Abstract
Benign prostatic hyperplasia (BPH), which is a common disorder in aging men, involves inflammation that is associated with an imbalance between cell proliferation and cell death. Because current BPH drug treatments have undesirable side effects, the development of well-tolerated and effective alternative medicines to treat BPH is of interest. Bee venom (BV) has been used in traditional medicine to treat conditions, such as arthritis and rheumatism, and pain. Although inflammation has been associated with BPH and BV has strong anti-inflammatory effects, the effects of BV on BPH are not fully understood. Therefore, in this study, we evaluated the efficacy of BV against testosterone-induced BPH in rats. BV decreased prostate weight compared to the untreated group. In addition, BV suppressed serum dihydrotestosterone concentration levels and the levels of proliferating cell nuclear antigen in the histological analysis. Furthermore, BV significantly decreased the levels of the apoptotic suppressors, Bcl-2 and Bcl-xL, and increased the levels of the proapoptotic factors, Bax and caspase-3 activation. These results suggested that BV suppressed the development of BPH and has good potential as a treatment for BPH.
Keywords: Benign prostatic hyperplasia, Bee venom, inflammation, apoptosis, Bcl-2/Bax ratio, caspase
Introduction
Benign prostate hyperplasia (BPH) is one of the most common diseases in aging men. Its incidence gradually increases with age, and about 50% of men over the age of 50 suffer from BPH symptoms, such as urinary urgency and retention.1 BPH develops from a simple micronodular hyperplasia to a microscopic volume enlargement and then to a clinical expression.2 Because the prostate enlarges under the influence of androgen hormones, 5α-reductase inhibitors, which block the activation of testosterone, are widely used to treat BPH.3 Although aging and androgens are the two established risk factors for the development of BPH, recent findings have highlighted the key role of inflammation.2,4 In fact, repeated tissue damage from chronic inflammation may provoke compensatory cellular proliferation with the risk of hyperplastic growth.2,5 These results suggest that reducing inflammation may play an important role in the treatment of BPH and lead to better clinical outcome.
Chronic inflammation can induce proliferative events in prostate tissue by affecting apoptotic protein expression. The major regulatory apoptotic proteins that have been identified include the antiapoptotic factor, Bcl-2, and the proapoptotic factor, Bax.6 Benign prostatic epithelium that has been subjected to various stresses, such as androgen ablation, demonstrates an overexpression of Bcl-2 in the surviving cells, which probably functions to block induced apoptosis.7 Bax collaborated with other proapoptotic Bcl-2 proteins to promote a rheostat of apoptosis sensitivity in that the ratio of these proteins influences a cell’s ability to respond to apoptotic signals.8 Therefore, it is reasonable to suggest that an effective treatment for BPH might involve regulating the balance of Bax and Bcl-2.
Bee venom (BV) therapy is the therapeutic application of honeybee venom in the treatment of various diseases. BV contains a variety of different peptides, including melittin, apamin, adolapin, and mast cell degranulating peptide.9 BV and its components have been used as a traditional medicine to treat a variety of conditions, such as arthritis, rheumatism, back pain, and skin diseases, by regulating inflammatory responses.10 Although several studies have demonstrated the antiproliferative effects of BV and its components in prostate cancer cells,11,12 the molecular mechanisms underlying BV action in BPH have not been described. In the present study, we investigated the antiproliferative effects of BV in a testosterone-induced BPH rat model through the regulation of the inflammatory response and apoptotic protein expression.
Materials and methods
Chemicals and reagents
BV, testosterone, phenylmethylsulfonylfluoride, Triton X-100, propidium iodide, Nonidet P-40, and the protein inhibitor cocktail were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA), and finasteride, a type II 5-reductase inhibitor, was obtained from Merck & Co., Inc. (Whitehouse Station, NJ, USA). 5α-reductase 2 and GAPDH oligonucleotide primers were purchased from Bioneer Corporation (Deajeon, Korea), and SYBR Premix Ex Taq was purchased from Takara Bio Inc. (Otsu, Japan). Antibodies for inducible nitric oxide synthase (iNOS; M-19), COX-2 (C-20), poly (ADP-ribose) polymerase-1 (PARP-1; F-2), caspase-3 (E-8), Bcl-2 (C-2), Bcl-xL (H-5), Bax (B-9), and β-actin (ACTBD11B7) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). An antibody for proliferating cell nuclear antigen (PCNA) was purchased from BD Biosciences (San Jose, CA, USA).
