Abstract
Increased levels of angiotensin II (Ang II) and activated matrix metalloproteinase 2 (MMP-2) produced by human aortic smooth muscle cells (human ASMCs) have recently been implicated in the pathogenesis of thoracic aortic aneurysm (TAA). Additionally, angiotensin II type 1 receptor (AT1R)-mediated extracellular signal-regulated kinase (ERK)1/2 activation contributes to TAA development in Marfan Syndrome. However, there is scant data regarding the relationship between Ang II and MMP-2 expression in human ASMCs. Therefore, we investigated the effect of Ang II on MMP-2 expression in human ASMCs and used Western blotting to identify the Ang II receptors and intracellular signaling pathways involved. Reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence data demonstrated that Ang II receptors were expressed on human ASMCs. Additionally, Ang II increased the expression of Ang II type 2 receptor (AT2R) but not AT1R at both the transcriptional and translational levels. Furthermore, Western blotting showed that Ang II increased MMP-2 expression in human ASMCs in a dose- and time-dependent manner. This response was completely inhibited by the AT1R inhibitor candesartan but not by the AT2R blocker PD123319. In addition, Ang II–induced upregulation of MMP-2 was mediated by the activation of ERK1/2, whereas p38 mitogen-activated protein kinase (p38 MAPK) and c-Jun N-terminal kinase (JNK) had no effect on this process. In conclusion, these results indicate that Ang II can increase the expression of MMP-2 via AT1 receptor and ERK1/2 signaling pathways in human ASMCs and suggest that antagonists of AT1R and ERK1/2 may be useful for treating TAAs.
Keywords: Angiotensin II, renin angiotensin system, matrix metalloproteinase-2, extracellular signal-regulated kinase 1/2, human aortic smooth muscle cells
Introduction
Thoracic aortic aneurysms (TAAs) tend to expand asymptomatically until aortic rupture or dissection occurs, but once they rupture, the mortality rate is at least 94%.1 Because there are no validated medical therapies for TAAs, treatment is confined to open or endovascular surgical repair. Thus, there is an urgent need to characterize the pathogenic mechanisms of this disease and develop effective pharmacological therapies.
Matrix metalloproteinases (MMPs) are a family of structurally related, zinc-containing endopeptidases implicated in the degradation of extracellular matrix and connective tissue proteins.2,3 Among MMPs, MMP-2 and MMP-9 have been found to be elevated in thoracic aortic aneurysmal tissue.4–6 In the thoracic aorta, the major source of MMP-9 is inflammatory cells such as macrophages and B lymphocyte cells in the media and at the adventitial-medial junction,7 whereas MMP-2 is mainly produced by vascular smooth muscle cells (VSMCs) in the tunica media of the aorta.8–10 However, the detailed mechanisms of MMP-2 upregulation in VSMCs remain unknown in TAAs.
Angiotensin II (Ang II) is a multifunctional octapeptide with diverse effects, including modulating vasomotor tone, cell migration, cell growth, apoptosis, and extracellular matrix deposition.11 Recently, accumulating evidence has demonstrated that Ang II is strongly associated with the formation and progression of TAAs in human and mouse models.12–15 However, the causal role of Ang II in the development of human TAAs remains to be clarified. VSMCs have been demonstrated to play important synthetic roles during development and vascular remodeling.16,17 Furthermore, it has become evident that in vitro, Ang II can stimulate rat aortic smooth muscle cells (ASMCs) and human umbilical vein endothelial cells to produce, secrete, and activate MMP-2.18–20 However, Browatzki et al.21 reported Ang II induced the expression of MMP-1, MMP-3, and MMP-9, but not MMP-2, in human VSMCs obtained from human saphenous veins.
Although individual studies have recently demonstrated that Ang II upregulates MMP-2 expression in VSMCs, these conclusions are based on rat ASMCs.18,22 Because rat and human VSMCs respond differently to Ang II,23 those conclusions cannot be definitively applied to human ASMCs. Furthermore, the mechanisms of Ang II–stimulated MMP-2 expression have not yet been clarified. Thus, we investigated Ang II–induced effects on MMP-2 expression in human ASMCs and the signaling pathways required for Ang II–induced MMP-2 expression in human ASMCs. Our results demonstrate that Ang II stimulates increased MMP-2 expression in human ASMCs through extracellular signal-regulated kinase (ERK)1/2 activation but not through p38 mitogen-activated protein kinase (p38 MAPK) or c-Jun N-terminal kinase (JNK).
