Abstract
Vascular remodeling due to excessive proliferation of endothelial and smooth muscle cells is a hallmark feature of pulmonary hypertension. microRNAs (miRNAs) are a class of small, non-coding RNA fragments that have recently been associated with remodeling of pulmonary arteries, in particular by silencing the bone morphogenetic protein receptor type II (BMPR2). Here we identified a novel pathway involving the concerted action of miR-125a, BMPR2 and cyclin-dependent kinase inhibitors (CDKN) that controls a proliferative phenotype of endothelial cells. An in silico approach predicted miR-125a to target BMPR2. Functional inhibition of miR-125a resulted in increased proliferation of these cells, an effect that was found accompanied by upregulation of BMPR2 and reduced expression of the tumor suppressors CDKN1A (p21) and CDKN2A (p16). These data were confirmed in experimental pulmonary hypertension in vivo. Levels of miR-125a were elevated in lung tissue of hypoxic animals that develop pulmonary hypertension. In contrast, circulating levels of miR-125a were found to be lower in mice with pulmonary hypertension as compared to control mice. Similar findings were observed in a small cohort of patients with precapillary pulmonary hypertension. These translational data emphasize the pathogenetic role of miR-125a in pulmonary vascular remodeling.
Keywords: Pulmonary hypertension, microRNAs, endothelial cell proliferation, anti-miRs, BMPR2
Background
Pulmonary hypertension (PH) is defined by an elevation of the mean pulmonary arterial pressure ≥ 25 mmHg.1 This condition might be the consequence of several different entities as summarized in the clinical classification system of PH.2 The small pulmonary arteries in most of these diseases are characterized by vascular remodeling, i.e. by excessive proliferation of the vascular smooth muscle cell and endothelial layer resulting in increased vascular wall thickness and luminal occlusion.
Many of these clinical disorders share one pathogenetic hallmark: genetic or non-genetic dysregulations of the bone morphogenetic protein receptor type II (BMPR2). This growth factor receptor has been found mutated in hereditary pulmonary arterial hypertension (PAH)3,4 and, in addition, downregulated in several other forms of the disease including PH due to congenital heart disease,5,6 infection with the human immunodeficiency virus,7 hypoxia-induced PH,8,9 and in postcapillary elevation of the pulmonary pressure in heart failure patients.6 As evidenced in vitro and by therapeutic approaches in experimental models, the downregulation of BMPR2 might be due to the action of microRNAs (miRNAs),10–12 which, moreover, have recently been associated with silencing of tumor suppressor genes and, as such, might further increase proliferation of vascular smooth muscle and endothelial cells. miRNAs are endogenously expressed, non-coding RNA fragments of 18–22 nucleotides that bind to a complementary mRNA sequence of a target gene, a mechanism that inhibits further translation and results in post-transcriptional gene silencing. miRNAs have emerged in PH as important regulators in the pathogenesis of the pulmonary vascular remodeling, as therapeutic targets and, finally, as potential biomarkers.
In this translational study, we conducted a screening for miRNAs that potentially interact with BMPR2 and identified altered expression of miR-125a in lung tissue samples of experimental PH. miR-125a was experimentally proven to regulate the expression of BMPR2 and the tumor suppressor CDKN1A in human endothelial cells resulting in a proproliferative phenotype of these cells. Levels of circulating miR-125a were further analyzed in experimental PH and in the serum of patients.
Materials and methods
Patients
Recruitment of patients was performed from July 2012 to March 2013 during regular visits in our outpatient clinic. All patients with confirmed diagnosis of precapillary PH were asked to participate. A total of 51 patients were included, 41 with PAH and 10 with CTEPH. Sample collection was made at any time point during follow up in our outpatient clinic unrelated to the latest right heart catheterization or echocardiography. Follow up and monitoring of patients was provided by regular visits in our outpatient clinic unrelated to the study design. Healthy adult control subjects were recruited from staff members at the University Hospital Zurich without matching between patients and control subjects. All patients gave their written informed consent. The study protocol was approved by Kantonale Ethikkommission (KEK), Zürich (approval number KEK–ZH–Nr 2010-0221). Table 1 summarizes the characteristics of patients and healthy subjects.
Table 1.
Patients' characteristics
| Patients | Controls | |
|---|---|---|
| Number | 51 | 41 |
| female:male | 35:16 | 22:19 |
| PAH | 41 | |
| Idiopathic/ hereditary PAH | 29 | |
| Collagen-vascular diseases | 7 | |
| Congenital heart diseases | 5 | |
| CTEPH | 10 | |
| PH target therapy* | ||
| Endothelin-receptor-antagonists | 44 | |
| Phosphodiesterase-5-inhibitors | 22 | |
| Prostacyclines** | 9 |
Several patients are treated with combination therapy.
