Abstract
Cisplatin, Cis-diamminedichloroplatinum (CDDP), is a platinum-based chemotherapy drug, and its chemotherapeutic use is restricted by nephrotoxicity. Inflammatory and apoptotic mechanisms play a central role in the pathogenesis of CDDP-induced acute kidney injury (AKI). The aim of this study was to compare the therapeutic potential of candesartan, angiotensin II receptor blocker, versus bone marrow-derived mesenchymal stem cells (BM-MSCs) in a rat model of CDDP-induced nephrotoxicity. Adult male Wistar rats (n = 40) were divided into four groups; Normal control: received saline injection, CDPP group: received CDDP injection (6 mg/kg single dose), Candesartan group: received candesartan (10 mg/kg/day) for 10 days + CDDP at day 3, and Stem cells group: received CDDP + BM-MSCs intravenously one day after CDDP injection. The rats were sacrificed seven days after CDDP injection. Significant elevation in serum creatinine and urea, renal levels of tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1, renal expressions of nuclear factor kappa B (NF-κB), p38-mitogen-activated protein kinase (MAPK), caspase-3 and Bcl-2-associated x protein (Bax) were found in CDDP-injected rats when compared to normal rats. Both candesartan and BM-MSCs ameliorated renal function and reduced significantly the inflammatory markers (TNF-α , NF-κB, p38-MAPK and MCP-1) and apoptotic markers (caspase-3 and Bax) in renal tissue after CDDP injection. Candesartan as well as BM-MSCs have anti-inflammatory and anti-apoptotic actions and they can be used as nephroprotective agents against CDDP-induced nephrotoxicity. BM-MSCs is more effective than candesartan in amelioration of AKI induced by CDDP.
Keywords: Angiotensin receptor blocker, stem cells, renal injury, inflammation, apoptosis
Introduction
Cisplatin or Cis-diamminedichloroplatinum (CDDP) is an inorganic platinum-based antineoplastic drug used to treat solid tumors.1 However, its effectiveness is restricted by its nephrotoxicity. Administration of high-dose CDDP can cause acute kidney injury (AKI) in about 30% of patients who show various degrees of renal dysfunction.2 The mechanism for CDDP-induced AKI is not well understood but appears to involve renal tubular epithelial cells inflammation, apoptosis and oxidative stress.1
Angiotensin II, the principal effector of the renin–angiotensin system, has been reported to have a crucial role in the pathogenesis of renal injuries.3 A few studies have suggested that angiotensin receptor blockers (ARBs) may exert a protective role toward drug-induced nephrotoxicity.4,5 To our knowledge, there was no previous study to explore the role of candesartan in CDDP-induced nephrotoxicity.
In addition, stem cell therapy provides a hopeful prospective for injured tissues and organs repair.6 The therapeutic effect of mesenchymal stem cells (MSCs) was questioned in different animal models of renal disease, including CDDP-induced AKI.7,8 Among the different types of stem cells, bone marrow-derived MSCs (BM-MSCs) have gained great popularity. The proposed mechanism of MSCs action could either be directly by replacing the damaged cells or indirectly through induction of cell regeneration8 leading to improvement in renal function and structure.9 However, the mechanism of MSCs against CDDP-induced AKI remains to be elucidated and explained.
The aim of our study was to compare the protective effects of angiotensin II receptor blocker, candesartan versus BM-MSCs against CDDP induced AKI and to emphasize the role of inflammatory and apoptotic markers in monitoring these therapeutic regimens.
Materials and methods
Animals
Forty male Wistar rats weighing 200–250 g were housed in an environmentally controlled room temperature (25 ± 2 ℃) with a 12-h light/dark cycle and given standard chow and water ad libitum. This experimental procedure was approved by the ethical committee in Mansoura Experimental Research Center, Mansoura University.
Harvesting and culturing of MSCs
Bone marrow was obtained from the femurs and tibia of adult Wistar rats10 weighing 180–210 g and cultured in basic media Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO, USA). The effluent was collected in sterile tubes. Gentle pipetting resulted in obtaining of a single cell suspension. Bone marrow cells were counted and plated with a concentration of 10 × 106/mL in T-75 flasks. The cells were then cultured in DMEM containing 10% FBS, penicillin (100 U/mL) and streptomycin (100 µg/mL) at 37 ℃ in a humidified atmosphere that contained 5% CO2. The medium was changed after four days and every three days thereafter. Non-adherent hematopoietic cells were removed when the medium was changed. After a mean of seven days, cells reached subconfluence and was detached with trypsin/EDTA, reseeded at 4 × 103 cells/cm2, and used for transplantation after the third passage. MSCs' features were demonstrated by typical spindle-shaped morphology.
