Abstract
The purpose of this study was to detect the urinary podocytes and its related protein, nephrin, in the urine of the children with glomerular disease in order to analyze the relationship of the clinical testing with the significance of the glomerular disease. A total of 65 children with nephrotic syndrome were selected for this study. The podocytes and nephrin were detected in the urinary sediment by indirect immunofluorescence, enzyme-linked immunosorbent assay, and Western blotting. The urinary podocytes and nephrin positive rates were 53.8% and 50.8%, respectively, in the children with glomerular disease. The serum total protein and albumin decreased in the podocyte-positive children, while the urine total protein at 24 h, urinary albumin/creatinine ratio, blood urea nitrogen, and serum creatinine were significantly elevated as compared to those of the podocyte-negative patients. Furthermore, the results were the same in the patients with positive nephrin as compared to that of the patients with negative nephrin. The podocyte number and nephrin level were significantly higher in the lupus nephritis group as compared to those of the other groups. Likewise, the podocyte number and nephrin level dramatically increased in the focal segmental glomerulosclerosis group as compared to those of the mesangial proliferative glomerulonephritis and minimal change disease groups. In addition, the podocyte numbers and nephrin expression were significantly higher in severe proteinuria group as compared to those of the mild proteinuria group. The urinary nephrin expression was positively related to podocyte and urinary albumin/creatinine ratio. We concluded that the detection of the urinary podocytes and nephrin could be taken as markers for children with glomerular disease, reflecting the type of the disease. Therefore, this can be used as a noninvasive method to evaluate the severity of the kidney disease in children.
Keywords: Podocyte, nephrin, glomerular disease
Introduction
Podocyte biology has been focused on in the past few years. The emerging understanding in podocyte biology has improved our knowledge about molecular mechanisms of many glomerular diseases. Urinary podocyte count and measurement of urinary podocyte-specific markers (nephrin and podocalyxin) have been reported to be able to detect podocyte injury.1,2 The slit diaphragm (SD) between the foot processes of the podocyte is an important component of the glomerular filtration barrier. Therefore, the podocyte damage followed by structural protein changes is considered to be the main cause of the proteinuria. Since podocytes are terminally differentiated cells with a limited replication potential, they are very difficult to restore, once destroyed.3,4
Podocyte damage is the key factor of glomerular disease. The decrease or loss of the podocyte is an important index for assessing the degree of the glomerular damage and kidney sclerosis progression. Detecting the urinary podocytes and its related protein molecules is a way to understand the severity of the glomerular disease.
Multiple recent studies have confirmed that the SD proteins such as nephrin, podocin, and CD2AP constitute the main barrier of the glomerular selective filtration to plasma composition. Nephrin is the protein related to the podocyte and was first found in the glomerular SD. The shifts, expression reduction, molecular damage, charge abnormality, and amino acid composition changes that occur in nephrin as a result of various causes can affect the skelemin rearrangement in the podocytes. This in turn could affect the function of the related protein molecule, which could lead to the destruction of the SD and production of the proteinuria.5 However, little is known about the nephrin loss in the urine of the children with glomerular diseases.
In this study, we focused on the podocytes that were lost in the urine as a direct indication of the glomerular injury and studied the expression behavior of nephrin in the children with nephrotic syndrome. The aim of this study was to analyze the relationship between urinary podocyte and nephrin in these patients and to evaluate the activity and severity of the glomerular disease.
Patients and methods
Patients
The selected subjects were 65 hospitalized children who were diagnosed with the glomerular disease from the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou Women and Children’s Medical Center, and Guangzhou First People’s Hospital. There were 40 males and 25 females, aged 7.06 ± 2.73 years. They had not yet adopted the hormone or other immunosuppressive agent treatments once admitted to our hospitals. There were a total of 10 healthy children in the normal group (Control), aged 6.64 ± 1.49 years with seven males and three females. In all cases, the fresh morning urine was collected with clean, dry, and sterile test tubes and was stored at −20℃.
