbits were sacrificed and hematoxylin and eosin (HE) staining was performed to confirm ONFH. The remaining 55 rabbits received no treatment (model group, N = 15), bilateral CD group (N = 20) or CD in combination with local DFO administration (DFO group, N = 20). Six weeks later, the rabbits were sacrificed and the femoral head of rabbits in each group were obtained and assessed by histological, ink artery infusion angiography, micro-CT scanning, and immunohistochemistry analysis.
Surgical procedure
Strict aseptic technique was observed throughout the procedure. After the animals were anesthetized with pentobarbital sodium, the greater trochanter of the femoral head was exposed for the CD. A drill with an outer diameter of 1.5 mm was inserted from the flare of the greater trochanter to the femoral neck and into the femoral head, without crossing the boundary surface of the femoral head cartilage. The location of the drill was confirmed radiographically (Figure S1). For the rabbits in DFO group, 500-μM DFO (Sigma, St. Louis, MO, USA) loaded in gelatin sponge (BIOT Biology, Wuxi, Jiangsu Province, China) was slowly plugged into the tunnel made by drill in the femoral head. Isometric saline loaded in gelatin sponge was slowly injected in the CD group. Then the hole was sealed by bone wax and the incision was closed layer by layer.
Ink artery infusion angiography and analysis
Six weeks after the surgery, five rabbits of each group were anesthetized with pentobarbital sodium and used to perform ink artery infusion angiography to investigate the blood supply of the femoral head. After anesthetized, the abdominal aorta and inferior vena cava were exposed and ligated immediately. A tube was punctured into the abdominal aorta at distal end of ligation, used to affuse ink. Another tube was distally inserted in the inferior vena cava for drainage. The abdominal aorta was irrigated with heparinized saline (25,000 units in 250 ml of 0.9% sodium chloride), until the liquid outflowed from the inferior vena cava was clear. The abdominal aorta was then injected continuously with a solution of 10% gelatin/Indian ink (20 g of gelatin in 100 ml of Indian ink and 100 ml of water) at pressure of 90 mmHg, until the skin and nails of the bilateral crura completely turned black. Then animals were euthanized and 24 h after the refrigeration at 4℃, the bilateral thighbones were dissected and harvested. The samples were fixed, decalcified, embedded, cut into slices of 20-µm thick, and stained by HE. The blood distribution of the femoral head was observed under a stereomicroscope.
Histopathology
Six weeks after the surgery, the remaining rabbits of each group were euthanized and bilateral thighbones were dissected and observed by macrography. Bilateral femoral head was obtained for histological analyses after micro-CT scanning. For HE staining, briefly, after fixed with 10% neutral-buffered formalin, bone samples were decalcified with 10% ethylene diamine tetraacetic acid neutralized with sodium sulfate buffer, then embedded in paraffin, cut along the coronal plane into 4-µm thick sections, and stained with HE. The microscopic changes in the femoral heads were observed under a light microscope. During the observation of the trabeculae at 100 times magnification, 10 fields were chosen randomly and 50 bone lacunae in each field were counted. The number of empty lacunae in 10 sections was counted and the percentage of empty lacunae was calculated by researchers who were blind to assure that all the work was honestly reported.
Immunohistochemistry
To analyze microvessels and osteogenic potential in the femoral head, immunohistochemistry was performed using antibodies specific for: HIF-1α (Upstate Biotechnology, Lake Placid, New York, USA), VEGF (Upstate Biotechnology, Lake Placid, New York, USA), vWF (Santa Cruz Biotechnology, Dallas, Texas, USA), osteocalcin (OCN) (Santa Cruz Biotechnology, Dallas, Texas, USA), and bone morphogenetic protein-2 (BMP-2) (Santa Cruz Biotechnology, Dallas, Texas, USA). Briefly, sections were immersed in 3% hydrogen peroxide in 0.01 M phosphate-buffered saline (PBS) for 10 min to block endogenous peroxidase activity and then were rinsed several times in PBS. After being blocked with 10% goat normal antiserum (Vector, Burlingame, California, USA) for 30 min at room temperature, sections were treated with a primary antibody overnight at 4℃ and incubated with the biotinylated secondary antibody for 30 min, followed by streptavidin peroxidases for 30 min at room temperature. To reveal the immunoreactivity (IR), the sections were then reacted with diaminobenzidine solution in the dark. Finally, the sections were treated with hematoxylin and mounted. Sections without primary antibodies processing were used as the negative control. The sections were observed and photographed by the eclipse 50i optical microscope imaging system (Nikon Co., Ltd., Tokyo, Japan). The positive staining images were quantitatively analyzed by using the Image-Pro® Plus 6.0 image analysis software and calculating the mean optical density using a single blind method. Ten randomly fields in bone marrow cavities in each section were selected and the positive staining was quantitated according to the integrated optical density (IOD) value. The mean optical density was defined as the ratio of IOD to the corresponding cavity area. To calculate the mean optical density, eight sections for each protein from each group were analyzed.