Flow cytometric analysis of cluster of differentiation (CD) markers
The cells were centrifuged at 1200 rpm for 5 min and then solved in phosphate buffered saline (PBS) at the concentration of 1 × 106/mL). The cells were stained with different fluorescently labeled monoclonal antibodies (mAb). In brief, 100 μL of cell suspension was mixed with 10 μL of the fluorescently labeled mAb and incubated in the dark at room temperature for 30 min. Washing with PBS containing 2% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA) was done twice and the pellet was resuspended in PBS and analyzed immediately on flow cytometry. The monoclonal antibodies used were fluorescein isothiocyanate (FITC)-conjugated CD45 and phycoerythrin-cyanine-5 (PeCY5)-conjugated CD90 (Serotec, Oxford, UK). The immunophenotyping was performed on EPICS-XL flow cytometry (Coulter, Miami, Florida). The cells were analyzed with the most appropriate gate FBS using the combination of forward and side scatters. The viability of stem cells was checked by Trypan blue exclusion according to the method of Maclimans et al.11
Experimental design
Four groups of animals, 10 in each group, were randomly assigned as experimental groups. In the first group, the rats were intraperitoneal (ip) injected with the appropriate volume of normal saline, and this group served as a normal control. In the second group (Cisplatin, CDDP), the rats were ip injected with a single dose (6 mg/kg) of CDDP (Hospira Co, Warwickshire, UK).12 In the third group (Candesartan), the rats were supplemented with 10 mg/kg candesartan (AstraZeneca AB, Södertälje, Sweden)13 for 10 days plus a single dose of CDDP (6 mg/kg) ip at day 3,14 while in the fourth group (stem cells), the rats received the same ip single dose of CDDP and was administered BM-MSCs (2 × 106 cells) by tail vein injection15 24 h after the CDDP injection.
Sample collection
All animals were sacrificed on day 7 after CDDP injection. Blood samples were collected and serum was separated by centrifugation at 3000 rpm for 10 min for the determination of renal function while, kidneys were removed rapidly, one stored in 10% neutral-buffered formalin for immunohistochemistry, while the other kidney frozen immediately in liquid nitrogen and transferred to a –80 ℃ for real time-PCR technique and preparation of renal homogenate.
Preparation of renal homogenate
Each frozen renal sample was homogenized in PBS solution (pH 7.4) using a Teflon homogenizer and then centrifuged for 10 min at 4 ℃ to remove debris. The supernatant was divided into portions, deeply frozen at −80 ℃ for enzyme linked immunosorbent assay (ELISA) technique.
Determination of serum creatinine and urea
Serum levels of creatinine and urea were assayed enzymatically using commercially available kits provided by Bio-Diagnostic Company, Giza, Egypt.
Measurement of tumor necrosis factor (TNF)-α and monocyte chemoattractant protein-1 (MCP-1)
Levels of TNF-α and MCP-1 were determined by ELISA technique in renal tissue homogenate using a commercially available ELISA kit (Uscn Life Science Inc., Houston, USA) in accordance with the manufacturer's instructions.
Real-time quantitative analysis for renal nuclear factor kappa B (NF-κB), p38-mitogen activated protein kinase (MAPK), and caspase-3 gene expressions
For RNA extraction, total RNA was isolated from renal tissue homogenates using RNeasy Purification Reagent (Qiagen, Valencia, CA, USA) according to the manufacturer's instruction. Concentration of the RNA was assessed using spectrophotometer and the integrity of the RNA was studied by gel electrophoresis on a 1% agarose gel. cDNA was produced from 2 μg of total RNA samples using Oligo(dT)12-18 and SuperScript Reverse Transcriptase (Life Technologies, Breda, the Netherlands). The primers for real-time PCR were: NF-κB forward primer: 5′-GCTTACGGTGGGATTGCATT-3′; NF-κB reverse primer: 5′-TTATGGTGCCATGGGTGATG-3′; p38-MAPK forward primer: 5′-ACTCAGATGCCGAAGATGAAC-3′; p38-MAPK reverse primer: 5′-GTGCTCAGGACTCCATCTCT-3′; caspase-3 forward primer: ′5-TGTCATCTCGCTCTGGTACG-3′: caspase-3 reverse primer: 5′-AAATGAC-CCCTTCATCACCA-3′; Beta actin forward primer: 5′-TCT GGC ACC ACA CCT TCT-ACA ATG-3′; Beta actin reverse primer: 5′-AGC ACA GCC TGG ATA GCA ACG-3′. Real-time quantitative PCR was done by using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). PCR reactions were performed on step one plus Real Time PCR system (Applied Biosystems). Relative expression of the studied genes was calculated using the comparative threshold cycle method. All values were normalized to the beta actin genes.16
Immunohistochemistry
Samples of kidney tissues were collected, and were fixed in a 4% formaldehyde solution for 24 h. Immunohistochemical analyses were performed on 5 μm thick paraffin sections cut from a paraffin block of kidney. Sections were incubated with monoclonal anti-Bax (Uscn Life Science Inc., Houston, USA) at 4 ℃. Slides were counterstained with hematoxylin and examined under microscope using PC-driven digital camera (Olympus E-620).
Statistical analysis
Data are presented as mean ± SD. One way analysis of variance (ANOVA) followed by Bonferroni multiple tests was used to determine the differences between means of different groups. The level of significance was set at p ≤ 0.05. Statistical computations were done using the computer software SPSS version 20 (Chicago, IL, USA).
