Abstract
Resveratrol (RSV), a natural compound, is known for its effects on energy homeostasis. Here we investigated the effects of RSV and possible mechanism in insulin secretion of high-fat diet rats. Rats were randomly divided into three groups as follows: NC group (animals were fed ad libitum with normal chow for 8 weeks), HF group (animals were fed ad libitum with high-fat diet for 8 weeks), and HFR group (animals were treated with high-fat diet and administered with RSV for 8 weeks). Insulin secretion ability of rats was assessed by hyperglycemic clamp. Mitochondrial biogenesis genes, mitochondrial respiratory chain activities, reactive oxidative species (ROS), and several mitochondrial antioxidant enzyme activities were evaluated in islet. We found that HF group rats clearly showed low insulin secretion and mitochondrial complex dysfunction. Expression of silent mating type information regulation 2 homolog- 1 (SIRT1) and related mitochondrial biogenesis were significantly decreased. However, RSV administration group (HFR) showed a marked potentiation of glucose-stimulated insulin secretion. This effect was associated with elevated SIRT1 protein expression and antioxidant enzyme activities, resulting in increased mitochondrial respiratory chain activities and decreased ROS level. This study suggests that RSV may increase islet mitochondrial complex activities and antioxidant function to restore insulin secretion dysfunction induced by high-fat diet.
Keywords: Resveratrol, insulin secretion, mitochondrial
Introduction
The proportion of people with type 2 diabetes (T2DM) and obesity has been continuously and rapidly increasing in Asian countries including China, where it was recently reported that the prevalence of diabetes and prediabetes were 9.7 and 15.5%, respectively, of the adult population.1,2 One possible reason for this phenomenon is considered to be at least partly the contribution of a “Western diet.”3,4
The “Western diet” is characterized by high intakes of high-fat foods.5 A large number of experimental studies in laboratory animals strongly support the notion that high-fat diets are associated with impaired insulin sensitivity, and a deficiency in insulin secretion induced by beta-cell failure, which eventually leads to T2DM.6 Significant insulin resistance has been observed in rats fed with high-fat diet (59% fat, 20% carb) only for 3 weeks.7 Maria Sörhede Winzell and Bo Ahrén demonstrated that after 3 weeks of high-fat feeding, mice were observed no significant glucose elimination from minute 15 to 60 in oral glucose tolerance test, which suggests severe glucose intolerance.8 A reduction of beta-cell mass was reported after high-fat diet for 16 weeks in mice.9
Insulin resistance and pancreatic beta-cell failure have been identified as being pivotal in the pathogenesis of T2DM. However, growing number of studies have found that pancreatic beta-cell failure is the most critical factor in the development and progress of this disease.10 One prospective study in Britain clearly pointed out that the development of T2DM is associated more with the decline of pancreatic beta-cell function, rather than with the severity of insulin resistance.11 It has also been observed that transplanting functional islet cells to a T2DM animal model could maintain normal blood glucose levels.12 These observations suggest that functional islet beta cells play a pivotal role in T2DM development and restoration of beta-cell function maybe one of key strategy for treatment of T2DM.
Resveratrol (3,5,4-trihydroxystilbene) (RSV) is a natural compound found in grape skins.13 Results of many studies have provided evidence for its beneficial effects, including anticancer,14,15 antioxidant,16 cardioprotective,17,18 and lifespan.19 In addition, there is growing evidence that RSV improves insulin sensitivity in type 2 diabetic animals or patients.20,21 Brasnyo reported that patients who were treated with oral RSV twice daily (in gelatin capsules containing 5 mg RSV) for 4 weeks have significantly improved insulin resistance with increased pAkt:Akt ratio in platelets. However, studies of RSV in vitro in insulin-secreting cells have exhibited converse results. Some have shown enhanced exocytosis,22 while others have displayed inhibition of insulin secretion.23 Since the effects of RSV on beta cell are so far not clear, in this study, we investigated the effects of RSV treatment on beta-cell insulin secretion function in vivo and attempted to explore the possible mechanisms.
Materials and methods
Animals and diets
Eight-week-old male Sprague-Dawley rats (n = 54) were purchased from the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology. Rats were housed individually in a temperature-controlled room (22℃ ± 3℃, 50–60% relative humidity) with a 12-h light/dark cycle and were maintained on a normal chow diet with free access to water for a 1-week period of adaptation before the start of the study. After acclimatization, the rats were randomly divided into three groups as follows: NC group (animals were fed ad libitum with normal chow for 8 weeks), HF group (animals were fed ad libitum with high-fat diet for 8 weeks), and HFR group (animals were treated with high-fat diet and administered with RSV for 8 weeks). The normal chow (containing 13.68% fat, 64.44% carbohydrate, and 21.88% protein) was provided by the Laboratory Animal Center mentioned earlier. The high-fat diet (containing 59% fat, 20% carbohydrate, and 21% protein) was made as described by Chalkley et al.24 The rats in the HFR group received an intragastric administration of RSV (Sigma-Aldrich, St Louis, MO; 100 mg/kg body weight per day) in physiological saline, while rats in the NC and HF groups were treated with physiological saline only. The body weights were determined every week. At the end of the experiment all animals were killed by decapitation. All the experimental procedures performed were approved by the Animal Ethics Committee in our university and in accordance with the Hubei Province Laboratory Animal Care Guidelines for the use of animals in research.