Animals
Ten-week-old male Sprague-Dawley rats (200 ± 20 g) were purchased from Daehan Biolink Co. (Daejeon, Korea). The animals were housed under conditions that were in accordance with the guidelines for the care and use of laboratory animals, which were adopted and promulgated by the Institutional Animal Care Committee of Sangji University (Reg. No. 2014-06). The rats were acclimatized to the laboratory conditions for 2 weeks before starting the experiment.
BPH was induced in the rats by intramuscular injections of testosterone propionate after castration. Briefly, rats were divided into four groups (n = 6 each): Group 1 – control group (Con, normal prostate with vehicle : 200 µl corn oil; p.o.); Group 2 – BPH-induced group (BPH); Group 3 – BPH-induced group treated with finasteride 5 mg/kg/day; p.o. (Fina); Group 4 – BPH-induced group treated with BV 0.1 mg/kg/day; i.p.(BV). The rats in the control group were cut open and then sewn up without removing the testicles after anaesthetization with zoletil® 50 (i.p., 20 mg/kg); rats in the other groups were castrated. After three days recovery, BPH-induced groups were injected testosterone propionate (10 mg/kg/day) alone or along with BV or finasteride daily for four weeks. Twenty-four h after the last administration, all rats were sacrificed after anaesthetization with zoletil® 50 (i.p.,20 mg/kg). Blood samples were drawn from the caudal vena cava and serum was separated by centrifugation and stored at −80℃. The entire prostate gland was removed and weighed.13
Serum concentrations of dihydrotestosterone analysis
The serum concentrations of DHT were determined with commercial ELISA kits (ALPCO Diagnostics, Salem, NH, USA). The assays were performed according to the manufacturer’s instructions.
Histological analysis
The prostatic tissue in each group was fixed in 4% formalin and embedded in paraffin, and the tissue was then cut into 4-µm sections. The sections were stained with hematoxylin and eosin (H&E) for the histological examination. Images were acquired with a SZX10 microscope (Olympus Corporation, Tokyo, Japan). The thickness of epithelium tissue from prostate (TETP) was measured using Leica Application Suite (LAS, ver.3.3.0) software for histological analysis.
Quantitative real-time polymerase chain reaction analysis
The prostatic tissue from each animal was homogenized, and the total RNA was isolated with Easy-Blue® Reagent (iNtRON Biotechnology, Inc., Gyeonggi-do, Korea) according to the manufacturer’s instructions. Total RNA was quantified with an Epoch® microvolume spectrophotometer system (BioTek Instruments, Inc. Winooski, VT, USA). Total RNA from the prostate was converted to cDNA with a high-capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY, USA). Polymerase chain reaction amplification was performed with the incorporation of SYBR green (Life Technologies). The oligonucleotide primers for 5α-reductase 2 that were designed from rat were ATG GGG ACC CTG ATC CTG TG (forward) and CGA CAC CAC AAA GGA AGG CA (reverse) and for GAPDH that were used as a housekeeping gene and that were designed from rat were TGA TTC TAC CCA CGG CAA GT (forward) and AGC ATC ACC CCA TTT GAT GT (reverse). Reverse transcription was conducted with a thermo cycler (Gene Amp® PCR system 9700, Life Technologies), and the results were expressed as the ratio of optimal density to GAPDH.
