Abstract
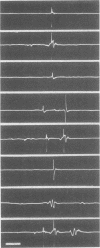
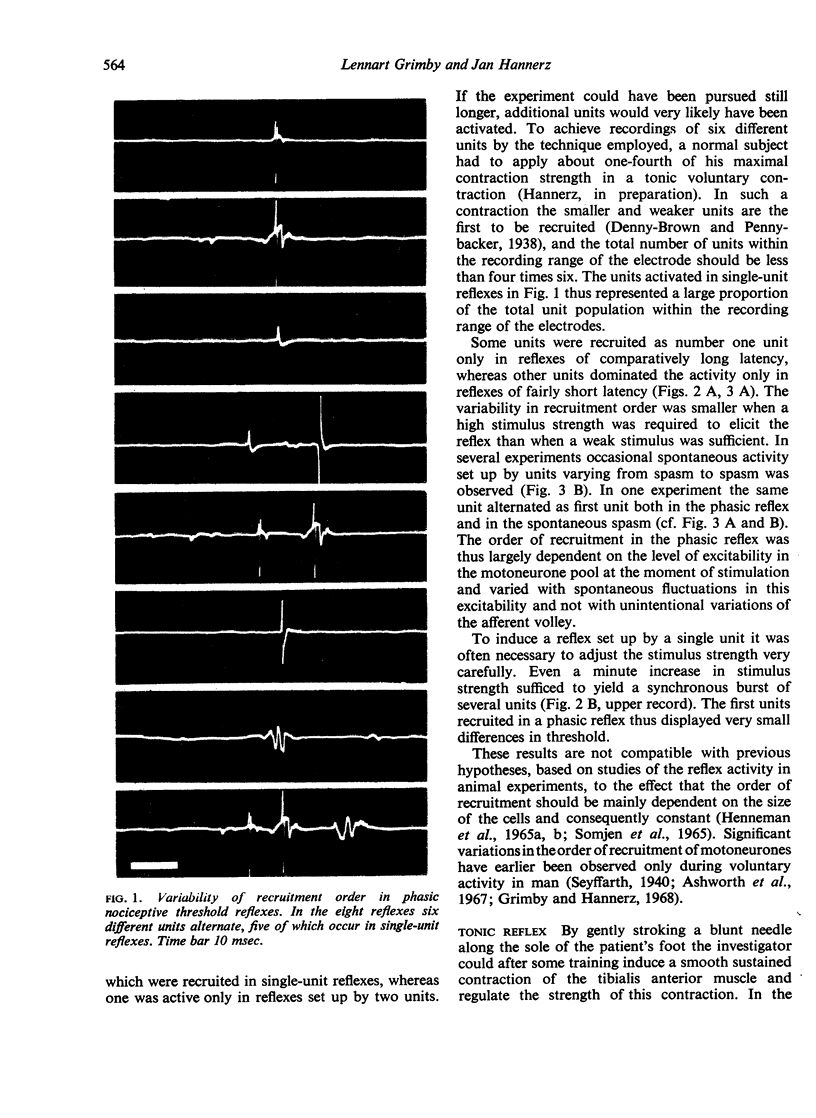
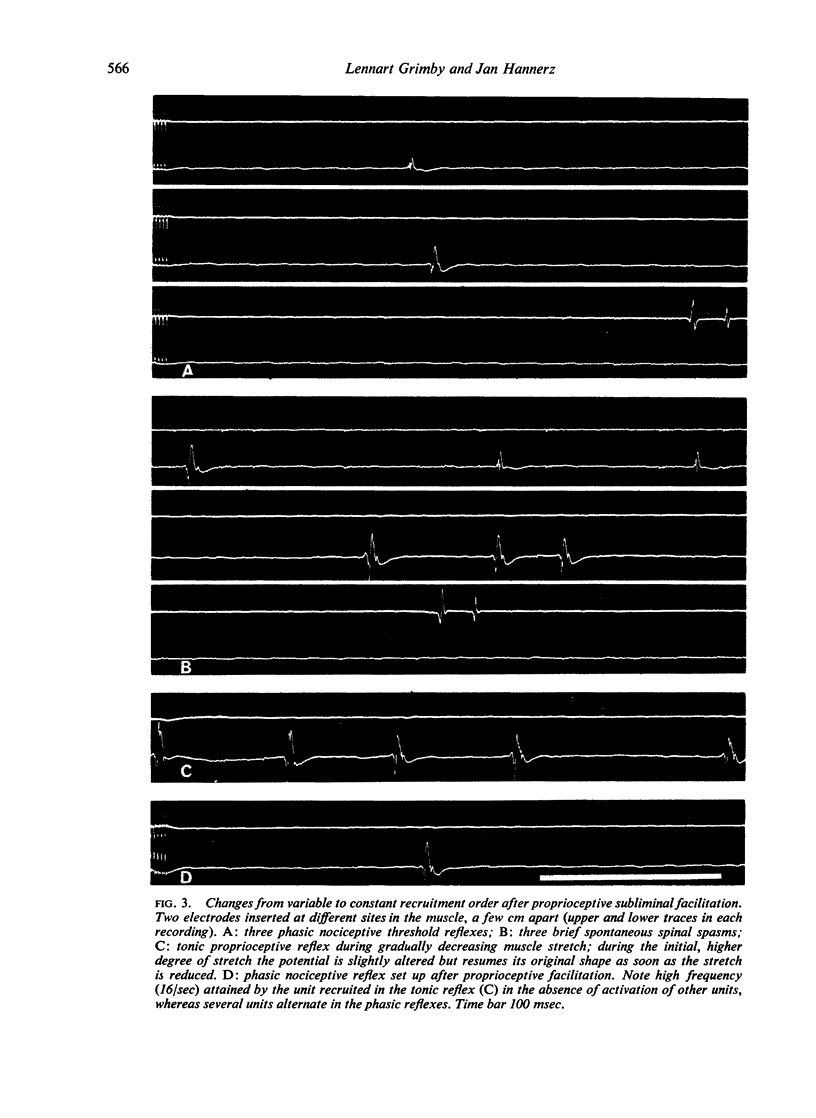
The recruitment order of motoneurones in muscle contractions has been held to be largely constant and determined by the size of the cell. However, as shown in a previous investigation using electromyographic techniques, the order in which different motor units are activated during voluntary muscle contractions changes in normal human subjects on shifts from phasic to tonic contraction. In order to investigate these two types of activity also in cases in which the cerebral influence on the motoneurone pool is blocked, an analysis was made of the recruitment order in phasic and tonic flexion reflexes in 10 patients with total interruption of the spinal cord. The following four principles were found to apply and presumed to be generally valid for the isolated human spinal cord: (1) in the phasic exteroceptive reflex, the order of recruitment varies despite application of a standardized stimulus; (2) in the tonic reflex, the first unit to be recruited is usually the same even with widely different types of stimuli; (3) a shift from phasic to tonic reflex activation may result in considerable changes in recruitment order; (4) after facilitation by a subliminal long-lasting stimulus, the first unit to be recruited in the phasic reflex is also the first to be recruited in the tonic reflex. It is suggested that a tonic influence on the motoneurone pool is required for the presupposed constancy of the recruitment order.