After immuohistochemical staining for vWF, microvessel was analyzed by light microscopy. Any single vWF-positive endothelial cell or cluster of vWF-positive endothelial cells which were clearly separated from adjacent microvessels was regarded as one microvessel. Ten random areas in each section were counted and the microvessels number was calculated. Ten sections in each group were analyzed. The evaluation was performed by researchers who were blind regarding the identity of three groups.
Micro-CT scanning
Bone structure of the femoral head was analyzed by micro-CT (eXplore Locus SP, GE, Fairfield, Connecticut, USA) using a high-resolution system (MS-8) six weeks after the surgery. Briefly, each femoral head was scanned continuously with a slice thickness and increment of 14 µm at 80 kVp with an exposure time of 3000 ms/frame. The voxel size was 14 × 14 × 14 µm3. Along the center line of the femoral neck, a 1.5 mm × 3 mm × 5 mm cuboid region of interest (ROI) containing the bone tunnel generated by CD was selected with a semiautomatic contouring method in the center of the femoral head. Three-dimensional reconstruction images of the cuboid ROI were acquired by Reconstruction Utility with 16-bit gray levels. Microstructural parameters of ROI including bone mineral density (BMD; expressed as mg/cm3), bone volume fraction (BVF; expressed as a percentage), trabecular thickness (Tb.Th; expressed as mm), trabecular number (Tb.N; expressed as 1/mm), and trabecular separation (Tb.Sp; expressed as mm) were calculated from the 3-D images by using Advanced Bone Analysis software (GE Health Care).
Statistical analysis
All the data were described as mean ± standard deviation. One-way analysis of variance was performed to analyze the statistical data. The Statistical Package for the Social Sciences 17.0 software was used for analysis. P < 0.05 was considered as statistically significant.
Results
Histological analysis
In the subchondral bone of the femoral head, the model group showed sparser trabecular bone with more empty lacunae (29.0 ± 5.75%), surrounded by few spindle-shaped osteoblasts and more marrow fat cells which dominantly occupied the marrow space. In the CD group, a somewhat decreased number of empty lacunae (24.6 ± 6.11%) in the subchondral bone of the femoral head was observed (P > 0.05, n = 10).
In the DFO group, fewer empty lacunae (10.8 ± 4.44%) were observed in the subchondral bone of the femoral head compared with that in the model group and CD group (P < 0.05, n = 10). Besides, there was abundant hematopoietic tissue in the bone marrow (Figure 1(a)). In the tunnels of both the CD group and the DFO group, the gelatin sponge was not observed. In the CD group, the tunnel made by CD was almost filled with fibrovascular tissue. However, in the DFO group, massive newly formed trabecular tissue filled the tunnel of the CD (Figure 1(b)). This was also confirmed by micro-CT scanning. Around the bone tunnel, the CD group showed more empty lacunae in the trabecular bone, which was surrounded with fibrous tissue. In the DFO group, less empty lacunae were found in the trabecular bone, which was surrounded with normal marrow (Figure 1(c)). The histologic results indicated that DFO had the potential to improve bone repair.
Figure 1.