Western blot analysis
The prostatic tissue from each animal was homogenized in a commercial lysis buffer PRO-PREP® (iNtRON Biotechnology, Inc.) and incubated for 25 min on ice to induce cell lysis. Tissue extracts were centrifuged at 13,000 rpm (4℃) for 20 min, and the supernatant was transferred to a clean tube. Aliquots of each protein sample (30 µg) were separated on a 8–12% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane. The membranes were incubated 1 h with blocking solution and then incubated with a 1:1000 dilution of primary antibodies, including anti-iNOS, anti-COX-2, anti-PARP-1, anti-caspase-3, anti-Bcl-xL, anti-Bax, and anti-β-actin for overnight. The blots were washed three times with Tween-20/Tris-buffered saline, which was followed by incubation with the corresponding secondary antibodies (Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Blots were again washed three times with Tween-20/Tris-buffered saline, and the immunoreactive protein bands were visualized with enhanced chemiluminescence and exposed to X-ray film (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
Statistical analysis
All of the data are expressed as the mean ± standard error of the mean of six rats. The data were analyzed with one-way analysis of variance with Dunnett’s test. The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS, version 19.0; IBM Corporation, Armonk, NY, USA).
Results
Effects of BV on prostate weight in the BPH-induced rat model
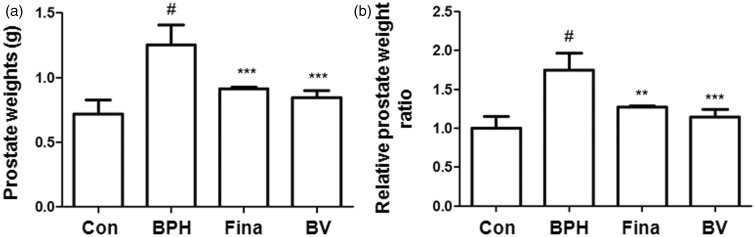
Prostate weights and relative prostate weight ratios are commonly used to evaluate the development of BPH. As shown in Figure 1(a), the prostate weight of rats in the testosterone-induced BPH group significantly increased compared to those in the other groups, which suggested that testosterone successfully induced BPH in the castrated rats. However, the finasteride- and BV-treated groups showed a significant trend for a reduction in the prostate weight (Figure 1a). The relative prostate weight ratio in the BPH-induced group was almost 1.75 times higher than that in the control group. In the finasteride- and BV-treated groups, the prostate weights were 1.27 and 1.14 times higher, respectively, than that of the control group (Figure 1b). In addition, the growth inhibition ratio of prostate in each group is shown in Table 1. In the finasteride- and BV-treated group, the growth inhibition ratio of prostate reduced by almost 64% and 76%, respectively.
Figure 1.
Effect of BV administration on prostate weight in BPH-induced rat models. Changes in prostate total weight (a) and relative prostate weights ratio (b). Con (control group); BPH (BPH-induced group); Fina (BPH-induced group treated with finasteride 5 mg/kg/day; p.o.); BV (BPH-induced group treated with BV 0.1 mg/kg/day; i.p.). The data shown represent mean ± SEM of six rats per group. #p < 0.05 vs. Con group; **p < 0.01, ***p < 0.001 vs. BPH group
Table 1.
Effect of treatments on prostate growth in rats treated with testosterone
| Group | Prostate weight (g) | Inhibition ratio (%) |
|---|---|---|
| Control | 0.72 ± 0.11 | |
| BPH | 1.25 ± 0.15# | |
| Fina | 0.91 ± 0.01*** | 63.98 ± 1.86 |
| BV | 0.82 ± 0.07*** | 75.78 ± 9.38 |
Con, control group; BPH, BPH-induced group; Fina, BPH-induced group treated with finasteride 5 mg/kg/day, p.o.; BV, BPH-induced group treated with BV 0.1 mg/kg/day; i.p..
#p < 0.05 vs. Con group.
p < 0.001 vs. BPH group.