Serum parameters
Blood glucose levels were measured using a glucometer (One Touch® Ultra, Lifescan, Milpitas, CA). Serum insulin was quantified with a rat insulin enzyme linked immunosorbent assay (ELISA) kit (Linco Research, St Charles, MO). Insulin resistance was assessed with the homeostasis model assessment for insulin resistance (HOMA-IR), according to the following equation: as HOMA-IR = [fasting blood glucose (mg/dl) × fasting insulin (µU/ml) ]/405.25
Hyperglycemic clamp
Five rats per group were caged in our rat restrainer for at least 30 min/day for 6 days before the experiments to acclimate them to the limited space. Hyperglycemic clamp studies were performed in awake, unstressed, 12-h postprandial rats, as described previously with some modifications.26,27 All rats were immobilized in the restrainers mentioned earlier and received local anesthesia by a subcutaneous injection of lidocaine (50 mg/kg body weight) in the tail root instead of general anesthesia. Tail artery and vein catheterizations were performed with intravenous integrated catheters (24 G × 19 mm; Weihai Jierui Medical Products Co. Ltd). Catheters were flushed with small amounts of heparinized saline (25 U/mL), after which the venous line was connected for the infusion of test substances and blood replacement. The arterial line was used for sampling. After a 60-min equilibration phase (−60 to 0 min; basal period), a glucose bolus (375 mg glucose/kg body weight) was infused through the catheter for the first 1 min of the clamp, and 25% glucose was administered through the catheter to maintain the serum glucose levels at 13.5 mmol/L (steady state). Serum glucose and insulin levels were measured from the blood collected from the tail artery at 0, 5, 10, 15, 30, 45, and 60 min during the clamp.28
Islet isolation
The isolation of rat pancreatic islets was carried out by injecting Hank's Balanced Salt Solution (HBSS) (Invitrogen, No. 14175-095) into the pancreatic duct and digestion by collagenase V (Sigma-Aldrich, No. C9263). A discontinuous Ficoll 400 (Sigma-Aldrich, No. F4375) solution was applied to the purification of the islets.
RNA isolation
Total RNA was extracted from frozen isolated islets using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). According to the manufacturer’s protocol, 1 mL TRIzol reagent was added to homogenize 100 mg frozen isolated islets by using a glass-teflon. The homogenized islets were incubated for 5 min at room temperature to permit complete dissociation of the nucleoprotein complex. Then 0.2 mL of chloroform was added into tube per 1 mL of TRIzol reagent used for homogenization. After shaking the tube vigorously by hand for 15 s and incubating again at room temperature for 2 min, the islet samples were centrifuged at 12,000 × g for 15 min at 4℃. Next, the aqueous phase of the sample was removed into a new tube. Ten micrograms of RNase-free glycogen was added to the new tube. A total of 0.5 mL of 100% isopropanol was also added to the new tube per 1 mL of TRIzol reagent used for homogenization. Incubation was at room temperature for 10 min before centrifuge at 12,000 × g for 10 min at 4℃. The supernatant was removed from the tube, leaving only the RNA pellet. The pellet was washed with 1.5 mL of 75% ethanol. Then RNA pellet was air dried for 5 min. Finally, the RNA pellet was resuspended in 20 µL RNase-free water and incubated in a heat block set at 55–60℃ for 10 min.
cDNA synthesis
The RNA concentration was determined by spectrophotometry (1:100 diluted in DEPC H2O and read at 260 nm). Complementary DNA was prepared by using a two-step reverse transcription PCR method (PrimeScript RT reagent Kit, Perfect Real Time; TaKaRa, Otsu, Japan). Firstly, 5 × PrimeScriptTM buffer, PrimeScriptTM RT enzyme mix I, oligo dT primer l, Random 6 mers, total RNA, RNase Free dH2O, and Gene Specific Primer were mixed together to incubate at 37℃ for 15 min for reverse transcription. The reverse transcription reaction was inactivated at 85℃ for 5 s. Then target genes were analyzed by RT-PCR using a SYBR Green I dye (Catalog No. 4309155). The reaction reagents include SYBR Premix Ex Taq TM (2X), PCR Forward Primer (10 μM), PCR Reverse Primer (10 μM), ROX Reference Dye, RT reaction solution (cDNA solution), and dH2O (satirized distilled water). Temperature cycles were programed as follows: 95℃ for 30 s, followed by 42 cycles of 95℃ for 5 s, and 60℃ for 30 s. The SYBR green fluorescence was measured at the end of each cycle to monitor the amount of PCR product formed during that cycle. The sequences of the primers were as follows:
pancreatic and duodenal homeobox 1 (PDX1), forward 5′-TGCCAGAGTTCAGTGCTAATCC-3′ and reverse 5′-TTCCCTGTTCCAGCGTTCC-3′;
Insulin, forward 5′-ACCTTTGTGGTCCTCACCTG-3′ and reverse 5′-GTGCAGCACTGATCCACAAT-3′,
SIRT1, forward 5′-GGATCCTTCATTTATCAGAGTTGCCACC-3′ and reverse 5′-CTTCGAGGTTCTTCTAAACTTGGACTCTGG-3′,
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1a), forward 5′-GAGAACAAGACTATTGAGCGAAC-3′ and reverse 5′-GTGGAGTGGCTGCCTTGGGT-3′;
uncoupling protein 2 (UCP2), forward 5′-TGCTGGGCACCATCCTAACC-3′ and reverse 5′-CCTGGAAGCGGACCTTTACC-3′;
Actin, forward 5′-CGTTGACATCCGTAAAGACCTC-3′ and reverse 5′-TAGGAGCCAGGGCAGTAATCT-3′.