Full text
PDF




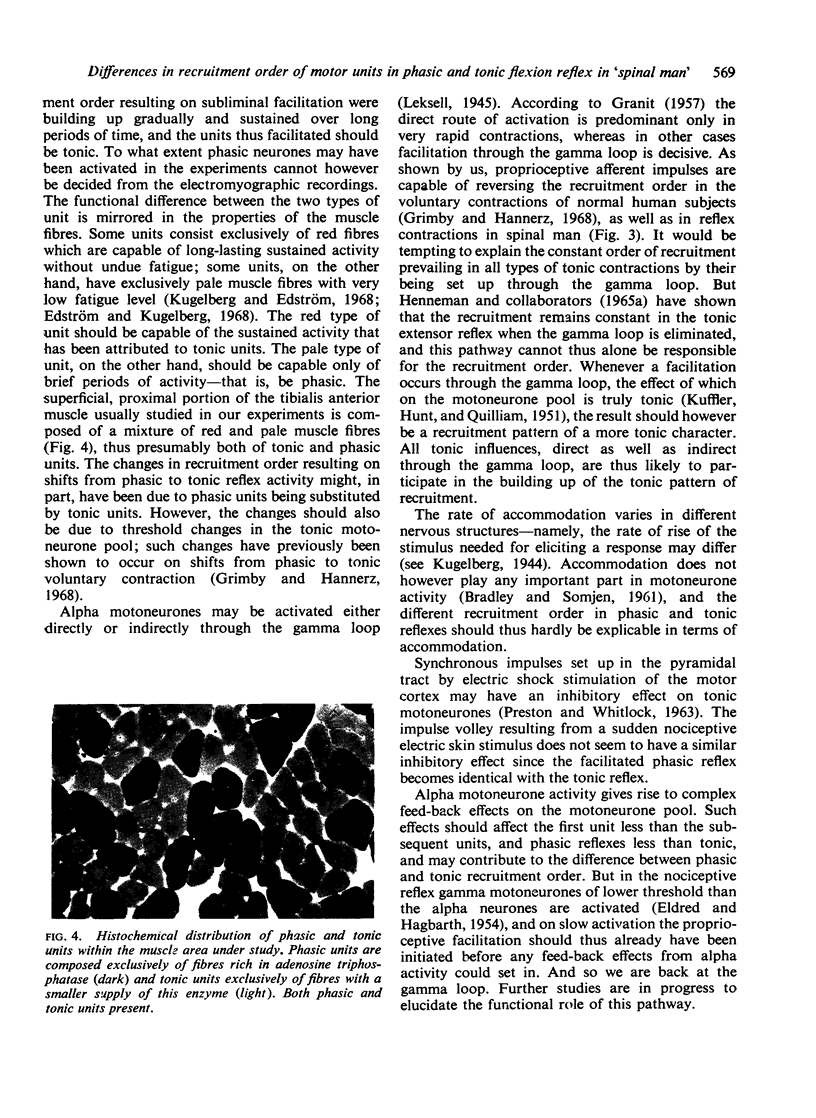

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth B., Grimby L., Kugelberg E. Comparison of voluntary and reflex activation of motor units. Functional organization of motor neurones. J Neurol Neurosurg Psychiatry. 1967 Apr;30(2):91–98. doi: 10.1136/jnnp.30.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K., Somjen G. G. Accommodation in motoneurones of the rat and the cat. J Physiol. 1961 Apr;156(1):75–92. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol. 1968 Jun;196(3):631–654. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., HAGBARTH K. E. Facilitation and inhibition of gamma efferents by stimulation of certain skin areas. J Neurophysiol. 1954 Jan;17(1):59–65. doi: 10.1152/jn.1954.17.1.59. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- Grimby L., Hannerz J. Recruitment order of motor units on voluntary contraction: changes induced by proprioceptive afferent activity. J Neurol Neurosurg Psychiatry. 1968 Dec;31(6):565–573. doi: 10.1136/jnnp.31.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTON J. B., WHITLOCK D. G. A comparison of motor cortex effects on slow and fast muscle innervations in the monkey. Exp Neurol. 1963 Apr;7:327–341. doi: 10.1016/0014-4886(63)90079-7. [DOI] [PubMed] [Google Scholar]
- SASAKI K., TANAKA T. PHASIC AND TONIC INNERVATION OF SPINAL ALPHA MOTONEURONS FROM UPPER BRAIN CENTERS. Jpn J Physiol. 1964 Feb 15;14:56–66. doi: 10.2170/jjphysiol.14.56. [DOI] [PubMed] [Google Scholar]
- Somjen G., Carpenter D. O., Henneman E. Responses of motoneurons of different sizes to graded stimulation of supraspinal centers of the brain. J Neurophysiol. 1965 Sep;28(5):958–965. doi: 10.1152/jn.1965.28.5.958. [DOI] [PubMed] [Google Scholar]