Effects of BV on serum DHT concentrations and 5α-reductase 2 mRNA expression in BPH-induced rats
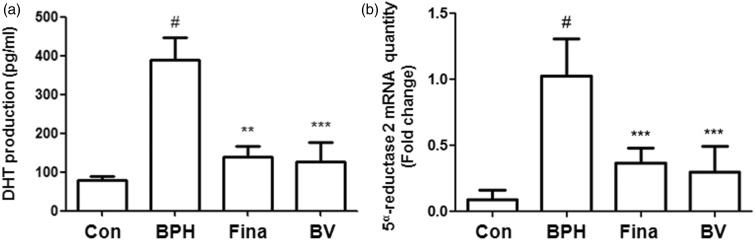
Androgens play a key role in the development and maintenance of normal and aberrant prostatic growth. DHT is synthesized from testosterone under the catalysis of the 5α-reductase isoenzymes. Therefore, it is well known that the inhibition of these isoenzymes is a rational approach for the development of pharmacological therapy for BPH.3 To demonstrate whether BV attenuated the production of DHT, we examined the effects of BV on serum DHT levels in the BPH-induced rats. As shown in Figure 2(a), a significant increase in serum DHT concentration was found in the BPH-induced group compared to the control group. In both the finasteride- and BV-treatment groups; however, serum DHT concentrations were significantly lower than that observed in the BPH-induced group. In addition, to determine if this effect of BV was associated with altered levels of the 5α-reductase 2 protein, we examined the expression levels of 5α-reductase 2 mRNA in prostatic tissue. As shown in Figure 2(b), BV and finasteride treatment significantly reduced 5α-reductase 2 mRNA levels in the prostate tissue of BPH-induced rats.
Figure 2.
Effect of BV administration on the serum DHT production and mRNA level of 5α-reductase 2 in prostate tissues of BPH-induced rat models. (a) Serum concentrations of DHT were determined using ELISA assay. (b) Expression of mRNA for 5α-reductase 2 in prostate tissue was analyzed by quantitative real-time PCR. Con (control group); BPH (BPH-induced group); Fina (BPH-induced group treated with finasteride 5 mg/kg/day; p.o.); BV (BPH-induced group treated with BV 0.1 mg/kg/day; i.p.). The data shown represent mean ± SEM of six rats per group. #p < 0.05 vs. Con group; **p < 0.01, ***p < 0.001 vs. BPH group
Effects of BV on the histological analysis and cell proliferation in BPH-induced rats
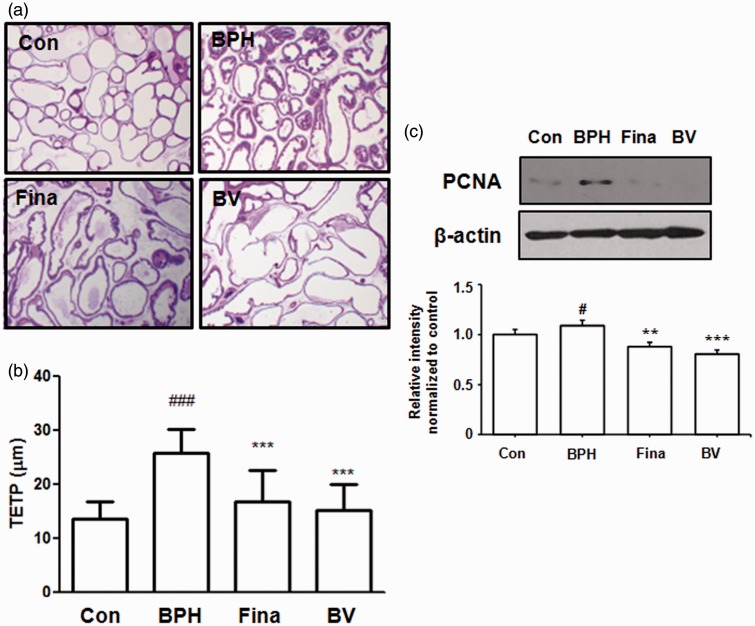
The effects of BV on prostate gland morphology were investigated with prostatic histological examinations (Figure 3). The BPH-induced rats showed the typical histologic changes of glandular hyperplasia with epithelial proliferation, vacuolated cytoplasm pointing into the glandular lumen, and decreased glandular luminal area (Figure 3a). However, finasteride and BV treatment suppressed these typical hyperplastic patterns, which represent histologic changes of normal prostatic tissue into prostatic hyperplasia. The luminal volume was increased, and the grandular epithelial height markedly reduced in these groups. As shown in Figure 3(b), the TETP was significantly higher in the BPH-induced group than in the control group. In the BV-treated group, although TETP was higher than that recorded for the control group, it was significantly lower than that observed in the BPH-induced group, suggesting a marked recovery of prostate hyperplasia.