Protein isolation
The islet was placed in eppendorf tubes and immersed in liquid nitrogen to “snap freeze.” Lysis buffer was added to the tube (60 µL lysis buffer/mg piece of tissue). Mixture was homogenized with an electric homogenizer by rinsing the blade twice with another 2 × 300 µL lysis buffer. Constant agitation was maintained for 2 h at 4℃. After centrifuge for 20 min at 12,000 r/min at 4℃, supernatant was aspirated and pellet was discarded. Finally, proteins were measured by the bicinchoninic acid method.
Analysis of protein expression by Western blot
PDX1, SIRT1, and UCP2 protein expression was determined by Western blotting, which was performed according to standard procedures. About 10 µg of protein were electrophoresed on 5 and 10% sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) for separation. Then samples were transferred onto a nitrocellulose membrane. The membrane was next incubated in 5% milk in mixture of tris-buffered saline and Tween 20 (TBST) for 1 h at room temperature to block the membrane. And the proteins were incubated with the primary antibody overnight at 4℃ (PDX1, 1:1000, cell signal NO.5679; SIRT1, 1:1000, cell signal NO. 8469; UCP2, 1:1000, abcam NO.ab67241; β-Actin, 1:1000, cell signal NO. 4970). After washing the membrane 5 times in TBST, the membrane was incubated with secondary antibody for 30 min at room temperature. Finally, the protein was detected with electrochemiluminescence (ECL) Western blotting reagents.
Measurement of activities of individual complexes of the mitochondrial respiratory chain in islet mitochondria
Islet mitochondrial electron transport chain activities of complexes I, II, III, and IV were measured using a WFJ7200 spectrophotometer (Unico; Shanghai Instrument, Shanghai, China). Oxidation of reduced form of nicotinamide adenine dinucleotid (NADH) by complex I (NADH dehydrogenase) was recorded using ubiquinone as the electron acceptor. The decrease in absorbance was measured at 340 nm at 30℃ in both the absence and presence of 5 µg/mL rotenone. Enzyme activity was measured for 3 min, with values recorded every 10 s after initiation of the reaction. The extinction coefficient used for NADH was 6.22 (mmol/L)/cm. Complex I activity was then calculated as the difference between the total rate and the rate in the presence of rotenone. Complex II (succinate dehydrogenase) activity was determined by measuring the reduction of 2,6-dichlorophenol indophenol (DCPIP) by the absorbance at 600 nm at 30℃. The extinction coefficient used for DCPIP was 21 (mmol/L)/cm. The oxidation of reduced ubiquinone (CoQH2) by complex III (cytochrome c reductase) was determined using cytochrome c as an electron acceptor. The reaction was started with 10 µg of mitochondrial protein, and enzyme activity was measured at 550 nm at 30℃. The extinction coefficient used for cytochrome c was 18.5 (mmol/L)/cm. Finally, complex IV activity was determined by measuring the oxidation of cytochrome c at 550 nm at room temperature. Briefly, the assay mixture for complex IV consisted of 10 mmol/L phosphate buffer (pH 7.4) and 20 mmol/L reduced cytochrome c.
Fluorescence measurement of mitochondrial ROS
Islet mitochondrial ROS production was measured using the ROS-sensitive dye 6-chloromethyl-2′,7′- dichlorodihydrofluorescein diacetate according to the manufacturers’ instructions (Genmed Scientifics, Wilmington, DE). Fluorescence intensity was determined by using a microplate fluorescence spectrophotometer (Bio-Tek Instruments, Winooski, VT) with excitation wavelength at 490 nm and emission wavelength at 520 nm.
Assessment of oxidative islet damage
Islet damage caused by oxidative stress was quantified by measuring malondialdehyde (MDA) in freshly isolated islets. Briefly, isolated islets were disintegrated at 4℃ by sonication (Sonifier250; Branson, London, UK) in 0.5 mL phosphate-buffered saline. The protein content of the homogenate was estimated using a protein detection kit (Beyotime Inst. Biotech, Peking, PR China). Lipid peroxidation was assayed using the 2-thiobarbituric acid method as described previously16 and expressed as nmol/L MDA/g protein.
Determination of mitochondrial antioxidant enzyme activities
The total superoxide dismutase (Cu/Zn-, Mn-, and Fe-SOD) activity in islets was measured by determining the reduction of nitrite, which was produced by Xanthine/XOD and inhibited by SOD (Nanjing Jiancheng Technology Company, China). The activities of glutathione peroxidase [GPx] and catalase [CAT] were determined according to previous studies (Nanjing Jiancheng Technology Company, China).29,30
Data analysis
The data were represented by the means ± standard error of mean. Comparisons were assessed using one-way analysis of variance followed by a Student–Newman–Keuls post hoc analysis for multiple comparisons. Differences with a P < 0.05 were considered to be statistically significant. All analyses were performed with the SPSS statistical software 15.0 (SPSS, Chicago, IL).
Results
RSV prevented dietary obesity
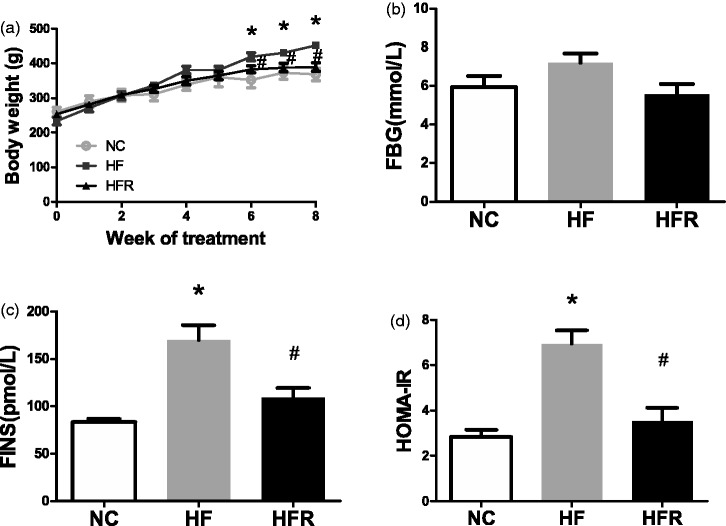
The high-fat diet group rats gained weight after high fat feeding. As expected, rats treated with RSV (group HFR) gained significantly less weight. Despite no change in food intake, rats in the HFR group weighted 389.61 ± 39.04 g as compared with 452.37 ± 16.82 g (P < 0.01) after 8 weeks of HFD feeding (Figure 1(a)).