Based on the diagnostic standards,6 the selected cases were confirmed. According to the disease type, the cases were divided into four groups, including 42 children with the primary nephrotic syndrome (PNS), eight children with IgA nephropathy (IgAN), six children with Henoch-Schönlein purpura nephritis (HSPN), and nine children with lupus nephritis (LN). Among these, 25 cases were diagnosed through renal biopsies. According to the pathological type, the cases were divided into three categories, including seven cases with minimal change disease (MCD), 10 cases with mesangial proliferative glomerulonephritis (MSPGN), and eight cases with focal segmental glomerulosclerosis (FSGS). According to the urine protein excretion, the cases were divided into three categories, including 13 cases with mild proteinuria (urinary total protein at 24 h [24 h UTP] <50 mg/(kg·d)), 17 cases with moderate proteinuria (50 mg/(kg·d) ≤24 h UTP <100 mg/(kg·d)), and 35 cases with severe proteinuria groups (24 h UTP ≥100 mg/(kg·d)).
Indirect immunofluorescence
A total of 30 mL urine was collected and centrifuged in a centrifugal tube. The supernatant was discarded and repeatedly suctioned with a straw. All the cells at the bottom were washed into the liquid phase, sucked off with a pipette, and transferred into a 1.5-mL centrifugal tube with pointed bottom. A mixture of glacial acetic acid, ethanol, and double-distilled water was added up to a total of 1 mL (2:1:7). It was then centrifuged for 5 min at 2000 rpm. The supernatant was discarded, and the sediment was sucked off and was uniformly smeared from the slide center by circling clockwise to the outside. The slide was then incubated with goat anti-human Podocalyxin (PCX) polyclonal antibody (1:200, Santa Cruz Biotechnology, Inc.) at 4℃ overnight.
After washing, the slides were incubated with fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat IgG (1:200) for 30 min at room temperature. The nuclei of the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The slides were observed with an immunofluorescence microscope at 510 nm of blue fluorescence and 340 nm of ultraviolet wavelengths in five different positions (upper left, lower left, upper right, lower right, and middle parts). The number of podocytes was recorded under 20 high-power fields.
Enzyme-linked immunosorbent assay
One milliliter of the sample diluent was added to the human nephrin enzyme-linked immunosorbent assay (ELISA) kit (Huamei, Wuhan) standard product (10 ng) to obtain the final concentration of 10 ng/mL. We then took a series of doubling dilutions and divided the diluent into six 1.5-mL Eppendorf tube (EP) with 5 ng/mL, 2.5 ng/mL, 1.25 ng/mL, 0.625 ng/mL, 0.312 ng/mL, and 0.156 ng/mL. The blank, standard, and sample wells were assigned with 100 µL of sample diluent, standard product, and test sample, respectively. The ELISA plate was incubated at 37℃ for 120 min. After discarding the liquid, the plate was incubated with 100 µL of Horseradish Peroxidase (HRP) avidin working solution (1:100) at 37℃ for 60 min. After the incubation, the solution was suctioned. A total of 90 µL of the substrate solution was added, and the plate was kept in the dark for 15 min at 37℃ for color development. We then added 50 µL of the stopping solution in order to terminate the reaction. The light density (OD value) was tested with a microplate reader at the wavelength of 450 nm, and the standard curve was prepared, based on which we calculated the nephrin concentration in the samples.
Western blotting
The cell lysis buffer was added into the centrifuged sediment of urine specimen with LN, PNS, IgAN, HSPN, and Control. The protein concentration was determined by BCA method. A total of 30 µg of the total protein was run on the SDS-PAGE for 90 min and wet transferred to a PVDF membrane. The membrane was blocked with 5% nonfat milk at room temperature for 30 min and was then incubated with goat anti-human nephrin (1:500; Santa Cruz Biotechnology, Inc.) polyclonal antibody at 4℃ overnight. It was then subsequently incubated with the HRP-labeled rabbit anti-goat IgG (1:500) at room temperature for 60 min. After being washed, the membrane was visualized with an enhanced chemiluminescence reagent and was exposed to the X-ray films. The expression of the nephrin protein was analyzed according to the protein band intensity.
The clinical data
The related biochemical information was collected from the patients. These included serum total protein (TP), albumin (ALB), total cholesterol (TC), serum creatinine (Scr), blood urea nitrogen (BUN), urine total protein at 24 h (UTP/24 h), urinary albumin/creatinine ratio (uALB/Cr), and urine creatinine (Cr).
Statistical analysis
The data were expressed as the mean ± standard deviation. Statistical analysis was performed using analysis of variance. The least significant difference test (LSD) test was used for the pairwise comparison of various samples and the spearman analysis was used for the correlation analysis using SPSS16.0 (SPSS, Inc., Chicago, USA). A P level of <0.05 was accepted as statistically significant.