Materials and methods
Regents and antibodies
The following main regents and antibodies were used in this study: Angiotensin II was purchased from Sigma (St. Louis, MO, USA). MAPKs inhibitors SB203580, PD98059, SP600125, Ang II receptors inhibitors candesartan and PD123319 were purchased from Selleck Chemicals (Houston, TX, USA). Fetal bovine serum (FBS), cell culture media and TRIzol reagent were purchased from Life Technologies (Grand Island, NY, USA). BCA protein assay reagents were purchased from Pierce (Rockford, IL, USA); anti-MMP-2 was purchased from Bioworld Technology (St. Louis Park, MN, USA), rabbit anti-c-Jun amino kinase terminal kinase (JNK), anti-phospho-JNK1/2, anti-extracellular-signal-regulated kinase (ERK)1/2, anti-phospho-ERK1/2, anti-p38, anti-phospho-p38 from Cell Signaling Technology (Beverly, MA, USA); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Santa Cruz Biotechnology (Dallas, TX, USA); antismooth muscle α-actin, anti-Ang II type 1 receptor (AT1R), and Ang II type 2 receptor (AT2R) from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG were purchased from Cell Signaling Technology (Beverly, MA, USA).
HASMC culture
Primary human ASMCs were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL penicillin G, 100 mg/mL streptomycin, and 10% FBS at 37℃ in a humidified 5% CO2 incubator. The purity of the human ASMCs was greater than 97% (data not shown), as confirmed by immunocytochemical staining of α-smooth muscle actin (α-SMA, Abcam). For the experiments, Cells from 3 to 8 subcultures were plated in six-well plates at a density of approximately 5 × 105 cells per well and grown to 80–90% confluence. The ASMCs were cultured in serum-free DMEM (serum-starved) for 24 h. Subsequently, the medium was replaced with DMEM supplemented with 0.1% FBS. Cells were then treated with one of the following protocols: (1) ASMCs were treated with various Ang II concentrations (0.01, 0.1, 1, or 10 µM) for 24 h or with various Ang II stimulation time courses (12, 18, 24, or 48 h) to test for MMP-2 expression. (2) ASMCs were stimulated with Ang II for various incubation times (2, 5, 10, 30, or 60 min) for assessing MAPK phosphorylation. (3) ASMCs were treated with or without 1 µM Ang II for 48 h for evaluating ATR expression. (4) Cells were pretreated with 0.1 µM candesartan, an AT1R antagonist, or 1 µM PD123319, an AT2R antagonist, for 30 min, followed by exposure to 0.1 µM Ang II for 2 min. (5) Cells were pretreated with 1 µM candesartan and 10 µM PD123319 for 60 min, followed by treatment with 0.1 µM Ang II for 24 h. (6) Cells were pretreated for 60 min with 20 µM SB203580, a p38 MAPK inhibitor, 10 µM PD98059, an ERK1/2 inhibitor, or 10 µM SP600125, a JNK inhibitor. At the end of each treatment, the cells were collected for protein extraction, and protein concentrations were determined using the BCA Protein Assay Kit.
Immunofluorescent staining
Human ASMCs were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.1% Triton X-100 for 20 min at room temperature. Then, the cells were incubated with the primary antibodies (AT1R 1:100; AT2R 1:100) overnight at 4℃ followed by incubation with anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA, USA) conjugated with Alexa 488 at a dilution of 1:400 for 1 h at room temperature. Cell nuclei were counterstained with 4′, 6-diamidino-2-phenylindol (DAPI, Beyotime, Jiangsu, China) for 5 min. Slides were mounted with antifading reagent (Beyotime, Jiangsu, China) and examined with a Leica TCS SP8 confocal spectral microscope (Wetzlar, German). Representative images were automatically taken using a SPOT digital camera.
Reverse transcription polymerase chain reaction
Total cellular RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was quantified using a spectrophotometer (NanoDrop ND-3300, Thermo Scientific, Waltham, MA, USA) at the absorbance of 260 nm/280 nm. Total RNA (1 µg) was reverse-transcribed to the cDNA using RevertAid First Strand cDNA Synthesis Kit (# K1622 Thermo Scientific). The expression of AT1R and AT2R mRNA was assessed by reverse transcription polymerase chain reaction (RT-PCR). The single-stranded cDNA was amplified by PCR using 35 cycles. PCR conditions were 15 s of denaturation at 94℃, 20 s of annealing at 58℃, and 40 s of extension at 72℃. As an internal control, GAPDH was co-amplified. The number of cycles was 35, the sequences of the oligonucleotide primers used for PCR and the sizes of the predicted PCR products are shown in Table 1. The PCR product (10 µL) was electrophoresed on 2.0% agarose, stained with ethidium bromide, and visualized by UV absorption. Densitometric signals were quantified by using Quantity One 4.6.2 Software (Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequences and product sizes for AT1R, AT2R, and GAPDH
| Gene | Primer | Sequence (5′ to 3′) | PCR product length |
|---|---|---|---|
| AT1R | Forward | GATTGTCCCAAAGCTGGAAG | 104 bp |
| Reverse | ATCACCACCAAGCTGTTTCC | ||
| AT2R | Forward | TTCCCTTCCATGTTCTGACC | 191 bp |
| Reverse | AAACACACTGCGGAGCTTCT | ||
| GAPDH | Forward | CAGGGCTGCTTTTAACTCTGG | 180 bp |
| Reverse | TCCTGGAAGATGGTGATGGG |
AT1R: angiotensin II type 1 receptor; AT2R: angiotensin II type 2 receptor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
The human ASMCs were collected by centrifugation at 14,000 g for 10 min at 4℃ for protein extraction using RIPA lyses buffer (50 mM TriseHCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1.2% Triton-X-114, 1 mM NaF, 200 mM NaVO4) and 1 tablet/10 mL of protease inhibiter cocktail tablets 9 (Roche Diagnostics, Mannheim, Germany). Protein concentrations were determined by the BCA Protein Assay Kit. For Western blotting, equal amounts of protein extracts (20 µg) were loaded into 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Non-specific binding sites were blocked by incubating the membranes in TBS-0.1% Tween-20 with 5% skimmed milk for 1 h at room temperature, the membranes were incubated with primary antibodies (1:1000 dilution for MMP-2, AT1R, AT2R, t-p38, p-p38, t-ERK1/2, p-ERK1/2, t-JNK1/2, p-JNK1/2, GAPDH) overnight at 4℃, and then washed three times with TBS-T, followed by incubation with a dilution of 1:5000 of horseradish peroxidase-conjugated polyclonal anti-rabbit antibody for 1 h at room temperature. The membrane was detected with an enhanced ECL chemiluminescence system (Millipore, Billerica, MA, USA). Densitometric signal intensity was then measured using Quantity One 4.6.2 software. Equal protein loading of the samples was further verified by staining with GAPDH-specific monoclonal antibody.