Figure 1.
Effect of oral administration of resveratrol on insulin sensitivity. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a) Body weight growth curves. (b) The blood glucose levels after an overnight fast. (c) The overnight fasting serum insulin levels. (d) HOMA-IR calculated from overnight fasting blood glucose and insulin. The data shown are the means ± SE. n = 5 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
RSV restored systemic insulin sensitivity
There were no differences in the glucose levels among three groups (Figure 1(b)). However, compared to the NC group, the fasting blood insulin concentrations (35%, P < 0.01, Figure 1(c)), as well as the HOMA-IR (49%, P < 0.05, Figure 1(d)), were significantly higher in the HF groups, which indicated severe insulin resistance. Eight weeks treatment of RSV restored systemic insulin sensitivity as shown by a decrease in fasting serum insulin levels and HOMA-IR compared with HF rats (P < 0.05, Figure 1(c) and (d)).
RSV improved insulin secretion of beta cells
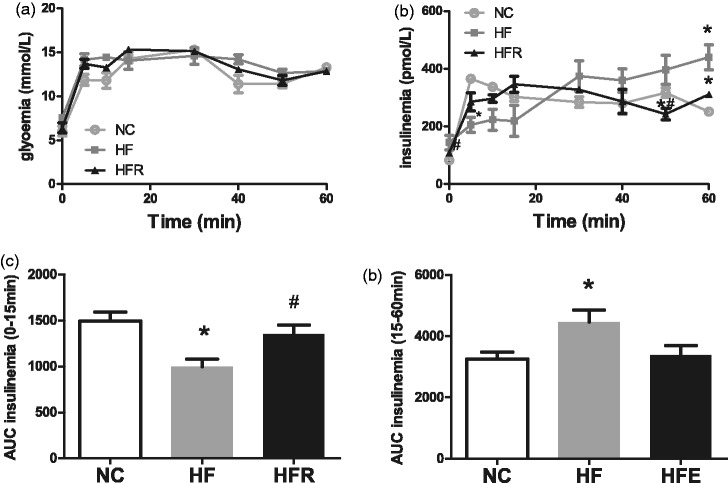
A hyperglycemic clamp was performed in overnight fasted conscious male rats to investigate the consequence of RSV treatment on insulin secretion of beta cells. Conscious HFR rats showed no change in basal glycemia (Figure 2(a)) but had lower insulinemia (109.3 ± 17.3 versus 169.6 ± 6.3 pmol/L, P < 0.05, Figure 2(b)) than the HF group rats. During the clamp, the glucose infusion rate was adjusted to maintain blood glucose at 13 mmol/L (Figure 2(a)). Insulin secretion in response to hyperglycemia was slightly increased in HFR group rats compared with HF group rats at 5–20 min (Figure 2(b)). Calculation of the area under the curve (AUC) for the first 15 min of the clamp, subtracting basal insulinemia, indicated that first phase glucose stimulated insulin secretion (GSIS) was increased by 26% in HFR group rats (Figure 2(c)). However, the AUC for the second phase GSIS (15–60 min of the clamp) of HFR group rats was significantly decreased compared with HF group rats (Figure 2(d)). These data confirm that the insulin secretion function of pancreatic beta cells in the HFR group rats almost recovered to normal.
Figure 2.
Evaluation of the insulin secretion abilities of beta cell by hyperglycemic clamp. Rats were fasted overnight before the test. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a) The blood glucose levels during the clamp. (b) The serum insulin levels during the clamp. (c) First phase insulin secretion in response to elevated glucose expressed as area under the curve of insulin (AUC) from 0 to 15 min. (d) Second phase insulin secretion in response to elevated glucose expressed as AUC from 15 to 60 min. The data shown are the means ± SE. n = 5 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
RSV increased pancreatic beta-cell-related gene expression
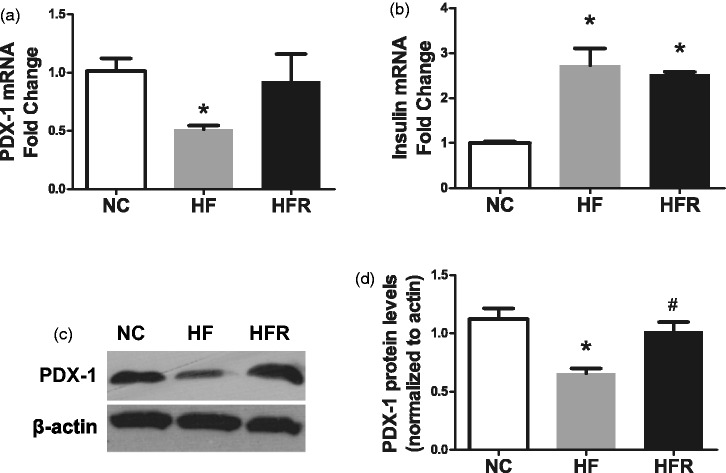
To determine a potential reason for improved beta-cell function due to RSV treatment, we measured the expression of PDX1 and insulin, which play a key role in beta-cell differentiation and function, in the isolated islet. The HF rats showed a reduced expression of PDX1 mRNA (48.4%, P < 0.01, Figure 3(a)) and protein (42.2%, P < 0.01, Figure 3(c)) than the NC group. A significantly increased expression of PDX1 on both mRNA (79.3%, P < 0.05, Figure 3(a)) and protein level (54.6%, P < 0.05, Figure 3(c)) was found in HFR group rats after treating with RSV for 8 weeks. Conversely, the insulin mRNA expression was higher in the HF group, as well as the HFR groups, compared with the NC group. (P < 0.01) (Figure 3(b)).