Results
Properties of MSCs
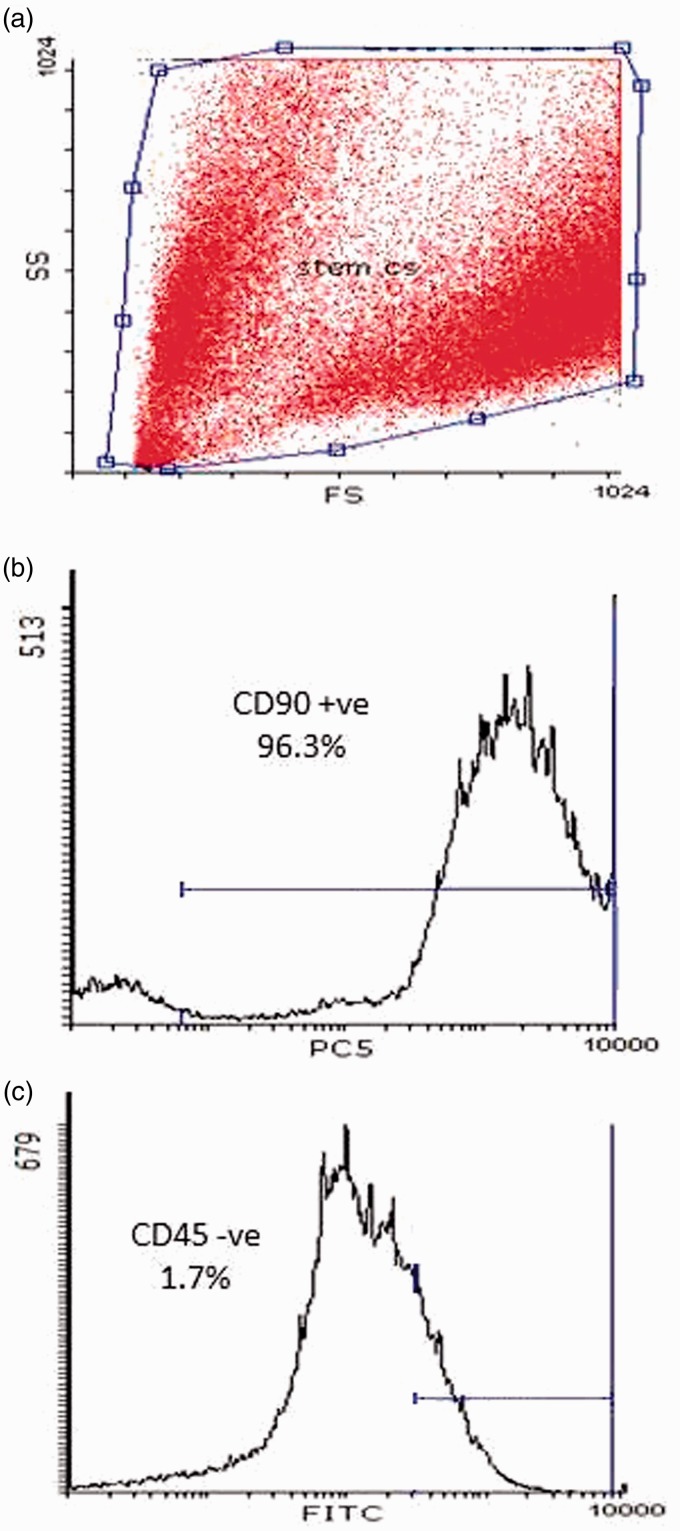
The MSCs were identified by surface marker CD90 (+ve) and CD45 (−ve) detected by flow cytometry as illustrated in Figure 1.
Figure 1.
Characteristics of rat BM-MSCs. Cells were stained with CD90 and CD45 antibody and analyzed by flow cytometry. BM-MSCs are shown as a dot plot (a). The expression levels of CD90 + ve (b) and CD45 –ve (c) of BM-MSCs are presented as a histogram. The percentage of expression of the indicated markers is defined in the figure. (A color version of this figure is available in the online journal.)
Effect of candesartan and stem cells on serum creatinine and urea levels
Injection with cisplatin in animals induced AKI as indicated by significant increase in serum creatinine and urea when compared with the normal control rats (p < 0.05). Both candesartan-treated rats and rats receiving BM-MSCs showed a significant reduction in levels of serum creatinine as well as urea in comparison to cisplatin-treated rats (p < 0.05) (Table 1). More reduction was observed in BM-MSCs group than Candesartan group but the difference was not statistically significant.
Table 1.
Effect of candesartan and stem cells on renal function tests of cisplatin-treated rats
| Group | Normal | Cisplatin | Candesartan | Stem Cells |
|---|---|---|---|---|
| Creatinine (mg/dL) | .4 ± .03 | 1.72 ± .05* | .87 ± .02† | .6 ± .01† |
| BUN (mg/dL) | 28.31 ± .44 | 79.76 ± .75* | 47.27 ± .41† | 44.15 ± 1.07† |
Note: All results were expressed as Mean ± SD.