Figure 3.
Effect of BV administration on the prostatic cell proliferation. Hematoxylin and eosin (H&E) staining of prostatic tissue from BPH-induced rat models (a) and (b) the thickness of epithelium tissue from prostate (TETP); original magnification 40×. (c) The expression level of PCNA protein was determined by western blotting using specific antibodies. β-actin was used as internal controls. Densitometric analysis was performed using Bio-rad Quantity One® Software. Con (control group); BPH (BPH-induced group); Fina (BPH-induced group treated with finasteride 5 mg/kg/day; p.o.); BV (BPH-induced group treated with BV 0.1 mg/kg/day; i.p.). The data shown represent mean ± SEM of six rats per group. #p < 0.05, vs. Con group; **p < 0.01; ***p < 0.001 vs. BPH group. (A color version of this figure is available in the online journal.)
In order to evaluate the effects of BV on the proliferation of prostatic epithelial cells, we examined the protein levels of PCNA, which is a proliferation marker, in the prostatic tissue of BPH-induced rats. As shown in Figure 3(c), a western blot analysis indicated an increase in PCNA protein levels in the BPH-induced group compared to the levels in the control group. Compared to the BPH-induced group, however, the finasteride- and BV-treated groups exhibited less of an increase in the levels of PCNA protein, indicating antiproliferation effects in BPH.
Effects of BV on inflammatory proteins in BPH-induced rats
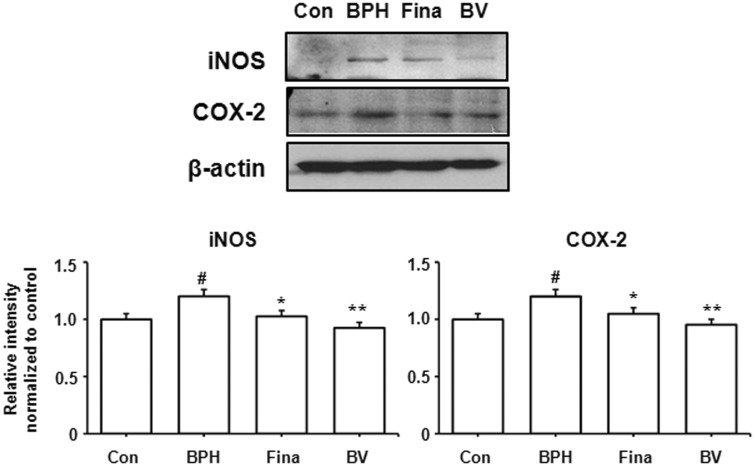
Inflammation plays an important role in the prostatic epithelial cell growth and proliferation processes. In all of the inflammatory cells that arrive in the prostate, iNOS is the principal factor that generates the reactive nitrogen species that can cause cell damage.14 In addition, COX-2 has been detected in all inflammatory cells in the epithelium and interstitial space, and it is upregulated in proliferative inflammatory lesions, generating pro-inflammatory prostaglandins.4 Based on these findings, we examined the protein levels of iNOS and COX-2 in the prostatic tissue of BPH-induced rats in order to investigate the effects of BV on inflammation. As shown in Figure 4, a western blot analysis revealed an increase in iNOS and COX-2 protein levels in the BPH-induced group compared to the control group. Compared to the BPH-induced group, however, the finasteride- and BV-treated groups exhibited less of an increase in the levels of iNOS and COX-2 protein, thus suggesting anti-inflammatory effects in BPH.
Figure 4.