Statistical analysis
Data are presented as means ± SME of at least three individual experiments and triplicate wells in each experiment. Group differences were analyzed by analysis of variance (ANOVA), and individual group differences were tested by the post hoc Fisher’s protected least significant difference test. P value less than 0.05 was considered statistically significant. All data were analyzed with SPSS 21.0 software.
Results
Ang II induces protein expression of MMP-2 in human ASMCs
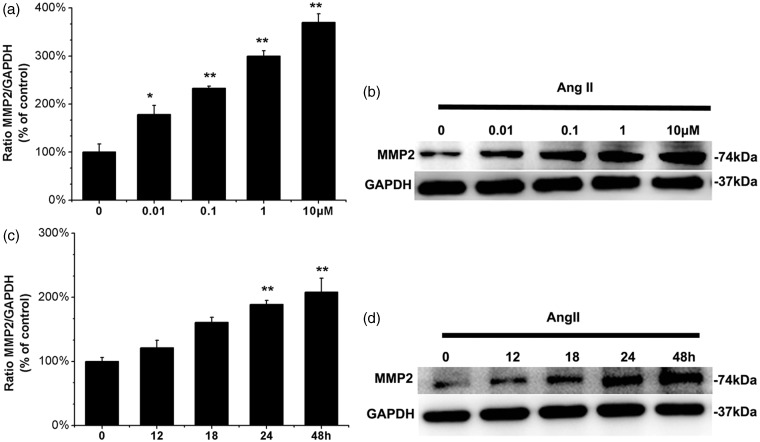
Compared with the control group, Ang II stimulation of human ASMCs induced a significant increase in MMP-2 protein expression, at concentrations as low as 0.01 µM for 24 h (P = 0.041, Figure 1a and b) and with further increases upon stimulation with 1 µM (P < 0.001, Figure 1a and b) or 10 µM for 24 h (P < 0.001, Figure 1a and b). Moreover, incubation with 0.1 µM Ang II for 12 h elevated the MMP-2 protein level (P = 0.134, Figure 1c and d), and greater increases in MMP-2 protein level were observed upon treatment with 0.1 µM Ang II for 24 h and 48 h (P < 0.001, Figure 1c and d). These results demonstrate that Ang II upregulates the protein expression of MMP-2 in a dose- and time-dependent manner in cultured human ASMCs.
Figure 1.