Figure 3.
Effect of resveratrol on beta-cell biogenesis related genes. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a) PDX-1 mRNA expression level. (b) Insulin mRNA expression level. (c) PDX-1 protein expression level. The data shown are the means ± SE. n = 4 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
RSV increased beta cell and mitochondrial biogenesis genes and SIRT1 protein expression
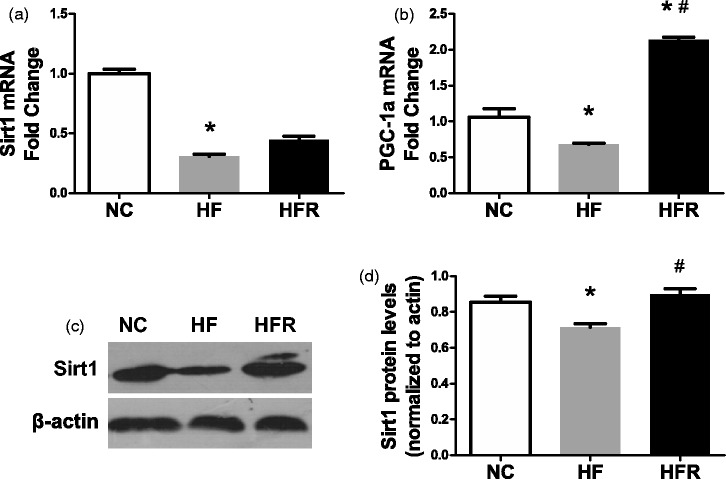
RSV is known for activating SIRT1, which, in turn, may up-regulate mitochondrial biogenesis genes. Thus, we performed real-time PCR and western blot on isolated islets. Comparing with NC group, the HF group displayed about a 70% decrease (P < 0.01) in SIRT1 mRNA expression and a 16.4% reduction (P < 0.05) in PGC-1a mRNA expression (Figure 4(a) and (b)). Meanwhile the HFR group rats showed a 1.5-fold increase (P < 0.01) of PGC-1a mRNA expression (Figure 4(b)). Although no change was found in the SIRT1 mRNA expression level, the SIRT1 protein level was up-regulated 20% (P < 0.05) in the HFR group (Figure 4(c)).
Figure 4.
Effect of resveratrol on mitochondrial biogenesis related genes. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a) SIRT1 mRNA expression level. (b) PGC-1a mRNA expression level. (c) SIRT1 protein level in isolated islet was measured by western blotting. The data shown are the means ± SE. n = 4 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
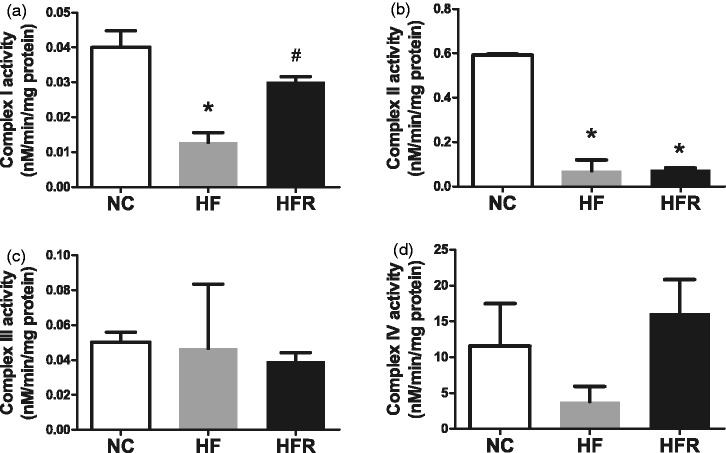
RSV increased mitochondrial respiratory chain activities
Mitochondrial respiratory chain activities of islets were also determined at the end of the treatment, as expressed by activities of mitochondrial complex I to IV, respectively. In the HF group activities of complexes I and II (P < 0.01, Figure 5(a) and (b)) were declined. This difference was statistically significant compared with the NC group. After 8 weeks RSV treatment, activity of mitochondrial complex I reverted to normal state (P < 0.05, Figure 5(a)). However, the activity of mitochondrial complex II was not significantly elevated compared to the HF group. Neither activities of complex III nor IV were changed by high-fat diet or RSV treatment (Figure 5(c) and (d)).
Figure 5.