Results
Counting the podocytes in the urinary sediment
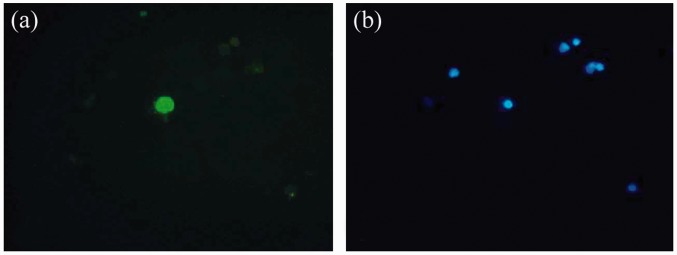
Under an immunofluorescence microscope, podocytes were round or oval-shaped with green fluorescence observed for the cell membrane. The podocyte nuclei were round and larger, centred, or tilted to the side. Only the nuclei of the cells were counterstained with blue in the negative controls (Figure 1). The results showed that the urinary podocytes and nephrin positive rates were 53.8% and 50.8% for the children with glomerular disease, respectively.
Figure 1.
Detection of the urinary podocyte with indirect immunofluorescence (×200). (a) The podocyte with cell membrane stained to be green. (b) The podocyte with the neuclus stained to be blue. (A color version of this figure is available in the online journal.)
Furthermore, we compared the clinical testing data for these patients. In the podocyte-positive children, the TP and ALB decreased, while the UTP/24 h, uALB/Cr, BUN, and Scr were significantly elevated as compared to those of the podocyte-negative patients. Likewise, the patients with the positive nephrin had significant differences as compared to the negative nephrin patients. There was no statistical significance for the TC levels (Table 1).
Table 1.
Comparison of the clinical biochemistry indices
| Indices | Podocyte |
Nephrin |
||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Expressions | 30/65 | 35/65 | 32/65 | 33/65 |
| Male/female | 18/12 | 22/13 | 20/12 | 19/14 |
| Age | 6.55 ± 2.34 | 7.37 ± 2.68 | 6.84 ± 2.87 | 7.26 ± 2.35 |
| UTP/24 h (g/kg) | 0.08 ± 0.02 | 0.12 ± 0.03* | 0.09 ± 0.03 | 0.11 ± 0.04* |
| TP (g/L) | 44.35 ± 6.82 | 32.24 ± 3.44* | 42.34 ± 4.35 | 32.74 ± 3.35* |
| ALB (g/L) | 27.43 ± 2.45 | 14.23 ± 3.24* | 26.38 ± 2.56 | 14.56 ± 3.35* |
| TC (mmol/L) | 9.34 ± 2.54 | 10.78 ± 2.25 | 9.78 ± 2.74 | 10.64 ± 2.85 |
| BUN (mmol/L) | 7.33 ± 2.54 | 10.43 ± 2.37* | 7.47 ± 2.85 | 10.16 ± 2.44* |
| Scr (µmol/L) | 57.28 ± 7.85 | 85.43 ± 10.32* | 59.56 ± 5.63 | 83.67 ± 6.34* |
| uALB/Cr (µg/µmol) | 262.45 ± 35.23 | 652.34 ± 82.43* | 284.73 ± 42.53 | 623.32 ± 76.82* |
Data represent mean ± standard deviation. UTP: urine total protein; TP: total protein; ALB: albumin; TC: total choloestrol; BUN: blood urea nitrogen; Scr: serum creatinine; uALB/Cr: urinary albumin/creatinine ratio.
P < 0.05 as compared to the negative group.
Pathological types of sources
In eight IgAN patients, we found six patients with MSPGN and two patients with FSGS by renal biopsy, and IgA showed granular deposition in the glomerular mesangial region. The clinical manifestations of these patients were recurrent hematuria associated with proteinuria and increased serum IgA levels. Nine LN patients with antinuclear antibody and anti-dsDNA antibody positivity as well as decreased C3 complement were admitted due to edema and proteinuria. Among these nine patients, there were three cases of MCD, one case of MSPGN pathological type III, one case of MSPGN pathological type IV, and four cases of FSGS. Seven PNS patients received renal biopsy, and the results showed four cases of MCD, one case of MSPGN, and two cases of FSGS. The other PNS patients were diagnosed for the first time with simple-type nephrotic syndrome without other nephropathy. Six cases of HSPN patients showed skin purpura, hematuria, and proteinuria, and one patient of them was diagnosed with MSPGN.