Histological observations of the femoral head six weeks after the surgery (200 × magnification). (a) Representative photomicrograph of the subchondral bone of the femoral head in three groups. (a1) The model group showed sparser trabecular bone with more empty lacunae (29.0 ± 5.75%), surrounded by few spindle-shaped osteoblasts, few marrow cells, and more marrow fat cells. (a2) In the CD group, a somewhat decreased number of empty lacunae (24.6 ± 6.11%) in the CD group was observed (P > 0.05, n = 10). (a3) Compared with the model group and the CD group, the DFO group showed fewer empty lacunae (10.8 ± 4.44%) and abundant hematopoietic tissue in the bone marrow (P < 0.05, n = 10). (b) Representative photomicrograph of tissue within the bone tunnel. (b1) The tunnel made by core decompression was filled with fibrovascular tissue in the CD group. (b2) The tunnel was filled with massive newly formed trabecular tissue in the DFO group. (c) Representative photomicrograph of tissue around the bone tunnel. (c1) In the trabecular bone around the bone tunnel in the CD group, more empty lacunae were found. In addition, the trabecular bone was surrounded with fibrous tissue. (c2) In the DFO group, less empty lacunae were found in the trabecular bone and the trabecular bone was surrounded with normal marrow. (A color version of this figure is available in the online journal.)
Microvessel analysis
Ink artery infusion angiography of the femoral head showed that in the model group few ink-stained blood vessels were found. In the CD group, increased ink-stained blood vessels were observed. Compared with the model group and the CD group, more ink-stained blood vessels were present (Figure 2).
Figure 2.
Ink artery infusion angiography and analysis (200 × magnification). (a) Few ink-stained blood vessels were found in the model group. (b) Increased ink-stained blood vessels were observed in the CD group. (c) More ink-stained blood vessels were present in the DFO group compared with the model group and the CD group. (A color version of this figure is available in the online journal.)
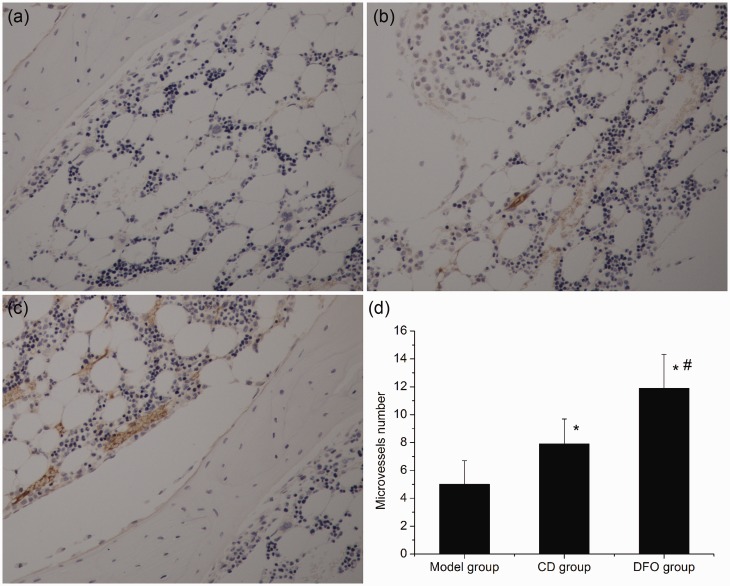
Microvessels were also analyzed by immuohistochemical staining for vWF and similar results were obtained. Few microvessels were observed in the model group (Figure 3(a)). In the CD group, increased microvessels were found compared with the model group (Figure 3(b)). In comparison with the model group and the CD group, significantly higher numbers of microvessels were present in the DFO group (Figure 3(c)).
Figure 3.
Immuohistochemical staining for vWF (200 × magnification) and microvessel analysis. (a) Representative photomicrograph of the model group showed that there were few microvessels in the femoral head. (b) In the CD group, increased microvessels were observed. (c) In the DFO group, significantly higher numbers of microvessels were present. (d) Bar graph shows the number of microvessels in each group. *P < 0.05 versus model group; #P < 0.05 versus CD group; n = 10. (A color version of this figure is available in the online journal.)
New bone formation analyzed by micro-CT scanning
Six weeks after the surgery, newly formed bone was present in the bone tunnel of both the CD group and the DFO group (Figure 4). Compared with the CD group, the DFO group formed significantly more new bone in the bone tunnel, which was also confirmed by histologic observation.
Figure 4.
Representative three-dimensional micro-CT scanning images. (a) In the CD group, few newly formed bone was present. (b) Compared with the CD group, significantly more newly formed bone was observed in the DFO group. (A color version of this figure is available in the online journal.)