Ang II induces MMP-2 protein expression in ASMCs. (a) Human ASMCs were incubated for 24 h in the presence of various concentrations of Ang II. (b) Human ASMCs were incubated in the absence or presence of 0.1 µM Ang II for the indicated times intervals. The cell lysates were then collected. Levels of MMP-2 protein in cell lysates were measured by Western blot. The group without Ang II incubation was used as control. Graph shows the relative MMP-2 protein levels normalized to GAPDH relative to control. Values are mean ± SEM of three individual experiments, *P < 0.05, **P < 0.01 vs. control
AT1R and AT2R expression in human ASMCs
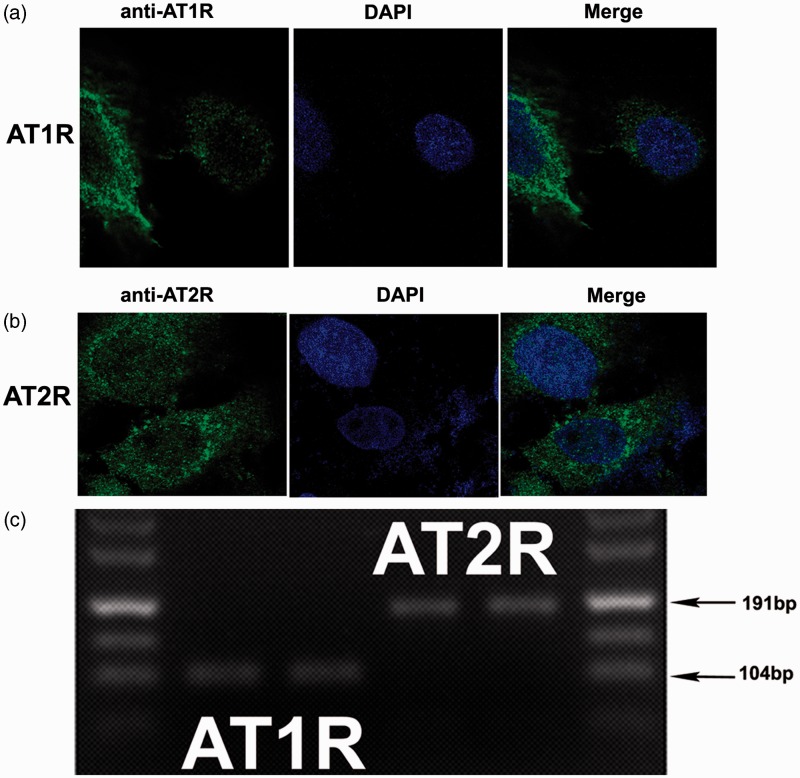
We assessed the expression of Ang II receptors in human ASMCs using RT-PCR and immunocytochemistry. Both AT1 and AT2 receptors are present in human ASMCs (Figure 2). Immunocytochemistry showed strong membrane and cytoplasmic expression of AT1R (Figure 2a) and obvious cytoplasmic as well as very weak membrane expression of AT2R (Figure 2b).
Figure 2.
AT1R and AT2R expression of human ASMCs. Immunofluorescence cytochemistry (a and b) was used to detect the AT1R and AT2R expression in ASMCs. These photomicrographs are representative of similar results obtained from three separate experiments. Sections were analyzed using a laser confocal microscopy (magnification 630×). RT-PCR (c) was used to detect mRNA for the AT1R and AT2R in human ASMCs. The AT1R (104 bp) and AT2R (191 bp) PCR product length is shown by an arrow. (A color version of this figure is available in the online journal.)
Ang II increases the expression of AT2R but not AT1R in human ASMCs
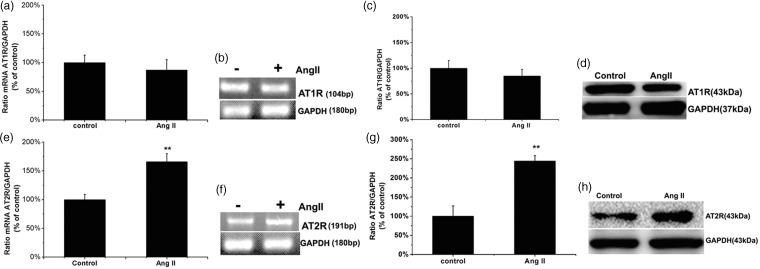
We evaluated the effects of Ang II on the overall transcriptional and translational expression of AT1R and AT2R in the total cell extracts using RT-PCR and Western blot analysis after exposure to 1 µM Ang II for 48 h. RT-PCR revealed no significant change in AT1R mRNA levels following pretreatment with Ang II (P > 0.05; Figure 3a and b), which was confirmed by the translational expression via Western blot (P > 0.05; Figure 3c and d). However, Ang II markedly stimulated both the transcriptional and translational expression of AT2R (P = 0.003, Figure 3e and f; P = 0.009, Figure 3g and h, respectively) in human ASMCs.
Figure 3.
Exposure of the human HASMCs with Ang II caused AT2R but not AT1R mRNA and proteins level to increase significantly. Human ASMCs were incubated in the absence or presence of 1 µM Ang II for 48 h. The cell lysates were then collected. Levels of AT1R and AT2R protein in cell lysates were respectively measured by RT-PCR and Western blot. (a, c, e, and g): Bar graphs corresponding to mean results of three independent experiments normalized to their corresponding loading controls. (b and f): Examples of qPCR for AT1R and AT2R mRNA. (d and h): Representative Western blots for evaluation of AT1R and AT2R protein in cell lysates. GAPDH protein is shown as the loading control. Values are expressed as mean ± SEM of three individual experiments. The cells without Ang II treatment were used as control. **P < 0.01 compared with control cells in the absence of Ang II stimulation
Ang II promotes activation of ERK1/2 but not p38 MAPK or JNK in human ASMCs
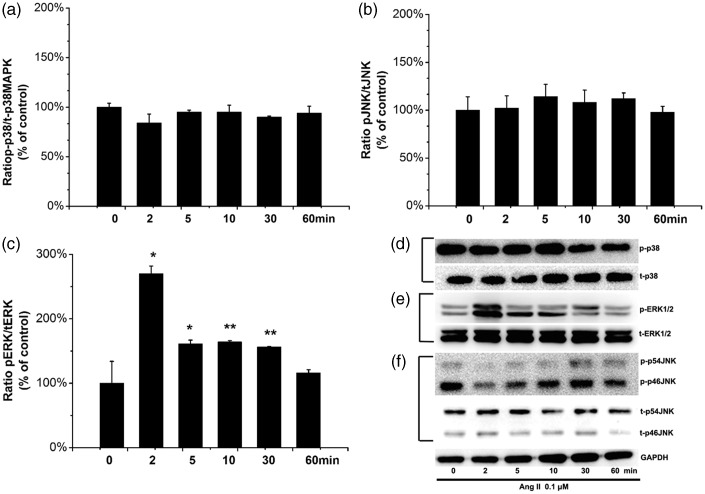
We examined whether Ang II stimulation affects MAPK activity in human ASMCs. Human ASMCs were stimulated with 0.1 µM Ang II for 0, 2, 5, 10, 30, or 60 min. As shown in Figure 4, our results demonstrate that in human ASMCS, ERK1/2 but not p38 MAPK or JNK was activated in response to treatment with Ang II, and ERK1/2 activation peaked at 2 min (P = 0.016, Figure 4b and e). Levels of p-p38 MAPK and p-JNK showed no statistically significant differences at any of the time points tested (P = 0.510 Figure 4a and P = 0.874 Figure 4c, respectively).