Analyses of mitochondrial ETC complexes I to IV in isolated islet. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a–d) The activity of the individual complexes of electron transport chain is expressed by nanomoles NADH per minute per milligram isolated islet protein. The data shown are the means ± SE. n = 4 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
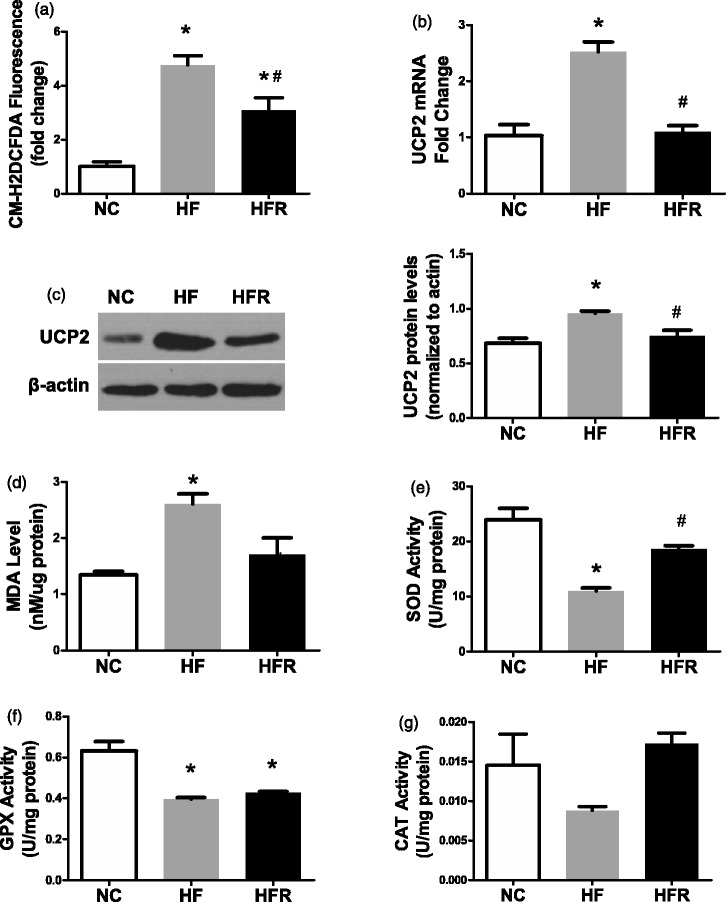
RSV protected islets from oxidative stress-induced cell damage
A high-fat diet for 8 weeks led to an increased ROS level (3.7-fold increase, P < 0.05, Figure 6(a)). However, RSV supplementation had a 35.3% decreased ROS level compared with the HF group (Figure 6(a)). The UCP2 mRNA and protein expression, as an important marker of mitochondria-derived reactive oxygen species,22,31–33 was consistent with ROS level (Figure 6(b) and (c)). We also measured MDA levels in freshly isolated islets to assess oxidative damage to islets. In the HF group, the MDA level was 2.61 ± 0.3 nmol/µg protein, while the level in the HFR group was 1.71 ± 0.5 nmol/µg protein (Figure 6(d)). All these data indicated that RSV significantly inhibited oxidative stress in islets.
Figure 6.
Assesses oxidative stress level in islet. NC: Rats that were fed the normal chow diet ad libitum for all 8 weeks. HF: Rats that were fed the high-fat diet ad libitum and received vehicle (control) for 8 weeks. HFR: Rats that were fed the high-fat diet ad libitum and received resveratrol (100 mg/kg body weight daily) orally for 8 weeks. (a) Mitochondrial ROS production was quantified by CM-H2DCFDA fluorescence. (b) UCP2 mRNA level was expressed as another marker of oxidative stress damage. (c) UCP2 protein level was expressed as another marker of oxidative stress damage. (d) Islet damage caused by oxidative stress was expressed by MDA level in fresh isolated islet. (e) SOD activity level in isolated islet. (f) GPX activity level in isolated islet. (g) CAT activity level in isolated islet. The data shown are the means ± SE. n = 4 in each group. *P < 0.05 versus NC, #P < 0.05 versus HF
RSV elevated islet mitochondrial antioxidant enzyme activities
We next evaluated the islet mitochondrial antioxidant enzyme activities by using a spectrophotometer. The activities of SOD and GPx in HF rats were decreased 53.8 and 37.1%, respectively (P < 0.05, Figure 6(e) and (f)). RSV supplementation in HFR rats led to a 68.8% increase of SOD and a 7.8% enhancement of GPx activities, compared to HF rats (P < 0.05, Figure 6(e) and (f)). The concentration of CAT was too low to identify the difference in activity among the three groups (Figure 6(g)).
Discussion
The current study demonstrated that RSV supplementation was resistant to diet-induced obesity and insulin resistance, elevated the first phase of GSIS in pancreatic islets, restored the damage to islet mitochondria, and reduced oxidative stress in high-fat-diet fed rat islets. These findings are important since pancreatic beta cells play a key role in T2DM development.
RSV, as a potent SIRT1 activator,31 has been widely studied in both in vivo and in vitro studies.19,34 Numerous beneficial effects of RSV have been demonstrated, including cardioprotective, anticancer, anti-inflammatory, and antioxidant effects. Recently, this broad spectrum of effects has been enlarged by new studies proving the significant potency of this compound in relation to obesity and diabetes. However, in vitro studies of RSV in insulin-secreting cells have revealed contradictory conclusions. Chen’s experiment showed that insulin secretion was increased in the presence of RSV (3, 10, 30, and 100 μM) in MIN6, Hit-T15, and RIN-m5F cells.22 Meanwhile, in another study, incubations of pancreatic islets with RSV (1–100 mM, 90 min) have revealed that the release of insulin induced by 6.6 and 16.6 mM glucose was substantially restricted by this compound in a concentration-dependent manner.23 Moreover, studies of RSV in vivo also had different observations. It has been reported that RSV (3 mg/kg, i.p.) increased insulin secretion associated with lower plasma glucose in normal rats, but not in streptozotocin-diabetic rats within the initial 60 min.22 However, in our experimental rat model, treatment with RSV (100 mg/kg) enhanced the impaired GSIS induced by high-fat diet, a finding which was supported by other previous studies.35,36 Young et al. observed that treatment of db/db mice with RSV (20 mg/kg) for 12 weeks significantly improved the glucose tolerance of db/db mice, resulting in a significantly lower blood glucose concentration at 120 min after glucose loading. Also, the mean value of the AUCg was significantly lower in the db/db RSV treated group than in the db/db control group during the glucose tolerance test.35 Zhang et al. found that a high-fat diet for 8 weeks followed by orally administered RSV at 400 mg/kg daily could increase islet insulin secretion.36 These contradictory data could be due to a differing dosage of RSV. Thus, the beneficial results in vivo were only displayed when the dosage of RSV was big enough. The dosage of 3 mg/kg RSV, used in Chen’s experiment, was too low to improve impaired insulin secretion.