Significant difference from Normal group at P < 0.05.
Significant difference from Cisplatin group at P < 0.05.
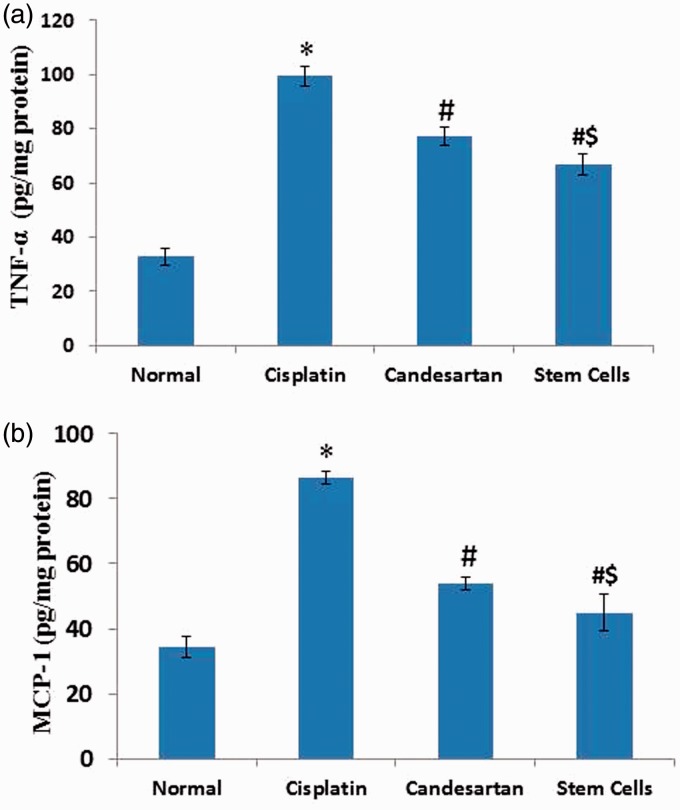
Effect of candesartan and stem cells on renal levels of tumor necrosis factor (TNF)-α and monocyte chemoattractant protein-1 (MCP-1)
Figure 2 showed a significant increase in renal levels of TNF-α by three fold (Figure 2a) and MCP-1 by 2.5 fold (Figure 2b) in cisplatin-treated rats in comparison with normal control rats. However, a significant decrease in renal levels of TNF-α and MCP-1 were observed in candesartan and MSCs groups in comparison with cisplatin-treated rats (p < 0.05). Moreover, BM-MSCs injection showed a more significant reduction in renal levels of TNF-α and MCP-1 than candesartan administration.
Figure 2.
Effect of cisplatin alone and its combination with candesartan or stem cells on renal levels of tumor necrosis factor (TNF)-α (a) and monocyte chemoattractant protein (MCP)-1 (b). *Significant difference as compared with normal group at P < 0.05. #Significant difference as compared with the cisplatin group at P < 0.05. $Significant difference as compared with the Candesartan group at P < 0.05. (A color version of this figure is available in the online journal.)
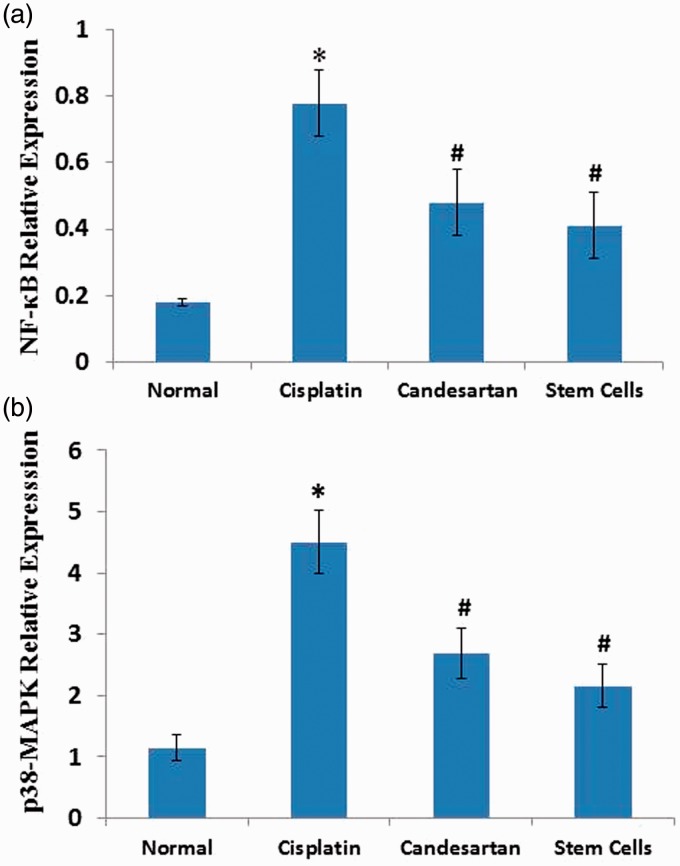
Effect of candesartan and stem cells on m-RNA gene expressions of nuclear factor kappa-B (NF-κB) and p38-mitogen activated protein kinase (MAPK) in renal tissue
Treatment with cisplatin presented a significant elevation (p < 0.05) in renal mRNA expressions of NF-κB (Figure 3a) and p38 MAPK (Figure 3b) as compared with the normal control. Administration of candesartan and MSCs injection exerted a significant reduction in renal gene expressions of NF-κB and p38 MAPK (p < 0.05) as compared with cisplatin-treated rats. More reduction was observed in BM-MSCs group than in the Candesartan group, but the difference was not statistically significant.