Figure 4.
Ang II activates ERK1/2, p38 MAPK, and JNK signaling pathway in ASMCs. Human ASMCs were stimulated with 0.1 µM Ang II for the times course indicated, and then cells were lysed and analyzed by Western blotting for (d) phosphorylated and total p38 (pp38 and tp38), (e) phosphorylated and total ERK1/2 (pERK1/2 and tERK1/2), and (f) phosphorylated and total JNK (pJNK and tJNK). (a–c): Bar graphs corresponding to mean results of three independent experiments normalized to their corresponding loading controls. (d–f): Representative Western blots for evaluation of phosphorylated protein in cell lysates. GAPDH protein is shown as the loading control. Values are expressed as mean ± SEM of at least 3 individual experiments. *P < 0.05 and **P < 0.01 compared with untreated control cells
Ang II stimulates ERK1/2 phosphorylation and the upregulation of MMP-2 expression via the AT1 receptor in human ASMCs
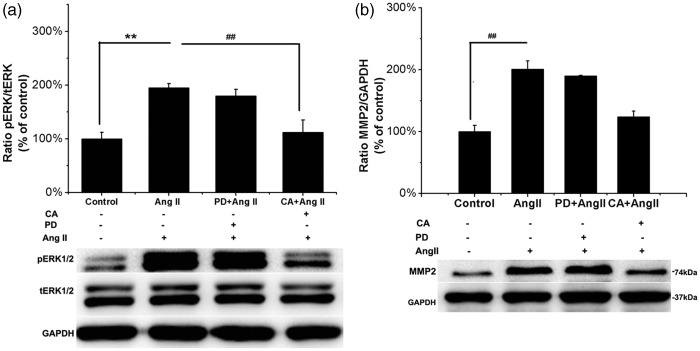
Because human ASMCs express both AT1R and AT2R, we examined which receptor was involved in Ang II–induced ERK1/2 phosphorylation and MMP-2 upregulation. We pretreated human ASMCs with the AT1 receptor antagonist candesartan (0.1 µM for 30 min) and the AT2 receptor blocker PD123319 (1 µM for 30 min) followed by incubation with Ang II (0.1 µM for 2 min). Phosphorylation of ERK1/2 was completely inhibited by candesartan (P = 0.004, Figure 5a). In contrast, PD123319 had no effect upon Ang II–induced ERK1/2 phosphorylation (P = 0.516, Figure 5a). In addition, human ASMCs were pretreated with candesartan (1 µM for 60 min) or PD123319 (10 µM for 60 min) and subsequently stimulated with Ang II (0.1 µM for 24 h). Candesartan completely inhibited the increase in MMP-2 protein expression induced by Ang II (P = 0.005, Figure 5b), but PD123319 did not (P = 0.439, Figure 5b). These results indicate that Ang II induced the phosphorylation of ERK and the upregulation of MMP-2 expression via AT1R in human ASMCs.
Figure 5.