In pancreatic beta cells, mitochondria play a central role in coupling glucose metabolism to insulin exocytosis, thereby ensuring strict control of GSIS. Numerous studies suggest that the beta cells mitochondrial dysfunction may be the key factor of islet beta cells dysfunction and apoptosis in diabetes.37 Mitochondria are the major site of ROS production. At the same time, mitochondria are also the targets of excess ROS production. In the present study, a high-fat diet significantly decreased the activity of mitochondrial complex I and complex II in rat pancreatic beta cells. After 8 weeks of RSV administration, the activity of mitochondrial complex I was dramatically elevated, whereas complex II activity was not affected. These results were associated with the change of ROS levels in rat pancreatic beta cells. The UCP2 expression and MDA level, as important controllers of mitochondria-derived reactive oxygen species,38–41 were also decreased in the HFR group. These data suggest that the high-fat diet-induced oxidative stress probably originated from an impaired complex I and complex II system, and the protective function of RSV might be through reducing the oxidative damage of complex I. However, Laurène et al. analyzed the five respiratory chain complexes by immunoblotting on mitochondria isolated from INS-1E cells after exposure to RSV for 24 h and found similar expression levels in control and treated cells for all tested subunits.42 These data do not align with our result. A possible reason might be the difference between in vitro and in vivo studies. It could also be that they examined the complexes’ protein expression level instead of activity. Our results are supported by the recent observation that activity of mitochondrial complex I was decreased in a diabetes animal model,41 and overexpression of mimitin, as a molecular chaperone for the mitochondrial complex I, could increase ATP production, and elevate basal and glucose-induced insulin secretion.43
In this study, we found that RSV has antioxidant effects in the pancreatic islet in rats, not only through the scavenging of ROS, but also through up-regulating the antioxidant enzyme activities including SOD and GPx. These results are in line with in vitro studies using pancreatic beta-cell lines (RIN-5F cells), in that RSV protected the cells from advanced glycation end product-induced oxidative stress and apoptosis.44
We then analyzed the possible molecular mechanisms underlying the RSV-induced benefit on pancreatic islets. We observed elevated expression of SIRT1 and PDX-1 on protein level. Some investigations have reported that SIRT1 positively regulates GSIS in beta cells.32,33 Mice with beta-cell-specific SIRT1 overexpression showed improved glucose tolerance and increased GSIS.33 In contrast, SIRT1 general knock-out animals had lower levels of circulating insulin, and their isolated islets showed blunted insulin secretion.32 Moreover, their studies showed that SIRT1 probably increased insulin secretion through transcriptional repression of UCP2 expression, which was consistent with our data. PDX-1 is a beta-cell master gene45 and was reported as a downstream target gene of SIRT1.46 It also has been shown to regulate GSIS47,48 through regulating TFAM expression.47 We therefore proposed that RSV might increase insulin secretion through activation of SIRT1 expression.
Moreover, SIRT1 has been implicated as a key regulator of energy homeostasis49 and mitochondrial biogenesis.50 In our study, SIRT1 protein and PGC-1a mRNA expression level were significantly increased in the RSV administration group, along with mitochondrial respiratory chain activities, especially mitochondrial complex I. SIRT1 increases mitochondrial respiratory chain activities, possibly depending on PGC-1α, which has been shown to induce transcription of nuclear-encoded mitochondrial subunits of the mitochondrial respiratory chain such as cytochrome c and cytochrome oxidase.50
In conclusion, we demonstrate the beneficial effects of RSV supplementation on GSIS in high-fat diet-induced obese male rats. RSV displays a wide range of roles in the potentiation of GSIS. Our data suggest that the mechanism of this beneficial effect is probably through increasing mitochondrial function by up-regulating SIRT1 and reducing oxidative stress.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30771035 and No.81170782).
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; WK, JZ, H-HZ, XH, T-SZ, and DH conducted the experiments. L-LC and WK contributed equally to this study.