The results of this study showed that there were significant differences in the urinary podocytes and nephrin among the three groups. Through the paired comparison for samples, the podocyte counts and nephrin expressions from the FSGS group were demonstrated to be significantly higher than those of the MCD and MSPGN groups. There was no statistical significance when we compared the difference between MSPGN and MCD groups (Table 2).
Table 2.
Podocyte count and nephrin expression from different pathological types of sources
| Groups | Cases | Podocytes (cells/20 HP) | Nephrin (ng/mL) | uALB/Cr (µg/µmol) |
|---|---|---|---|---|
| FSGS | 8 | 48.82 ± 5.86 | 10.24 ± 4.31 | 673.45 ± 83.72 |
| MCD | 7 | 15.36 ± 3.69* | 4.21 ± 1.85* | 325.23 ± 38.46* |
| MSPGN | 10 | 22.15 ± 4.58* | 5.67 ± 2.34* | 433.62 ± 42.31* |
Data represent mean ± standard deviation. FSGS: focal segmental glomerulosclerosis; MCD: minimal change disease; MSPGN: mesangial proliferative glomerulonephritis; uALB/Cr: urinary albumin/creatinine ratio.
P < 0.05 as compared to the FSGS group.
Different disease groups
In the experiment, the results showed that there were significant differences in the expression of the urinary podocytes and nephrin among these groups. In Control, no nephrin expression was detected, and the number of podocytes is 1.38 ± 0.42. Through the paired comparison of the samples, it was demonstrated that the urinary podocytes and nephrin expressions were significantly higher in the LN group as compared to those of the PNS, IgAN, and HSPN groups. The podocyte count and nephrin expression of the PNS group increased as compared to those of the IgAN and HSPN groups as well (Table 3).
Table 3.
Podocyte count and nephrin expression from different disease groups
| Groups | Cases | Podocytes (cells/20 HP) | Nephrin (ng/mL) |
|---|---|---|---|
| Control | 10 | 1.38 ± 0.42 | 0 |
| LN | 9 | 54.74 ± 8.25 | 6.12 ± 4.16 |
| PNS | 42 | 34.52 ± 7.41* | 4.53 ± 2.23* |
| IgAN | 8 | 17.34 ± 4.64*,† | 2.29 ± 1.22*,† |
| HSPN | 6 | 19.67 ± 3.48*,† | 3.47 ± 2.12* |
Data represent mean ± standard deviation. LN: lupus nephritis; PNS: primary nephrotic syndrome; IgAN: IgA nephropathy; HSPN: Henoch-Schönlein purpura nephritis.
P < 0.05 as compared to the LN group.
P < 0.05, as compared to the PNS group.
Different degrees of proteinuria
The results showed significant differences for the podocyte count and the nephrin expression among the three groups. There were significant differences of urinary podocyte count and nephrin expression between the heavy and mild proteinuria groups. The podocyte count and nephrin expression from moderate proteinuria group fell somewhere in the middle (Table 4).
Table 4.
Podocyte count and nephrin expression with different degrees of proteinuria
| Groups | Cases | Podocytes (cells/20 HP) | Nephrin (ng/mL) |
|---|---|---|---|
| Heavy proteinuria | 35 | 35.05 ± 6.07 | 5.18 ± 4.59 |
| Moderate proteinuria | 17 | 24.65 ± 5.85* | 3.32 ± 1.58* |
| Mild proteinuria | 13 | 13.82 ± 3.15* | 2.05 ± 2.19* |
Data represent mean ± standard deviation.
P < 0.05 as compared to the heavy proteinuria group.