Ang II induces MMP-2 protein expression and ERK phosphorylation ASMCs via AT1R. (a) ASMCs were pretreated with 0.1 µM candesartan (CA, selective AT1R inhibitor, 30 min) or 1 µM PD123319 (PD, selective AT2R inhibitor, 30 min) followed by stimulation with 0.1 µM Ang II for 2 min six-well plates. Western blot was performed to assess the levels of ERK phosphorylation in cell lysates. Top panel: Graphs show the relative p-ERK1/2 protein levels, respectively, normalized to total ERK1/2 relative to control. Bottom panel: Ratio of p-ERK1/2/t-ERK1/2 protein expression, respectively, evaluated using Western blot analysis from a representative experiment. Shown are mean ± SEM of three individual experiments. **P < 0.01 vs. untreated control cells; ##P < 0.01 vs. cells treated with Ang II. (b) ASMCs were incubated with 1 µM candesartan or 10 µM PD123319 for 60 min before stimulation with 0.1 µM Ang II for 24 h in six-well plates. Western blot was used for analyses of the levels of MMP-2 expression in cell lysates. Top panel: Graphs show the relative MMP-2 protein levels normalized to GAPDH relative to control. Bottom panel: Ratio of MMP-2/GAPDH protein expression from a representative Western blot experiment. Shown are mean ± SEM of three individual experiments. **P < 0.01 vs. untreated control cells; ##P < 0.01 vs. cells treated with Ang II
The Ang II–induced elevation of MMP-2 expression is mediated by ERK1/2 in human ASMCs
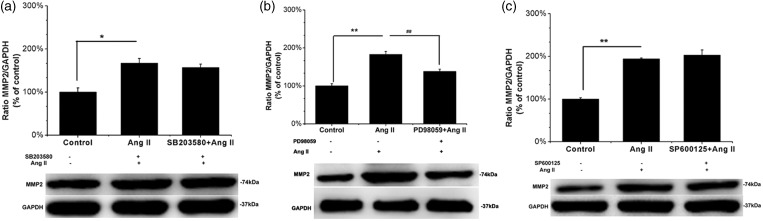
To determine whether the MAPK signaling pathway was involved in Ang II–induced MMP-2 expression, human ASMCs were stimulated with 0.1 µM Ang II for 24 h, with or without pretreatment with PD98059 (an ERK1/2 inhibitor, 10 µM for 60 min), SB203580 (a p38 MAPK inhibitor, 20 µM for 60 min), or SP600125 (a JNK1/2 inhibitor, 10 µM for 60 min). As shown in Figure 6, Ang II treatment led to a significant increase in MMP-2 levels that was completely abolished by PD98059 (P = 0.002, Figure 6b) but not by SB203580 or SP600125 (P = 0.719, Figure 6a and P = 0.347, Figure 6c). These data demonstrate that ERK1/2 was involved in Ang II–induced MMP-2 expression in human ASMCs.
Figure 6.
Inhibition of ERK1/2 but not p38 MAPK and JNK blocks Ang II–induced MMP-2 protein expression in ASMCs by the selective inhibitor of ERK PD98059. Human ASMCs were, respectively, pretreated in six-well plates with 10 µM PD98059 (an ERK inhibitor), 20 µM SB203580 (a p38 inhibitor), and 10 µM SP600125 (a JNK inhibitor) for 60 min followed by 0.1 µM Ang II for 24 h. MMP-2 protein levels in the cells were determined by Western blot. (a–c) Top panel: Ratio of MMP-2/GAPDH. (a–c) Bottom panel: Representative Western blot for MMP-2. Data are expressed as percentage of control, and are the mean ± SEM of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. untreated control cells, ##P < 0.01 vs. Ang II treatment
Discussion
In the present study, we show that Ang II increases MMP-2 expression in a dose- and time-dependent manner in human ASMCs (Figure 1a and b). This is consistent with the previous result using rat ASMCs as model cells.18 Moreover, Browatzki et al.21 reported that Ang II induced concentration-dependent expression of MMP-1 and MMP-3 in human VSMCs obtained from human saphenous veins. In addition, Guo et al.24 demonstrated that Ang II induces time-dependent MMP-9 expression via a NF-κB-dependent pathway in VSMCs cultured from rat aorta. Our findings and other results suggest that Ang II may induce an entire complement of MMPs within aorta tunica media, and together, these MMPs may degrade different components of the extracellular matrix and subsequently lead to thoracic aortic aneurysm formation.
RT-PCR and immunofluorescence showed that Ang II receptors (AT1R and AT2R) are expressed in the ASMCs (Figure 2). Ang II (1 µM, 48 h) induced increased AT2R expression at both the transcriptional and translational levels, whereas AT1R expression was not affected by Ang II (Figure 3). By contrast, de Godoy and Rattan25 demonstrated that Ang II (10 µM, 30 min) caused the movement of AT1R from the plasma membrane to the cytoplasm and had the opposite effect on AT2R but did not change the total levels of Ang II receptors. Thus, the mechanism of Ang II–induced increases in AT2R expression was unknown and required further investigation. Furthermore, it has been shown that increasing AT2R decreased the expression of MMP-2 in rat ASMCs and in Ang II–infused rat aortic tissues,26,27 which may contribute to slowing the progress of TAAs. To identify the receptor involved in the Ang II–induced increase in MMP-2 protein level, we examined the effects of candesartan (selective AT1R inhibitor) and PD123319 (selective AT2R blocker) on Ang II–induced MMP-2 expression. We found that candesartan completely inhibited Ang II–induced expression of MMP-2, whereas PD123319 did not (Figure 5b). These results indicate that Ang II–induced MMP-2 upregulation is mediated by AT1R in human ASMCs. Ford et al.28 obtained a similar result regarding Ang II–stimulated collagen synthesis in human arterial SMCs cultured from internal thoracic artery. Furthermore, the effect of Ang II on ERK phosphorylation in human ASMCs was also abolished by the AT1 antagonist candesartan, whereas the AT2R blocker PD123319 had no such effect (Figure 5a).