References
- 1.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004; 134: 205–10. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–101. [DOI] [PubMed] [Google Scholar]
- 3.Crescenzo R, Lionetti L, Mollica MP, Ferraro M, D’Andrea E, Mainieri D, Dulloo AG, Liverini G, Iossa S. Altered skeletal muscle subsarcolemmal mitochondrial compartment during catch-up fat after caloric restriction. Diabetes 2006; 55: 2286–93. [DOI] [PubMed] [Google Scholar]
- 4.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–8. [DOI] [PubMed] [Google Scholar]
- 5.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and French fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 2006; 83: 284–90. [DOI] [PubMed] [Google Scholar]
- 6.Sone H, Kagawa Y. Pancreatic beta-cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 2005; 48: 58–67. [DOI] [PubMed] [Google Scholar]
- 7.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 1991; 40: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 8.Maria SW, Bo A. The high-fat diet–fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004; 53: S215–9. [DOI] [PubMed] [Google Scholar]
- 9.Linn T, Strate C, Schneider K. Diet promotes beta-cell loss by apoptosis in prediabetic nonobese diabetic mice. Endocrinology 1999; 140: 3767–73. [DOI] [PubMed] [Google Scholar]
- 10.Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 2004; 53: 624–32. [DOI] [PubMed] [Google Scholar]
- 11.Polonsky KS. Dynamics of insulin secretion in obesity and diabetes. Int J Obes Related Metab Disord 2000; 24: S29–31. [DOI] [PubMed] [Google Scholar]
- 12.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 2005; 36: 197–209. [DOI] [PubMed] [Google Scholar]
- 13.Landcake P, Price RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol 1976; 9: 77–86. [Google Scholar]
- 14.Atten MJ, Attar BM, Milson T, Holian O. Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C-mediated mechanism. Biochem Pharmacol 2001; 62: 1423–32. [DOI] [PubMed] [Google Scholar]
- 15.El-Mowafy AM, Alkhalaf M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: A novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis 2003; 24: 869–73. [DOI] [PubMed] [Google Scholar]
- 16.Miller N, Rice-Evans CA. Antioxidant activity of resveratrol in red wine. Clin Chem 1995; 41: 1789–1789. [PubMed] [Google Scholar]
- 17.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res 2000; 47: 549–55. [DOI] [PubMed] [Google Scholar]
- 18.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009; 77: 1053–63. [DOI] [PubMed] [Google Scholar]
- 19.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le- Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444: 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011; 106: 383–9. [DOI] [PubMed] [Google Scholar]
- 21.Timmers S, Hesselink MK, Schrauwen P. Therapeutic potential of resveratrol in obesity and type 2 diabetes: New avenues for health benefits? Ann N Y Acad Sci 2013; 1290: 83–89. [DOI] [PubMed] [Google Scholar]
- 22.Chen WF, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol 2007; 568: 269–77. [DOI] [PubMed] [Google Scholar]
- 23.Szkudelski T. Resveratrol inhibits insulin secretion from rat pancreatic islets. Eur J Pharmacol 2006; 552: 176–81. [DOI] [PubMed] [Google Scholar]
- 24.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol 2002; 282: 1231–8. [DOI] [PubMed] [Google Scholar]
- 25.Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, Nakajima N, Nagashima Y, Wada K, Nakagama H, Nakajima A. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci 2008; 99: 2136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin 2008; 29: 698–706. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z, Zhou L. Dual tail catheters for infusion and sampling in rats as an efficient platform for metabolic experiments. Lab Anim 2003; 32: 45–48. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Park CH, Jang JS. Antecedent intake of traditional Asian-style diets exacerbates pancreatic beta-cell function, growth and survival after Western-style diet feeding in weaning male rats. J Nutr Biochem 2006; 17: 307–18. [DOI] [PubMed] [Google Scholar]
- 29.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 1991; 196: 143–51. [DOI] [PubMed] [Google Scholar]
- 30.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973; 179: 588–90. [DOI] [PubMed] [Google Scholar]
- 31.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–6. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic β-cells against cytokine toxicity by suppressing the nuclear factor-κB signaling pathway. Diabetes 2009; 58: 2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006; 4: e31–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109–22. [DOI] [PubMed] [Google Scholar]
- 35.Lee YE, Kim JW, Lee EM, Ahn YB, Song KH, Yoon KH, Kim HW, Park CW, Li G, Liu Z, Ko SH. Chronic resveratrol treatment protects pancreatic islets against oxidative stress in db/db Mice. PLoS One 2012; 7: e50412–e50412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Chen LL, Zheng J, Zeng TS, Li H, Xiao H, Deng X, Hu X. The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diab Res Clin Pract 2012; 97: 474–82. [DOI] [PubMed] [Google Scholar]
- 37.Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diab Res 2012; 2012: 703538–703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polonsky KS, Semenkovich CF. The pancreatic beta cell heats up: UCP2 and insulin secretion in diabetes. Cell 2001; 105: 705–7. [DOI] [PubMed] [Google Scholar]
- 39.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–20. [DOI] [PubMed] [Google Scholar]
- 40.Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr Diab Rev 2009; 5: 120–44. [DOI] [PubMed] [Google Scholar]
- 41.Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhatacharjee A, Prentice KJ, Sereda SB, Shirihai OS, Wheeler MB. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 2011; 60: 2710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurène V, Thierry B, Laurianne G, Domenico B, Pierre M. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1Eβ-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem 2011; 286: 6049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanzelka K, Skalniak L, Jura J, Lenzen S, Gurgul-Convey E. Effects of the novel mitochondrial protein mimitin in insulin-secreting cells. Biochem J 2012; 445: 349–59. [DOI] [PubMed] [Google Scholar]
- 44.Minakawa M, Kawano A, Miura Y, Yagasaki K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic b-cells. J Clin Biochem Nutr 2011; 48: 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev 2008; 22: 1998–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laurène V, Pierre M. Resveratrol-activated SIRT1 in liver and pancreatic β-cells: A Janus head looking to the same direction of metabolic homeostasis. Aging (Albany NY) 2011; 3: 444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauthier BR, Wiederkehr A, Baquié M, Dai C, Powers AC, Kerr-Conte J, Pattou F, MacDonald RJ, Ferrer J, Wollheim CB. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab 2009; 10: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CVE, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 2002; 277: 11225–32. [DOI] [PubMed] [Google Scholar]
- 49.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature 2006; 444: 868–74. [DOI] [PubMed] [Google Scholar]
- 50.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem 2006; 97: 673–83. [DOI] [PubMed] [Google Scholar]