The histomorphometric parameters of ROI including BMD, BVF, Tb.Th, Tb.N, and Tb.Sp were significantly different between the CD group and the DFO group (Table 1). The DFO group had significantly higher values of BMD, BVF, Tb.N, and Tb.Th, and lower values of Tb.Sp than the CD group (P < 0.05 for each parameter, n = 10).
Table 1.
The comparison of histomorphometric parameters of region of interest
| Parameters significancea | CD group (n = 10) | DFO group (n = 10) | Statistical |
|---|---|---|---|
| BMD | 133.56 ± 17.47 | 168.37 ± 24.29 | P < 0.01 |
| BVF | 14.91 ± 3.71 | 20.15 ± 4.37 | P < 0.05 |
| Tb.N | 1.106 ± 0.329 | 1.501 ± 0.351 | P < 0.05 |
| Tb.Th | 0.098 ± 0.016 | 0.164 ± 0.017 | P < 0.01 |
| Tb.Sp | 0.740 ± 0.155 | 0.594 ± 0.114 | P < 0.05 |
Note: BMD: bone mineral density, expressed as mg/cm3; BVF: bone volume fraction, expressed as a percentage; Tb.N: trabecular number, expressed as 1/mm; Tb.Th: trabecular thickness, expressed as mm; Tb.Sp: trabecular separation, expressed as mm.
Statistical significance was evaluated by independent samples t-test.
Local expression of HIF-1α, VEGF, BMP-2, and OCN
The expression of angiogenic factors including HIF-1α and VEGF, and osteogenic factors including BMP-2 and OCN was analyzed by immunohistochemical staining.
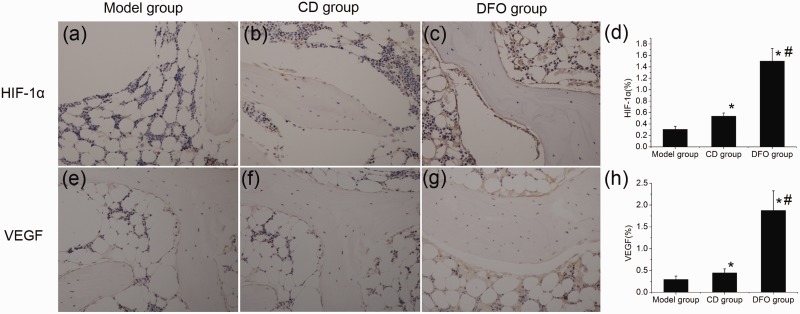
The appearance of yellow brown substance indicated positive staining. HIF-1α and VEGF IR were mainly observed in the osteoblasts and endothelial cells in each group. In the model group, a slight HIF-1α-IR and VEGF-IR was found (Figure 5(a) and (e)). The CD group showed increased level of HIF-1α-IR and VEGF-IR in osteoblasts and endothelial cells (Figure 5(b) and (f)). In comparison with the model group and the CD group, DFO group showed considerably strong HIF-1α-IR and VEGF-IR in osteoblasts and endothelial cells (Figure 5(c) and (g)).
Figure 5.
Immunohistochemical staining (200 × magnification) and semiquantitative analysis of HIF-1α and VEGF. HIF-1α and VEGF IR were mainly observed in osteoblasts and endothelial cells in each group. (a,e) Representative photomicrograph from the model group showed that HIF-1α-IR and VEGF-IR was weak. (b,f) Compared with the model group, the CD group showed increased level of HIF-1α-IR and VEGF-IR in osteoblasts and endothelial cells. (c,g) The DFO group showed strong HIF-1α-IR and VEGF-IR in osteoblasts and endothelial cells compared with other groups. (d,h) Bar graphs showed the mean optical density of HIF-1α and VEGF in bone marrow. Semiquantitative analysis was performed for eight sections (10 fields per section) in each group and the mean optical density was calculated. *P < 0.05 versus model group; #P < 0.05 versus CD group; n = 8. (A color version of this figure is available in the online journal.)
Slight BMP-2-IR and OCN-IR were observed in the model group which was concomitant with the number of osteoblasts (Figure 6(a) and (e)). Compared with the model group, the CD group showed increased BMP-2-IR, OCN-IR, and number of osteoblasts (Figure 6(b) and (f)). In the DFO group, the BMP-2-IR and OCN-IR significantly increased which was proportional to the increased number of osteoblasts (Figure 6(c) and (g)).