Figure 3.
Effect of cisplatin alone and its combination with candesartan or stem cells on renal gene expressions of nuclear factor (NF)-κB (a) and p38- mitogen-activated protein kinase (MAPK) (b). *Significant difference as compared with normal group at P < 0.05. #Significant difference as compared with the Cisplatin group at P < 0.05. (A color version of this figure is available in the online journal.)
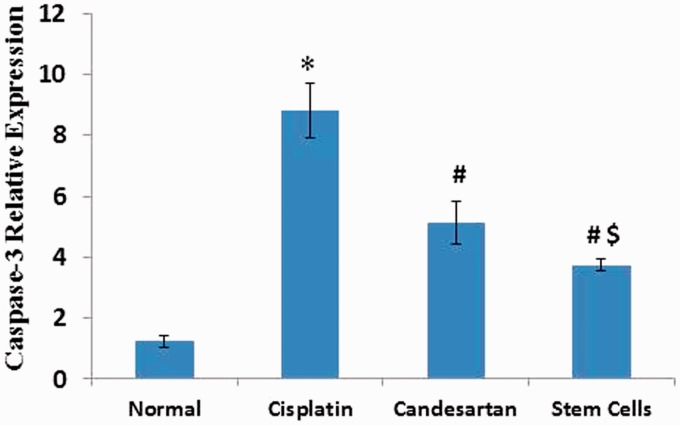
Effect of candesartan and stem cells on m-RNA gene expression of caspase-3
Figure 4 illustrated that administration of cisplatin resulted in a significant rise in renal m-RNA gene expression of caspase-3 when compared to the normal control group. Moreover, our investigation demonstrated a significant decline of renal gene expression of caspase-3 not only after candesartan treatment but also after MSCs injection when compared to rats injected with cisplatin. More significant reduction was observed in the BM-MSCs group than the Candesartan group.
Figure 4.
Effect of Cisplatin alone and its combination with candesartan or stem cells on renal gene expressions of caspase-3. *Significant difference as compared with normal group at P < 0.05. #Significant difference as compared with the cisplatin group at P < 0.05. $Significant difference as compared with the Candesartan group at P < 0.05. (A color version of this figure is available in the online journal.)
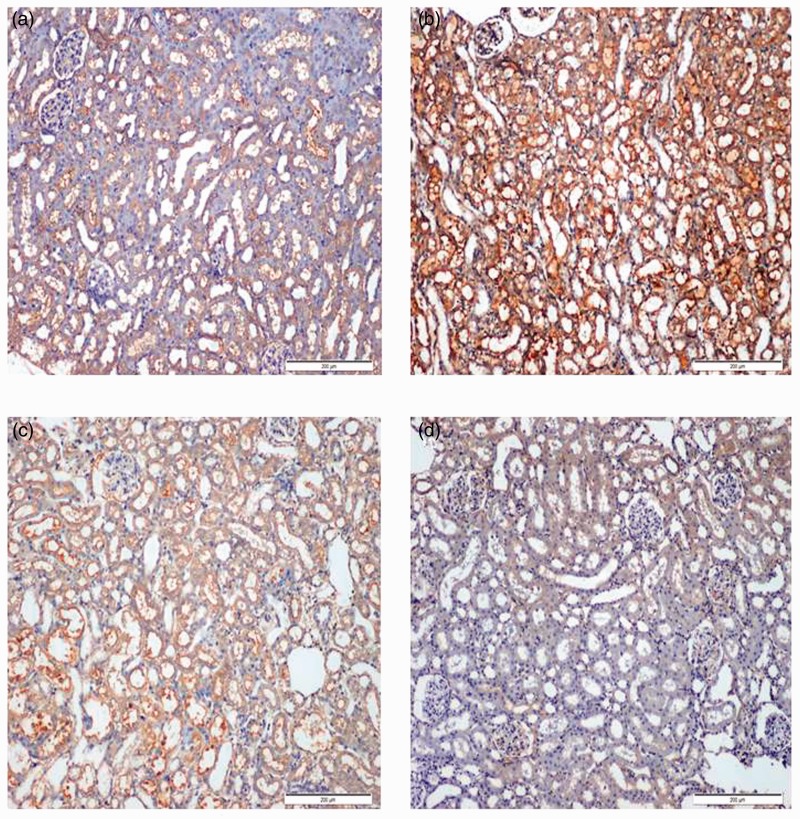
Effect of candesartan and stem cells on expression of Bcl-2 associated x protein (Bax)
Figure 5 showed the immunohistochemical staining of Bax in different studied groups: Bax expression was slightly expressed in the control group (Figure 5a), while a significant increase in Bax expression was noticed in the cisplatin group (Figure 5b). However, significant decrease in Bax expression was determined in both candesartan (Figure 5c) and BM-MSCs (Figure 5d) groups. More significant reduction in Bax expression was shown in the BM-MSCs group.