Effect of BV administration on the expression of iNOS and COX-2 in prostate tissues of BPH-induced rat models. The expression levels of iNOS proteins and COX-2 proteins were determined by western blotting using specific antibodies. β-actin was used as internal controls. Densitometric analysis was performed using Bio-rad Quantity One® Software. Con (control group); BPH (BPH-induced group); Fina (BPH-induced group treated with finasteride 5 mg/kg/day; p.o.); BV (BPH-induced group treated with BV 0.1 mg/kg/day; i.p.). The data shown represent mean ± SEM of six rats per group. #p < 0.05 vs. Con group; *p < 0.05, **p < 0.01 vs. BPH group
Effects of BV on apoptosis in BPH-induced rats
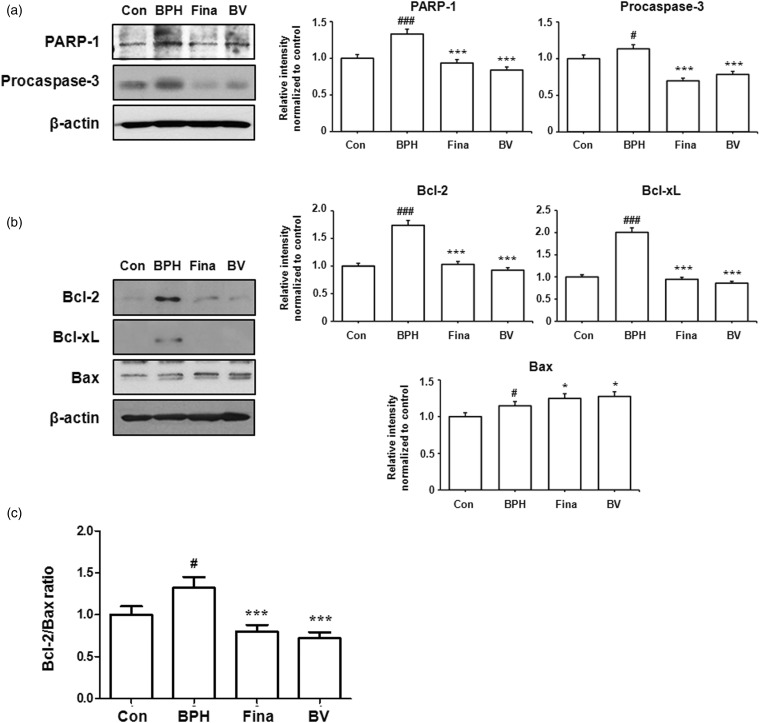
BPH arises from an imbalance between cell proliferation and cell death. Apoptosis refers to the death of cells, which occurs as a normal and controlled part of the growth of an organism. The activation of aspartate-specific cysteine proteases, which are also known as caspases, are an important biochemical event in apoptosis.15 As shown in Figure 5(a), the BV-treated group showed decreased protein levels of procaspase-3 and PARP-1 (an endogenous substrate of caspase-3) compared to the BPH-induced group.
Figure 5.
Effect of BV administration on the expression of apoptosis-related proteins in prostate tissues of BPH-induced rat models. (a) The expression levels of PARP-1 and procaspase-3 proteins were determined by western blotting using specific antibodies. (b) The expression levels of Bcl-2 family proteins were determined by western blotting using specific antibodies. β-actin was used as internal controls.(c) The densitometric analysis of Bcl-2 and Bax bands was performed, and the data (relative density normalized β-actin) were plotted as Bcl-2/Bax ratio. Densitometric analysis was performed using Bio-rad Quantity One® Software. Con (control group); BPH (BPH-induced group); Fina (BPH-induced group treated with finasteride 5 mg/kg/day; p.o.); BV (BPH-induced group treated with BV 0.1 mg/kg/day; i.p.). The data shown represent mean ± SEM of six rats per group. #p < 0.05, ###p < 0.001 vs. Con group; *p < 0.05, ***p < 0.001 vs. BPH group
Disturbances in apoptosis have been linked to a number of human medical diseases and conditions, including BPH.2 The Bcl-2 family plays a key role in the regulation of apoptosis through their ability to alter mitochondria membrane permeability.16 In order to determine whether BV induced apoptosis by modulating the expression of Bcl-2 proteins in the prostatic tissue of the BPH-induced animals, the levels of these proteins were examined by western blot analyses. As shown in Figure 5(b), the BV-treated group showed decreased protein levels of the antiapoptotic proteins Bcl-2 and Bcl-xL but increased expression of proapoptotic Bax compared to the BPH-induced group. Thus, the ratio of Bcl-2 to Bax significantly decreased after treatment with BV, suggesting involvement of the Bcl-2 family of proteins, which are an important mediator of the apoptosis pathway, in BV-induced apoptosis during BPH (Figure 5c).