Figure 6.
Immunohistochemical staining (200 × magnification) and semiquantitative analysis of BMP-2 and OCN. (a,e) The model group showed slight BMP-2 immunoreactivity (BMP-2-IR) and OCN immunoreactivity (OCN-IR), and few osteoblasts. (b,f) In the CD group, increased BMP-2-IR, OCN-IR, and number of osteoblasts were observed. (c,g) The DFO group showed intense BMP-2-IR and OCN-IR which was concomitant with the increase in the number of osteoblasts. (d,h) Bar graphs showed the mean optical density of BMP-2 and OCN in bone marrow. Semiquantitative analysis was performed for eight sections (10 fields per section) in each group and the mean optical density was calculated. *P < 0.05 versus model group; #P < 0.05 versus CD group; n = 8. (A color version of this figure is available in the online journal.)
Discussion
Corticosteroid-induced ONFH is a progressive disease that mostly affects 30–50 y old patients.14 Numerous methods have been taken to treat steroid-induced ONFH. Unfortunately, none of these methods is 100% successful, and the results have been variable. Recent studies indicated that HIF-1α and its downstream target VEGF play vital roles in regulation of angiogenic–osteogenic coupling during bone repair and regeneration.15 Activating HIF pathway by molecular HIFs PHIs has been used as an approach to accelerate bone healing following injury.11,12,16,17 However, the application of PHI in treating corticosteroid-induced ONFH has not been reported.
In the present study, we use DFO, a molecular inhibitor of HIFs prolyl hydroxylase, to activate HIFs and VEGF. We demonstrated that DFO enhances angiogenesis and increases bone repair in steroid-induced ONFH in rabbits.
Interruption to the bone microcirculation of the femoral head is considered as the final common pathway leads to corticosteroid-induced ONFH, which was generally agreed. Corticosteroid decreases expression of VEGF and disrupts the process of vascularization and growth of new blood vessels into the necrotic bone.18,19 HIF-1 is an important transcription factor which plays essential roles in mediating the adaptive response of cells to hypoxia. HIF-1α and its target gene VEGF play a central role in angiogenesis and neovascularization.20–22 DFO, an iron chelator in Food and Drug Administration, was also known as an inhibitor of HIFs prolyl hydroxylase. Previous studies have shown that DFO leads to HIF activation and promotes VEGF expression.8 In our study, we estimated local expression of HIF-1α and VEGF by immunohistochemical staining and it showed that DFO increased local level of HIF-1α and VEGF in the femoral heads. In the study of Shen et al.,11 DFO and DMOG, two inhibitors of HIFs prolyl hydroxylase which have small molecular weight, were directly injected at the fracture site to activate HIF-1α in a mice femur fracture model. It was found that DFO increases the vascularity as assessed by micro-CT angiography.11 Steward et al. found that the vascularity increased by using DFO combining with a biodegradable, weight-bearing scaffold in a femoral segmental defects model.12 In our study, ink artery infusion angiography and micro vessel density analysis after immuohistochemical staining for vWF showed more blood vessels in femoral heads of the DFO group, indicating that DFO improves angiogenesis.