Previous reports have shown that Ang II induces MMP-2 expression in a p47phox-dependent manner in mice ASMCs.18 However, to date, there have been no reports regarding the role of MAPK signaling pathways in the Ang II–induced expression of MMP-2 in human aortic SMCs. p38 MAPK, ERK1/2, and JNK are the three major members of the MAP kinase superfamily, which is composed of serine and threonine protein kinases that are involved in the regulation of numerous intracellular signal transduction pathways.29 To our knowledge, the present study is the first to show that Ang II induced MMP-2 expression in human ASMCs through ERK1/2 activation but not the p38 MAPK and JNK signaling pathways (Figures 4 and 6). Other than in human ASMCs, ERK1/2 is also involved in Ang II–induced MMP-2 expression and activation in human retinal pigment epithelium.30
Ghosh et al.31 demonstrated that phosphorylation of ERK is an important upstream event in MMP-2 activation in ERK1/2 knockout mice and mice ASMCs. Taken together, these data suggest that the mechanism of Ang II–induced MMP-2 expression, via the ERK1/2 signaling pathway, may play a critical role in the pathogenesis of TAAs. In addition, Touyz et al.32 showed that Ang II mediated contraction in VSMCs via ERK-dependent signaling pathways in a spontaneously hypertensive rat model. Inhibition of ERK activation also suppressed Ang II–induced abdominal aortic aneurysm formation in ApoE–knockout mice.33 Furthermore, the ERK1/2 inhibitor PD98059 significantly blocked Ang II–induced MMP-9 production in human non-syndromic thoracic aortic aneurysm walls.34
Other cytokines, including platelet-activating factor and IL-1β, have been implicated in MMP-2 expression in rat aortic smooth muscle cell via ERK activation.35,36 Additionally, through ERK phosphorylation, Ang II also induces the expression of platelet-derived growth factor B-chain in rat ASMCs.37 p38 MAPK and JNK do not appear to be involved in Ang II–induced MMP-2 upregulation in HASMCs (data not shown) because Ang II does not induce p38 MAPK and JNK phosphorylation (Figure 4a and c). However, p38 MAPK contributed to Ang II–stimulated migration of rat ASMCs.38 In addition, JNK is involved in Ang II–induced MMP-2 expression in human umbilical vein endothelial cells.20
In conclusion, Ang II induced an increase in MMP-2 expression via AT1R/ERK-dependent signaling pathways in human aortic smooth muscle cells. Manipulation of this pathway using selective pharmacological inhibitors may provide novel approaches for treating thoracic aortic aneurysms.
ACKNOWLEDGMENTS
This work was supported by a grant (2014-11) of Chinese National Center for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease.
Authors’ contribution
CW and XQ designed the research. CW and XS performed the experiments. CW, XQ, XS and QC analyzed the data. CW and QC wrote the manuscript. All authors approved the final version of the manuscript for publication.
References
- 1.Johansson G, Markstrom U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg 1995; 21: 985–8. [DOI] [PubMed] [Google Scholar]
- 2.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827–39. [DOI] [PubMed] [Google Scholar]
- 3.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008; 75: 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha I, Bethi S, Cronin P, Williams DM, Roelofs K, Ailawadi G, Henke PK, Eagleton MJ, Deeb GM, Patel HJ, Berguer R, Stanley JC, Upchurch GR., Jr A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg 2006; 43: 342–8. [DOI] [PubMed] [Google Scholar]
- 5.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan's syndrome. Circulation 1998; 98: 7–7. [PubMed] [Google Scholar]
- 6.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 2002; 110: 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 1995; 96: 318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowther M, Goodall S, Jones JL, Bell PR, Thompson MM. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg 2000; 32: 575–83. [DOI] [PubMed] [Google Scholar]
- 9.Patel MI, Melrose J, Ghosh P, Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg 1996; 24: 82–92. [DOI] [PubMed] [Google Scholar]
- 10.Lesauskaite V, Tanganelli P, Sassi C, Neri E, Diciolla F, Ivanoviene L, Epistolato MC, Lalinga AV, Alessandrini C, Spina D. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol 2001; 32: 1003–11. [DOI] [PubMed] [Google Scholar]
- 11.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol 2005; 90: 449–55. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Rateri DL, Bruemmer D, Cassis LA, Daugherty A. Involvement of the renin-angiotensin system in abdominal and thoracic aortic aneurysms. Clin Sci (Lond) 2012; 123: 531–43. [DOI] [PubMed] [Google Scholar]
- 13.Moltzer E, Essers J, van Esch JH, Roos-Hesselink JW, Danser AH. The role of the renin-angiotensin system in thoracic aortic aneurysms: clinical implications. Pharmacol Ther 2011; 131: 50–60. [DOI] [PubMed] [Google Scholar]