Figure 5.
Immunohistochemical staining of Bax in rat kidneys (200×). (a) Normal control rats showing a slight expression of Bax. (b) Significant increase in Bax expression was seen in renal tissue treated with Cisplatin group. (c) Significant decrease in Bax expression was observed in renal tissue of rats treated with Candesartan group. (d) Significant reduction in Bax expression was observed in renal tissue of rats treated with stem cells group. (A color version of this figure is available in the online journal.)
Discussion
Cisplatin is one of the important chemotherapeutic agents used for the treatment of various types of tumors with a major side effect of nephrotoxicity. Nephrotoxicity in experimental animals after CDDP injection was characterized by deterioration of renal function as evidenced by a significant increase in serum creatinine and urea as in line with the previous studies.13,17
The CDDP-induced renal injury has been associated with inflammation, oxidative stress, and apoptosis.18,19 Concerning inflammation, it was reported that TNF-α is a proinflammatory cytokine that acts as an important mediator of CDDP-induced inflammatory tissue damage.20 It is able to induce direct renal injury and causes induction of apoptosis and necrotic cell death.21 TNF-α also stimulates the release of other cytokines and chemokines, such as MCP-1,22 which attracts a variety of cells including leukocytes, monocytes and natural killer cells. Infiltration of damaged renal tissue with inflammatory cells is characteristic for CDDP-induced nephrotoxicity.20 Our experiment showed a significant elevation of renal TNF-α and MCP-1 gene expressions in CDDP group when compared to the normal group and this findings is in accordance with the study of Ueki et al.20 who reported a significant elevation of renal TNF-α and MCP-1 after CDDP injection.
The other cytokine that plays a key role in the inflammation process during nephrotoxicity is NF-κB.23 There is a growing body of evidence indicating that CDDP induces remarkable activation of NF-κB in kidneys with subsequent inflammatory reactions causing kidney damage.17,23 Coinciding with this findings, our results documented upregulation of NF-κB renal expression following CDDP injection. It was reported that NF-κB couples reactive oxygen species (ROS) to regulate CDDP nephrotoxicity, further exacerbating renal tissue damage.24
Moreover, it is postulated that p38-MAPK plays a critical role in producing pro-inflammatory cytokines and controlling cell apoptosis in renal injury.25 Our experiment showed a significant elevation of renal p38-MAPK expression in CDDP-treated rats in comparison with normal rats. CDDP induced activation of p38-MAPK that in turns mediates CDDP-induced renal injury and inflammation.26
Caspases are a family of enzymes that actively participate in apoptosis. The activation of caspase-3, the execution caspase, is involved in initiating apoptotic events.27 On the other hand, Bax is a pro-apoptotic protein that accumulates in mitochondrial membrane and hinders the protective effect of Bcl-2, which is responsible for cell survival.28 Furthermore, it was documented that CDDP-induced renal injury is mainly due to apoptosis of renal tubular cells.29 Our experiment showed a significant elevation of renal caspase-3 and bax expressions in CDDP-treated rats in comparison with normal rats which coincides with previous studies.23,30,31
To our knowledge, this is the first study to examine the effect of candesartan in CDDP-induced nephrotoxicity. Our results demonstrated that both candesartan and BM-MSCs reduced the levels of serum creatinine and urea in CDDP-treated animals. Losartan, the other member in the ARB group, showed a nephroprotective effect as evidenced by normalization of serum levels of creatinine and BUN in CDDP-treated rats when compared to the normal ones.14 Previous studies reported that losartan prevented nephrotoxicity caused by administration of a single dose of CDDP in male rats.13,14 On the contrary, a recent study of Rastghalam et al.32 showed that losartan could not improve renal dysfunction induced by continuous doses of CDDP as it increases renal blood flow which may lead to the accumulation of more cisplatin in the kidneys worsening the renal failure.
In addition, BM-MSCs have been reported to maintain renal function in several AKI models including gentamicin33 and cisplatinum9,27 induced nephrotoxicity.
Previous studies demonstrated that amelioration of CDDP nephrotoxicity can be achieved by inhibition of TNF-α production and NF-κB activation.20,23 Therefore, the modulation of renal inflammatory reactions and apoptotic pathways after CDDP injection may help to prevent CDDP-induced nephrotoxicity.20
Our study reported that candesartan reduced the renal TNF-α and MCP-1 levels and renal expression of NF-κB in CDDP-treated rats when compared to the normal rats. Conceding with our findings, candesartan suppressed the renal MCP-1 and NF-κB in a model of chronic kidney disease.34 Chen et al.35 showed the anti-inflammatory effects of candesartan through a direct antioxidant action independent of ARB. They postulated that candesartan has the ability to inhibit ROS production and to correct the redox imbalance induced by TNF-α.