These findings suggested that BV-induced apoptosis played a role in the effects of this compound on BPH and that this was mediated by the regulation of the expression of anti- and pro-apoptotic proteins.
Discussion
Despite the progress in its diagnosis and treatment, BPH remains the main prostate pathology and the most prevalent urologic health concern affecting men during their lifetime.17 BPH involves a growth of both epithelial and stromal cells from both the transition zone and periurethral areas. Like most chronic diseases, BPH is progressive. If left untreated, it often develops urine retention, nocturia, poor stream, urgency and frequency.18 To date, there are multiple theories on the cellular and molecular processes underlying the pathogenesis of BPH that leads to symptomatic disease. It is well known that androgens play a key role, but there are other pathways that may influence BPH and the associated voiding symptoms. In several reports, epidemiologic, histopathologic, and molecular pathologic studies have suggested that chronic inflammation may be involved in the development and progression of BPH.19
Nitric oxide and COX activity may play an important role in determining the association between inflammation and prostate growth. In prostatic inflammatory cells, iNOS is the principal factor that activates the reactive nitrogen species that can damage cells. Nitric oxide also enhances COX activity, which is the second factor. COX-2 activity has been detected in all inflammatory cells in the epithelium and interstitial spaces of human prostate tissue, and it is increased in proliferative inflammatory lesions, generating pro-inflammatory prostaglandins. In the present study, testosterone was injected into the animals for 4 weeks to induce BPH, and the levels of protein expression of iNOS and COX-2 were analyzed by western blot analyses. The levels of iNOS and COX-2 protein increased in the BPH-induced group compared to the levels in the control group. Compared to the BPH-induced group, however, the group treated with BV (0.1 mg/kg i.p.) did not exhibit increased levels (Figure 4). Therefore, these data indicated that the anti-BPH effects of BV might be related to its anti-inflammatory properties.
COX-2 inhibition can produce a significant increase in prostate cell apoptotic activity.20 The effect of COX-2 on prostate cell proliferation may be obtained either by modulating the inflammatory process or by directly upregulating Bcl-2 expression in order to decrease apoptosis.21 Bcl-2 proteins are defined both by their structure and function.8 The pro-apoptotic members (e.g. Bax) and anti- apoptotic members (e.g. Bcl-2 and Bcl-xL) may control apoptosis by the formation of heterodimers among them, which results in the mutual neutralization of bound pro- and anti-apoptotic proteins. The decision to initiate apoptosis is governed by the equilibrium between Bcl-2 and Bax, and drugs that stabilize the equilibrium can sustain cell survival, whereas interventions that shift the equilibrium toward free Bax promote the induction of apoptosis.22 In many systems, members of the Bcl-2 family modulate apoptosis with the Bcl-2/Bax ratio serving as a rheostat to determine cell susceptibility to apoptosis.23 In this study, we showed that BV markedly reduced the levels of antiapoptotic Bcl-2 protein levels and increased the levels of Bax protein in the rat BPH model. These data indicated that BPH was suppressed by an antiapoptotic mechanism that involved a decrease in the ratio of Bcl-2 to Bax in the BV-treated group. Moreover, caspase-3 activation by cell death signals may occur through three routes: the mitochondria (related to Bcl-2 and Bax), the endoplasmic reticulum, or death receptors (FAS, FAS-L).24 By suppressing the overexpression of Bcl-2 and the downregulation of Bax, BV modulated the Bcl-2/Bax equilibrium to induce the expression of caspase-3 in BPH-induced rats. These data further showed that BV promoted apoptosis probably through the mitochondrial pathway.
Hormonal imbalance has been proposed to trigger intraprostatic proliferation through inflammatory responses and reductions of apoptosis.4 In this study, the prostate weight ratio in the BPH-induced group increased, and this was accompanied by increased DHT and 5α-reductase 2 mRNA levels compared to the control group, indicating that testosterone successfully established BPH. However, BV treatment for 4 weeks was useful in the prevention of BPH, and these effects were comparable to finasteride. Considering all of these data, the preventive effects of BV were likely due to regulation of the inflammatory responses and the induction of apoptosis. Thus, BV is a potential therapeutic agent for the treatment of BPH.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1004790).