- 14.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE-/- mice. Clin Sci (Lond) 2010; 118: 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci 2011; 1245: 7–10. [DOI] [PubMed] [Google Scholar]
- 16.Touyz RM, Deng LY, He G, Wu XH, Schiffrin EL. Angiotensin II stimulates DNA and protein synthesis in vascular smooth muscle cells from human arteries: role of extracellular signal-regulated kinases. J Hypertens 1999; 17: 907–16. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Kawahara Y, Okuda M, Ueno H, Takeshita A, Yokoyama M. Angiotensin II stimulates mitogen-activated protein kinases and protein synthesis by a Ras-independent pathway in vascular smooth muscle cells. J Biol Chem 1997; 272: 16018–22. [DOI] [PubMed] [Google Scholar]
- 18.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 2005; 328: 183–8. [DOI] [PubMed] [Google Scholar]
- 19.Ham SA, Lee H, Hwang JS, Kang ES, Yoo T, Paek KS, Do JT, Park C, Oh JW, Kim JH, Han CW, Seo HG. Activation of peroxisome proliferator-activated receptor delta inhibits angiotensin II-induced activation of matrix metalloproteinase-2 in vascular smooth muscle cells. J Vasc Res 2014; 51: 221–30. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez E, Perez de la Blanca E, Urso L, Gonzalez I, Salas J, Montiel M. Angiotensin II induces MMP 2 activity via FAK/JNK pathway in human endothelial cells. Biochem Biophys Res Commun 2009; 380: 769–74. [DOI] [PubMed] [Google Scholar]
- 21.Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res 2005; 42: 415–23. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol 2005; 167: 1429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratt RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation 1996; 93: 848–52. [DOI] [PubMed] [Google Scholar]
- 24.Guo RW, Yang LX, Wang H, Liu B, Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul Pept 2008; 147: 37–44. [DOI] [PubMed] [Google Scholar]
- 25.de Godoy MA, Rattan S. Translocation of AT1- and AT2-receptors by higher concentrations of angiotensin II in the smooth muscle cells of rat internal anal sphincter. J Pharmacol Exp Ther 2006; 319: 1088–95. [DOI] [PubMed] [Google Scholar]
- 26.Jing T, Wang H, Srivenugopal KS, He G, Liu J, Miao L, He Y. Conditional expression of type 2 angiotensin II receptor in rat vascular smooth muscle cells reveals the interplay of the angiotensin system in matrix metalloproteinase 2 expression and vascular remodeling. Int J Mol Med 2009; 24: 103–10. [DOI] [PubMed] [Google Scholar]
- 27.Brassard P, Amiri F, Schiffrin EL. Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension 2005; 46: 598–606. [DOI] [PubMed] [Google Scholar]
- 28.Ford CM, Li S, Pickering JG. Angiotensin II stimulates collagen synthesis in human vascular smooth muscle cells. Involvement of the AT(1) receptor, transforming growth factor-beta, and tyrosine phosphorylation. Arterioscler Thromb Vasc Biol 1999; 19: 1843–51. [DOI] [PubMed] [Google Scholar]
- 29.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001; 410: 37–40. [DOI] [PubMed] [Google Scholar]
- 30.Pons M, Cousins SW, Alcazar O, Striker GE, Marin-Castaño ME. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol 2011; 178: 2665–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh A, DiMusto PD, Ehrlichman LK, Sadiq O, McEvoy B, Futchko JS, Henke PK, Eliason JL, Upchurch GR., Jr The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg 2012; 215: 668–80 e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res 1999; 84: 505–1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Naggar JC, Welzig CM, Beasley D, Moulton KS, Park HJ, Galper JB. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler Thromb Vasc Biol 2009; 29: 1764–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagasawa A, Yoshimura K, Suzuki R, Mikamo A, Yamashita O, Ikeda Y, Tsuchida M, Hamano K. Important role of the angiotensin II pathway in producing matrix metalloproteinase-9 in human thoracic aortic aneurysms. J Surg Res 2013; 183: 472. [DOI] [PubMed] [Google Scholar]
- 35.Kim YH, Lee SJ, Seo KW, Bae JU, Park SY, Kim EK, Bae SS, Kim JH, Kim CD. PAF enhances MMP-2 production in rat aortic VSMCs via a beta-arrestin2-dependent ERK signaling pathway. J Lipid Res 2013; 54: 2678–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrlichman LK, Ford JW, Roelofs KJ, Tedeschi-Filho W, Futchko JS, Ramacciotti E, Eliason JL, Henke PK, Upchurch GR., Jr Gender-dependent differential phosphorylation in the ERK signaling pathway is associated with increased MMP2 activity in rat aortic smooth muscle cells. J Surg Res 2010; 160: 18–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deguchi J, Makuuchi M, Nakaoka T, Collins T, Takuwa Y. Angiotensin II stimulates platelet-derived growth factor-B chain expression in newborn rat vascular smooth muscle cells and neointimal cells through Ras, extracellular signal-regulated protein kinase, and c-Jun N-terminal protein kinase mechanisms. Circ Res 1999; 85: 565–7. [DOI] [PubMed] [Google Scholar]
- 38.Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci 2007; 105: 74–8. [DOI] [PubMed] [Google Scholar]