Urinary nephrin expression in Western blotting
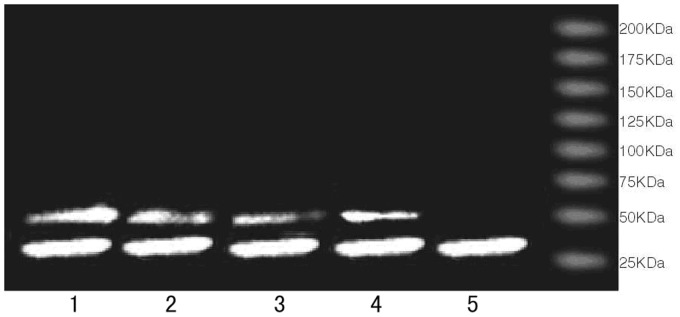
We analyzed the nephrin expression in the urine by Western blotting. According to the protein bands, nephrin was detected in the urine of the children with LN, PNS, IgAN, HSPN, and Control. Its molecular weight was low (about 43 KDa), and the sheared fragments of the nephrin protein were detected. The analysis was consistent with the results of the ELISA (Figure 2).
Figure 2.
Western blot analysis of the urinary nephrin. 1: lupus nephritis; 2: primary nephrotic syndrome; 3: IgA nephropathy; 4: Henoch-Schönlein purpura nephritis; 5: Control
Correlation analysis
Using the Spearman analysis method, the results showed that the nephrin expression in the urine of the children with glomerular disease had positive correlations with the urinary podocyte count (r = 0.996, P < 0.05) and uALB/Cr (r = 0.544, P < 0.05).
Discussion
In the past few years, the podocyte count has been focused on in the study of glomerular physiology, pathology, and disease progression. Podocytes are a unique cell both functionally and phenotypically. When the podocyte is damaged due to various reasons, a series of phenotypic changes could happen. The podocyte swelling, foot process retraction, and fusion have been observed with electron microscopy. These steps have been followed by podocyte shrinkage, pseudocyst formation, and anionic charge reduction. Finally, the podocyte was isolated from the glomerular basement membrane (GBM), stripped to the renal capsule, and was excreted from the body with urine.
After the damage and exfoliation of the podocyte, it can only rely on its surrounding. Therefore, the cell undergoes a compensatory hypertrophy in order to fill the bare area of the GBM. When the compensatory hypertrophy of the podocyte could not completely cover the GBM, the GBM without a podocyte support might be compressed and convexed into the renal capsule under the action of capillary hydrostatic pressure. Furthermore, it could adhere to the epithelial cell of the glomerular wall layer, cause cell proliferation, damage the extracellular matrix, and finally trigger and aggravate the glomerular sclerosis.7,8 Podocalyxin is the parietal epithelial molecule of the podocyte, which can induce the actin reorganization in podocyte, and so it can be used as the detection indicator of podocyte injury in the experiment.9,10 The indirect immunofluorescence could be conducted on the cell smear of the urine sediment as a specific method for testing the urinary podocyte count.11
Previous studies have reported the damage and exfoliation of the podocyte in various types of glomerular diseases; however, no podocyte was detected in the patients with tubulointerstitial nephritis (including pyelonephritis) and noninflammatory renal diseases.12–14
We further compared the clinical data among the patients in this study. For the podocyte-positive children, the serum total protein and albumin were decreased while the UTP/24 h, UALB/Cr, BUN, and Scr were significantly elevated as compared to those of the negative podocyte patients. Also, the patients with positive nephrin expression showed similar results as compared to the patients with negative nephrin expression. These findings suggested that the urinary podocyte count and nephrin expression could be used as markers in the children with glomerular disease and an increased urinary nephrin could potentially equate with an early podocyte injury.
In this study, the results showed that the podocyte count and nephrin expression from the LN group were significantly higher than those of the other groups. Likewise, the podocyte count and nephrin expression of the FSGS group dramatically increased as compared to those of the MSPGN and MCD groups. Furthermore, through the analysis of the nine LN patients’ pathological types, there were three cases of MCD, one case of MSPGN pathological type III, one case of MSPGN pathological type IV, and four cases of FSGS. These findings suggested that the podocyte count and nephrin expression in the urine were associated with the type of glomerular diseases, which were relevant to the LN group’s pathologic changes.
In addition, the podocyte count and nephrin expression from severe proteinuria group were significantly higher than those of the mild proteinuria group. The urinary nephrin expression was positively related to the podocyte count and uALB/Cr.