Authors' contributions
K-SC and H-JA carried out the study design, data collection and analysis, and draft of the manuscript. S-YC and K-RK performed the experiment. KHL was responsible for the study design, the funding, the data analysis, and the manuscript draft. All authors read and approved the final manuscript.
References
- 1.McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care 2006; 12: S122–8. [PubMed] [Google Scholar]
- 2.Sciarra A, Di Silverio F, Salciccia S, Autran Gomez AM, Gentilucci A, Gentile V. Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol 2007; 52: 964–72. [DOI] [PubMed] [Google Scholar]
- 3.Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab 2008; 22: 389–402. [DOI] [PubMed] [Google Scholar]
- 4.Sciarra A, Mariotti G, Salciccia S, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol 2008; 108: 254–60. [DOI] [PubMed] [Google Scholar]
- 5.Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, Maille P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate 2009; 69: 1774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yuan L, Xiong C, Yin C, Ruan J. Abacopteris penangiana exerts testosterone-induced benign prostatic hyperplasia protective effect through regulating inflammatory responses, reducing oxidative stress and anti-proliferative. J Ethnopharmacol 2014; 157: 105–113. [DOI] [PubMed] [Google Scholar]
- 7.Cardillo M, Berchem G, Tarkington MA, Krajewski S, Krajewski M, Reed JC, Tehan T, Ortega L, Lage J, Gelmann EP. Resistance to apoptosis and up regulation of Bcl-2 in benign prostatic hyperplasia after androgen deprivation. J Urol 1997; 158: 212–6. [DOI] [PubMed] [Google Scholar]
- 8.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 2004; 1644: 83–94. [DOI] [PubMed] [Google Scholar]
- 9.Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther 2007; 115: 246–70. [DOI] [PubMed] [Google Scholar]
- 10.Hider RC. Honeybee venom: a rich source of pharmacologically active peptides. Endeavour 1988; 12: 60–5. [DOI] [PubMed] [Google Scholar]
- 11.Park MH, Choi MS, Kwak DH, Oh KW, Yoon do Y, Han SB, Song HS, Song MJ, Hong JT. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate 2011; 71: 801–12. [DOI] [PubMed] [Google Scholar]
- 12.Russell PJ, Hewish D, Carter T, Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira D, Molloy PL, Werkmeister JA, Kortt AA. Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivo studies. Cancer Immunol Immunother 2004; 53: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo QL, Ding QL, Wu ZQ. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol Pharm Bull 2004; 27: 333–7. [DOI] [PubMed] [Google Scholar]
- 14.Baltaci S, Orhan D, Gogus C, Turkolmez K, Tulunay O, Gogus O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int 2001; 88: 100–3. [DOI] [PubMed] [Google Scholar]
- 15.Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 2005; 258: 479–517. [DOI] [PubMed] [Google Scholar]
- 16.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–56. [DOI] [PubMed] [Google Scholar]
- 17.Elkahwaji JE. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res Rep Urol 2012; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notara M, Ahmed A. Benign prostate hyperplasia and stem cells: a new therapeutic opportunity. Cell Biol Toxicol 2012; 28: 435–42. [DOI] [PubMed] [Google Scholar]
- 19.Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol 2011; 13: 147–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, Sciarra A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol 2003; 43: 164–75. [DOI] [PubMed] [Google Scholar]
- 21.Di Silverio F, Bosman C, Salvatori M, Albanesi L, Proietti Pannunzi L, Ciccariello M, Cardi A, Salvatori G, Sciarra A. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Eur Urol 2005; 47: 72–8. [DOI] [PubMed] [Google Scholar]
- 22.Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem 2004; 90: 1281–9. [DOI] [PubMed] [Google Scholar]
- 23.Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T, Dougenis D. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 2007; 21: 123–32. [PubMed] [Google Scholar]
- 24.Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke 2003; 34: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]