Recently, HIF-1α and its target gene VEGF were reported to promote bone repair and bone regeneration partially by inducing angiogenesis and they played critical roles in angiogenic–osteogenic coupling.7 Considering the role of HIF-1α and VEGF in angiogenic–osteogenic coupling, the use of HIF activators to increase vascularity and promote bone repair is attractive. In distraction osteogenesis model, Wan et al.16 demonstrated that HIF-1α activation by administering DFO into the distraction gap promotes angiogenesis and obviously accelerates bone regeneration.16 In our study, DFO was locally delivered into the tunnel made by CD in a rabbit model of steroid-induced ONFH. Six weeks later, bone repair was evaluated by HE staining and micro-CT scanning. Micro-CT scanning indicated that the DFO group had more new bone formation and better microstructural parameters than the CD group, which was also confirmed by HE staining. The volume of newly formed bone in both groups was directly proportional to the number of blood vessels. In addition, local expression of osteogenic factors including BMP-2 and OCN was invested in this study. Immunohistochemical results showed that expression of BMP-2 and OCN increased in the DFO group compared with the model group and the CD group. In the study of Shen et al.11 and Steward et al, it was also indicated that DFO improves angiogenesis and promotes bone repair.11,12
The precise cellular and molecular mechanisms of angiogenic–osteogenic coupling are still not clear. It seems to require interaction between vascular endothelial cells and osteoprogenitor cells. Studied indicated that VEGF and endothelial cells induces osteogenic differentiation of bone marrow-derived stromal cells.23,24 Also, osteoprogenitors have effects on endothelial cells. Mesenchymal stem cells may serve as trophic mediators that benefit migration and formation of tubular structures of endothelial cells.25 Moreover, mesenchymal stem cells promote the extracellular matrix invasion, migration, survival, and proliferation of endothelial cells.26 We propose that the mechanisms that couple angiogenesis to bone formation are: (1) the blood vessels deliver chemical signals that induce bone formation and carry more osteoprogenitors that subsequently mature and form new bone; (2) the endothelial cells of blood vessels induce osteoblast differentiation; (3) the osteoblast progenitors brought by blood vessels in turn promote the formation of blood vessels by promoting the extracellular matrix invasion, migration, survival, and proliferation of endothelial cells. Future studies should focus on exploring the cellular and molecular mechanisms of angiogenic–osteogenic coupling.
Because of the importance of neoangiogenesis and osteogenesis in bone repair, attention has been focused on angiogenic factors (e.g. VEGF and bFGF), osteogenic factors (e.g. BMP-2), and stem cells27 in steroid-induced ONFH. Local angiogenic factors, osteogenic factors, or stem cells delivery after CD has been successfully applied in animal ONFH models and in patients to increase vascularity and bone healing, but success has not been universal.28 Besides, considering the high price of recombinant proteins, the high death rate of stem cells after transplantation and limited sources, we thought that our strategy to promote angiogenesis and bone regeneration by targeting HIFs activation has advantages over angiogenic–osteogenic factors and stem cells therapy. First, vascular formation needs cooperative action of several angiogenic factors and only one proangiogenic factor is insufficient to form mature and functional vascular structures.29 HIF pathway can activate both VEGF and other associated angiogenic factors (e.g. angiopoeitins).22,30 Second, HIF pathway inhibits apoptosis, promotes metabolic flexibility, and induces recruitment and proliferation of stem cells.31–33 Also, local DFO injection is simpler and cheaper than methods involving recombinant growth factors and stem cells, avoiding the increased expense and biological safety concerns.
It was indicated that continuously systemic applying of HIFs PHIs may result in neoplasm formation, polycythemia, thrombosis, and serious cardiocerebrovascular diseases.34 In order to decrease the incidence of these side effects, DFO should be delivered and administrated locally in a short period of time which was adopted in our study. In our study and other researches in which DFO was used locally, potential side effects mentioned above were not present.
In summary, this study indicated that local DFO administration enhance angiogenesis and bone repair of early stage ONFH in rabbit model and it may offer an efficient, economic, and simple therapy for early stage ONFH.
Acknowledgements
The authors thank the grants provided by the National Nature Science Foundation of China (nos. 81101363 and 81371944).
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; JL and LF performed most experiments and contributed equally to this paper. ZY performed the HE and immunohistochemical staining. XD performed the intra-artery ink perfusion. JL and LF performed the surgery and micro-CT scanning. JL, LF, and KW analyzed the data. ZY and XD prepared figures. KW directed all the work. JL, LF, and KW wrote the manuscript.