Our study observed that there was a significant reduction in p38-MAPK, caspase-3 and Bax renal expressions indicating the nephroprotective effect of candesartan via its antiapoptotic effects. These encouraging results support those reported by Lv et al.36 who documented the antiapoptotic activity of candesartan by blocking the mesangial cell oxidative stress and apoptosis induced by angiotensin II.
Regarding our findings about BM-MSCs, we documented that injection of BM-MSCs showed a significant reduction in the renal levels of TNF-α and MCP-1 levels and renal expression of NF-κB in CDDP-treated rats when compared to the normal rats. It was found that BM-MSCs significantly lowered renal gene expression of TNF-α in a model of experimental diabetic nephropathy.6 Moreover, BM-MSCs significantly lowered the renal MCP-1 in rats treated with MSCs and gentamicin when compared to rats administered gentamicin alone in a model of genatmicine-induced AKI.32
The antiinflammatory and antifibrotic activities of BM-MSCs were indicated by attenuation of the TNF-α and NF-κB overexpression in a recent model of albumin-induced renal tubular inflammation and fibrosis.37 The MSCs have a prominent immunomodulatory property that leads to the downregulation of inflammatory reactions.38
Moreover, BM-MSCs also significantly suppressed the renal p38-MAPK, caspase-3 and Bax expressions in CDDP-treated rats. Our results are similar to these findings of Qi and Wu27 who observed downregulation of Bax, p38-MAPK and caspase-3 in CDDP-treated rats administered BM-MSCs as compared with the CDDP-treated rats administered saline.
The possibilities of the therapeutic potential of MSCs were either direct effect by replacing damaged cells or indirect effect through signals and induction of cellular regeneration.8,39 The transplanted MSCs was able to cause release of various factors with its beneficial antiapoptotic, anti-inflammatory, mitogenic or angiogenic properties.8,40
In conclusion, Candesartan and BM-MSCs are capable of improving renal injury in CDDP nephrotoxic rats through anti-inflammatory action via reduction of renal contents of TNF-α, NF-κB, p38-MAPK and MCP-1 and antiapoptotic action via downregulation of renal caspase-3 and Bax. Mesenchymal stem cells seem to have a better renoprotective effect than candesartan in amelioration of nephrotoxicity induced by CDDP.
Authors' contributions: SIO contributed in the study design, practical work, manuscript drafting and revision, and critical discussion. A-MLA contributed in the manuscript revision, critical discussion, and supervision. AAM contributed in collecting scientific materials, and SMA contributed in practical work and manuscript drafting. MHE contributed in the study design, manuscript revision and critical discussion. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
This research project was supported by a fund from the Deanship of Scientific Research, Princess Nourah bint Abdulrahman University, Riyadh, Kingdom of Saudi Arabia.
References
- 1.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins 2010; 2: 2490–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 2007; 334: 115–24. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan N. Angiotensin II-receptor blockers: will they replace angiotensin-converting enzyme inhibitors in the treatment of hypertension? J Human Hypertens 2000; 14: S87–90. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim MA, Ashour OM, Ibrahim YF, El-Bitar HI, Gomaa W, Abdel-Rahim SR. Angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol Res 2009; 60: 373–81. [DOI] [PubMed] [Google Scholar]
- 5.Yamagishi S, Takeuchi M. Telmisartan is a promising cardiometabolic sartan due to its unique PPAR-γ-inducing property. Med Hypotheses 2005; 64: 476–8. [DOI] [PubMed] [Google Scholar]
- 6.Aziz MTA, Wassef MAA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, Hussein RE, Abdelaziz M. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diab Metab Syndr 2014; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos N, Zhao J, Bouchentouf M, Forner K, Birman E, Yuan S, Boivin MN, Martineau D. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol-Renal Physiol 2010; 299: F1288–98. [DOI] [PubMed] [Google Scholar]
- 8.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cell 2008; 26: 2075–82. [DOI] [PubMed] [Google Scholar]
- 9.Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, Gupta S, Singhal PC. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Experiment Mol Pathol 2013; 94: 466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol 2006; 34: 1606–7. [DOI] [PubMed] [Google Scholar]
- 11.McLimans WF, Davis EV, Glover FL, Rake GW. The submerged culture of mammalian cells: the spinner culture. J Immunol 1957; 79: 428–33. [PubMed] [Google Scholar]
- 12.Helmy M, Helmy M, ALLAH D, ZAID A, EL-DIN MM. Role of nitergic and endothelin pathways modulations in cisplatin-induced nephrotoxicity in male rats. JPP 2014; 3: 08. [PubMed] [Google Scholar]