Nephrin is a transmembrane protein, which is located on the glomerular SD.15 It is very important to maintain the integrity of the foot processes and the function of the SD. Nephrin has been found in urine (nephrinuria) in several experimental studies and in human proteinuric diseases as detected by immunoblotting analysis.16 Several studies on the glomerular diseases in relation to proteinuria in human and rats have reported that there was podocyte damage detected in these diseases. Furthermore, they reported that the expression of nephrin and podocyte-associated molecules was changed. Moreover, nephrin was reported to be fallen off from the podocyte followed by being excreted from the body within the urine.17
Adopting affinity purification antibody, Pätäri et al.18 first reported the sheared fragments of the nephrin in the urine of the patients with type I diabetic nephropathy. Meanwhile, the typical full-length protein band of the nephrin was detected from the patients’ glomeruli. Therefore, it was hypothesized that some of the nephrin was being degraded during the collection and storage of the urine. In this study, the sheared fragments with the molecular weight of about 43 KDa were detected from the urine of the patients with glomerular disease by Western blotting. This might have been the soluble sheared variant of the nephrin in the urine.
This study showed that the podocyte count and nephrin expression in the urine could be used as biomarkers reflecting the type of the glomerular disease and its activity. The urinary detection of the podocyte injury might become a more sensitive indicator of the glomerular disease damage as compared to proteinuria.19 The urinary podocyte cytology is a potential noninvasive test with a good diagnostic value for glomerular disease. This could potentially compensate for the drawbacks of the renal biopsy for children whose renal biopsy is usually not performed in China.
ACKNOWLEDGEMENTS
This study was supported by the Natural Science Foundation of Guangdong Province (S2012010008588), Scientific and Technical Innovation Program for Higher School of Education Department of Guangdong Province (2012KJCX0088), and Scientific Research Program of Guangzhou Education Bureau (10A254).
Author contributions
PW and ZJ initiated and designed the project, analyzed the data, and edited the paper. PW, ML, QL, and BC performed the data collection and the molecular experiments and carried out the initial analyses.
REFERENCES
- 1.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998; 1: 575–82. [DOI] [PubMed] [Google Scholar]
- 2.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, Mc Quistan T, Furthmayr H, Farquhar MG. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 2001; 12: 1589–98. [DOI] [PubMed] [Google Scholar]
- 3.Liu YQ, Ji ZQ. Podocyte and podocytopathy. J Nephrol Dial Transplant 2011; 20: 54–61. [Google Scholar]
- 4.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 2002; 13: 3005–15. [DOI] [PubMed] [Google Scholar]
- 5.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 2001; 158: 1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Subspecialty Group of Nephrology, Society of Pediatrics, Chinese Medical Association. The clinical classification, diagnosis and treatment of children glomerular disease. Chin J Pediatr 2001; 39: 746–9. [Google Scholar]
- 7.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 2006; 69: 2131–47. [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 2001; 10: 543–9. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 2001; 108: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegner B, Al-Momany A, Kulak SC, Kozlowski K, Obeidat M, Jahroudi N, Paes J, Berryman M, Ballermann BJ. CLIC5A, a component of the ezrin- podocalyxin complex in glomeruli, is a determinant of podocyte integrity. Am J Physiol Renal Physiol 2010; 298: F1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi K, Yonemura S, Matsui T, Tsukita S. Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxylterminal threonine phosphorylated in cultured cells and tissues. J Cell Sci 1999; 112: 1149–58. [DOI] [PubMed] [Google Scholar]
- 12.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 2003; 285: F40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achenbach J, Mengel M, Tossidou I, Peters I, Park JK, Haubitz M, Ehrich JH, Haller H, Schiffer M. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant 2008; 23: 3138–45. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Sekizuka K, Ebihara I, Koide H. Urinary podocytes for the assessment of disease activity in lupus nephritis. Am J Med Sci 2000; 320: 112–16. [DOI] [PubMed] [Google Scholar]
- 15.Patari-Sampo A, Ihalmo P, Holthofer H. Molecular basis of the glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med 2006; 38: 483–92. [DOI] [PubMed] [Google Scholar]
- 16.Camici M. Urinary detection of podocyte injury. Biomed Pharmacother 2007; 61: 245–9. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Lai FM, Tam LS, Li KM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol 2007; 34: 2358–64. [PubMed] [Google Scholar]
- 18.Pätäri A, Forsblom C, Havana M, Taipale H, Groop PH, Holthöfer H. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes 2003; 52: 2969–74. [DOI] [PubMed] [Google Scholar]
- 19.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 2005; 16: 1733–41. [DOI] [PubMed] [Google Scholar]