References
- 1.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 2006; 88A: 1117–32. [DOI] [PubMed] [Google Scholar]
- 2.Leali A, Fetto J, Hale JJ. Biostructural augmentation for the treatment of osteonecrosis: rationale, technique, and case example. J South Orthop Assoc 2002; 11: 167–71. [PubMed] [Google Scholar]
- 3.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head – potential treatment with growth and differentiation factors. Clin Orthop Relat Res 1998; 355: S314–35. [PubMed] [Google Scholar]
- 4.Chiu KH, Shen WY, Ko CK, Chan KM. Osteonecrosis of the femoral head treated with cementless total hip arthroplasty – a comparison with other diagnoses. J Arthroplasty 1997; 12: 683–8. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JR. Core decompression for osteonecrosis of the hip. Clin Orthop Relat Res 2004; 418: 29–33. [DOI] [PubMed] [Google Scholar]
- 6.Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am 1998; 80A: 1270–5. [DOI] [PubMed] [Google Scholar]
- 7.Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1 alpha in angiogenic-osteogenic coupling. J Mol Med (Berl) 2009; 87: 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1 alpha, HIF-2 alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med 2005; 38: 1002–13. [DOI] [PubMed] [Google Scholar]
- 9.Li Y-X, Ding S-J, Xiao L, Guo W, Zhan Q. Desferoxamine preconditioning protects against cerebral ischemia in rats by inducing expressions of hypoxia inducible factor 1 alpha and erythropoietin. Neurosci Bull 2008; 24: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, Date I. Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery 2008; 62: 232–40. [DOI] [PubMed] [Google Scholar]
- 11.Shen X, Wan C, Ramaswamy G, Mavalli M, Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL, Gilbert SR. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res 2009; 27: 1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart R, Goldstein J, Eberhardt A, Chu G, Gilbert S. Increasing vascularity to improve healing of a segmental defect of the rat femur. J Orthop Trauma 2011; 25: 472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin L, Zhang G, Sheng H, Yeung KW, Yeung HY, Chan CW, Cheung WH, Griffith J, Chiu KH, Leung KS. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone 2006; 39: 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drescher W, Furst M, Hahne HJ, Helfenstein A, Petersen W, Hassenpflug J. Survival analysis of hips treated with flexion osteotomy for femoral head necrosis. J Bone Joint Surg Br 2003; 85B: 969–74. [DOI] [PubMed] [Google Scholar]
- 15.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis–angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 2009; 24: 1347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1 alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A 2008; 105: 686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donneys A, Deshpande SS, Tchanque-Fossuo CN, Johnson KL, Blough JT, Perosky JE, Kozloff KM, Felice PA, Nelson NS, Farberg AS, Levi B, Buchman SR. Deferoxamine expedites consolidation during mandibular distraction osteogenesis. Bone 2013; 55: 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XD, Jin L, Cui QJ, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int 2005; 16: 101–8. [DOI] [PubMed] [Google Scholar]
- 19.Harada I. The effects of glucocorticoids on angiogenesis in vitro. Nihon Seikeigeka Gakkai zasshi 1992; 66: 763–70. [PubMed] [Google Scholar]
- 20.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999; 77: 527–43. [DOI] [PubMed] [Google Scholar]
- 21.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 2002; 99: 9656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai ZQ, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 2003; 93: 1074–81. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wan C, Deng LF, Liu XM, Cao XM, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor a pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 2007; 117: 1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thebaud NB, Siadous R, Bareille R, Remy M, Daculsi R, Amedee J, Bordenave L. Whatever their differentiation status, human progenitor derived – or mature – endothelial cells induce osteoblastic differentiation of bone marrow stromal cells. J Tissue Eng Regen Med 2012; 6: e51–60. [DOI] [PubMed] [Google Scholar]
- 25.Gruber R, Kandler B, Holzmann P, Vogele-Kadletz M, Losert U, Fischer MB, Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng 2005; 11: 896–903. [DOI] [PubMed] [Google Scholar]
- 26.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007; 25: 1761–8. [DOI] [PubMed] [Google Scholar]
- 27.Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, Pullig O, Tuan RS, Rudert M, Noth U. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther 2012; 3: 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res 2004; 429: 139–45. [DOI] [PubMed] [Google Scholar]
- 29.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000; 6: 460–3. [DOI] [PubMed] [Google Scholar]
- 30.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res 2003; 93: 664–73. [DOI] [PubMed] [Google Scholar]
- 31.Fulda S, Debatin K-M. HIF-1-regulated glucose metabolism a key to apoptosis resistance? Cell Cycle 2007; 6: 790–2. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011; 117: 459–69. [DOI] [PubMed] [Google Scholar]
- 33.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE 2007; 2: e416–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents – time for a reevaluation. N Engl J Med 2010; 362: 189–92. [DOI] [PubMed] [Google Scholar]