- 13.Ashrafi F, Nematbakhsh M, Safari T, Talebi A, Nasri H, Khazaei M, Baradaran-Mahdavi MM, Jafapisheh A, Olia B, Pirhaji O, Hashemi-Nia SJ, Eshraghi F, Pezeshki Z, Mortazavi M. A combination of vitamin C and losartan for cisplatin-induced nephrotoxicity in rats. Iranian J Kidney Dis 2012; 6: 361–5. [PubMed] [Google Scholar]
- 14.Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy 2009; 55: 399–406. [DOI] [PubMed] [Google Scholar]
- 15.Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Experiment Cell Res 2013; 319: 2019–27. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 17.El-Garhy AM, El-Raouf A, Ola M, El-Sayeh BM, Fawzy HM, Abdallah DM. Ellagic acid antiinflammatory and antiapoptotic potential mediate renoprotection in cisplatin nephrotoxic rats. J Biochem Mol Toxicol 2014; 28: 472–9. . [DOI] [PubMed] [Google Scholar]
- 18.Francescato HD, Costa RS, Scavone C, Coimbra TM. Parthenolide reduces cisplatin-induced renal damage. Toxicology 2007; 230: 64–75. [DOI] [PubMed] [Google Scholar]
- 19.İş eri S, Ercan F, Gedik N, Yüksel M, Alican İ. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 2007; 230: 256–64. [DOI] [PubMed] [Google Scholar]
- 20.Ueki M, Ueno M, Morishita J, Maekawa N. D-ribose ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Tohoku J Experiment Med 2013; 229: 195–201. [DOI] [PubMed] [Google Scholar]
- 21.Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol 2003; 23: 1553–8. [DOI] [PubMed] [Google Scholar]
- 22.Banas B, Luckow B, MÖLLER M, Klier C, Nelson PJ, Schadde E, Brigl M, Halevy D, Holthöfer H, Reinhart B, Schlöndorff D. Chemokine and chemokine receptor expression in a novel human mesangial cell line. J Am Soc Nephrol 1999; 10: 2314–22. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Liu D, Feng L, Zhang Z, Jia X, Xiao W. Protective effect of standardized extract of Ginkgo biloba against cisplatin-induced nephrotoxicity. Evid-Based Complement Alternat Med 2013, pp. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Experiment Therapeut 2008; 327: 300–7. [DOI] [PubMed] [Google Scholar]
- 25.Adams JL, Badger AM, Kumar S, Lee JC. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Progr Med Chem 2001; 38: 1–60. [DOI] [PubMed] [Google Scholar]
- 26.Rashed LA, Hashem RM, Soliman HM. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomed Pharmacother 2011; 65: 474–80. [DOI] [PubMed] [Google Scholar]
- 27.Qi S, Wu D. Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis. Int J Mol Med 2013; 32: 1262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz A, Lorz C, Catalán MP, Danoff TM, Yamasaki Y, Egido J, Neilson EG. Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int 2000; 57: 969–81. [DOI] [PubMed] [Google Scholar]
- 29.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 2008; 73: 994–1007. [DOI] [PubMed] [Google Scholar]
- 30.Borrego A, Zamora ZB, González R, Romay C, Menéndez S, Hernández F, Berlanga J, Montero T. Ozone/oxygen mixture modifies the subcellular redistribution of Bax protein in renal tissue from rats treated with cisplatin. Archive Med Res 2006; 37: 717–22. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-α by p53 in cisplatin-induced renal cell apoptosis. Oncogene 2006; 25: 4056–66. [DOI] [PubMed] [Google Scholar]
- 32.Rastghalam R, Nematbakhsh M, Bahadorani M, Eshraghi-Jazi F, Talebi A, Moeini M, Ashrafi F, Shirdavani S. Angiotensin Type-1 receptor blockade may not protect kidney against cisplatin-induced nephrotoxicity in rats. ISRN Nephrology 2014; 2014: 479645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu P, Feng Y, Dong C, Yang D, Li B, Chen X, Zhang Z, Wang Y, Zhou Y, Zhao L. Administration of BMSCs with muscone in rats with gentamicin-induced AKI improves their therapeutic efficacy. PloS ONE 2014; 9: e97123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Gong R, Rifai A, Tolbert EM, Dworkin LD. Long-term, high-dosage candesartan suppresses inflammation and injury in chronic kidney disease: nonhemodynamic renal protection. J Am Soc Nephrol 2007; 18: 750–9. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int 2008; 74: 1128–38. [DOI] [PubMed] [Google Scholar]
- 36.Lv J, Jia R, Yang D, Zhu J, Ding G. Candesartan attenuates Angiotensin II-induced mesangial cell apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun 2009; 380: 81–6. [DOI] [PubMed] [Google Scholar]
- 37.Wu HJ, Yiu WH, Li RX, Wong DW, Leung JC, Chan LY, Zhang Y, Lian Q, Lin M, Tse HF, Lai KN, Tang SC. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PloS ONE 2014; 9: e90883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther 2010; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, Curtis LM. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol-Renal Physiol 2011; 300: F254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bussolati B, Hauser PV, Carvalhosa R, Camussi G. Contribution of stem cells to kidney repair. Current Stem Cell Res Ther 2009; 4: 2–8. [DOI] [PubMed] [Google Scholar]