Intravenous epoprostenol (two patients), inhalative iloprost (four patients), subcutaneous treprostinil (three patients).
Blood sampling
Peripheral venous blood samples were obtained from patients, who had precapillary PH confirmed by right-heart catheterization (patient clinical parameters, Table 1), and from healthy control subjects. After clotting of blood, samples were centrifuged at 300g for 10 min and the supernatant (serum) was collected and stored at −80 ℃.
Cell culture
Human pulmonary artery smooth muscle cells (HPASMC) and human pulmonary artery endothelial cells (HPAEC) were obtained from Gibco (Life Technologies, Zug, Switzerland) and were cultured in supplemented medium 231 and 200 (both from Gibco), respectively. Cells were cultured at 37 ℃ in a humidified atmosphere of 5% CO2. Exposure of cells to hypoxia was carried out at 37 ℃ in a humidified atmosphere of 5% CO2 and 1% O2.
Hypoxia-induced PH
Male mice (BL6) were obtained at the Institute for Veterinary Physiology at the University of Zurich. A total of 16 animals were distributed equally in two groups consisting of one normoxic control and one hypoxic group (10% oxygen for five weeks). Hypoxic conditions were provided in chambers connected to a gas mixer (Ruskinn Life Science, Bridgend, United Kingdom). After five weeks, mice were anesthetized and right heart catheterization was performed to measure the right ventricular pressure (RVP). In detail, a small skin incision was made in the neck of the mice and the right external jugular vein was isolated to insert a polyethylene (PE 10) catheter and forward it to the right ventricle of the heart. In each animal the blood pressure within the right ventricle was continuously recorded with a 1 kHz sampling frequency for at least 30 s using a piezoelectric pressure transducer and the PowerLab system (ADInstruments, Spechbach, Germany). After recording a 500 µl, venous blood sample was drawn from the right ventricle via the catheter. After blood collection, the animals were euthanized by cervical dislocation and lungs were collected. To isolate miRNAs from lung tissue samples, the miRNeasy Mini kit (Qiagen, Hombrechtikon, Switzerland) was used. Circulating miRNAs from murine plasma were purified as described in the section of purification of circulating miRNAs. All animal experiments were approved by the Swiss Cantonal Veterinary Office (license 151/2012, Zurich, Switzerland).
Purification of circulating miRNAs
miRNAs were purified from human serum or murine plasma as described before.13 Briefly, 100 µl of plasma and 600 µl of serum were denatured by the addition of 400 µl and 1200 µl of Qiazol (Qiagen), respectively. To control technical variabilities during RNA isolation procedure and since no specific miRNAs are recommended to serve as suitable endogenous control, synthetic Caenorhabditis elegans miRs, cel-miR-39, and cel-miR-238 (synthesized by Microsynth, Balgach, Switzerland) were added to the denatured sample (10 µl of a 5 fmol solution) and used as described in Kroh et al.13 After centrifugation (12,000g at 4 ℃ for 15 min) supernatants were mixed with ethanol and transferred to miRNeasy spin columns (Qiagen). RNA samples containing miRNAs were eluted in 32 µl RNase-free water and stored at −20 ℃.
Quantification of mature miRNAs
Mature miRNA sequences were detected with specific stem-loop primers and reverse transcribed using MultiScribe reverse transcriptase (Life Technologies, Zug, Switzerland) according to Chen et al.14 Quantification of complementary DNA (cDNA) was performed by SYBR Green quantitative PCT (qPCR, Applied Biosystem StepOnePlus system, Life Technologies). The reverse transcription (RT) and amplification primers used in this study are shown in Table 2. To enhance sensitivity, cDNA samples of murine plasma were pre-amplified prior qPCR analysis.13 Briefly, cDNA samples were mixed with the respective miRNA-specific forward primer and the universal reverse primer and were amplified with Phire polymerase (Thermo Scientific, Wohlen, Switzerland) for 14 cycles. qPCR data of circulating miRNAs were median-normalized to the mean cycle of threshold (ct) values of the spike-in controls, cel-miR-39 and cel-miR-238, and expressed as median-normalized ct values as recommended by Kroh et al.13 Median-normalized instead of absolute levels were used to control technical variabilities during RNA isolation.
Table 2.
Primer sequences
| miRNA expression primer | |
|---|---|
| miR-125a RT (hsa, mmu) | 5′ – GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT CAC AG – 3′ |
| miR-125a fwd (hsa, mmu) | 5′ – TCC CTG AGA CCC TTT AAC C – 3′ |
| cel-miR-39 RT | 5′ – GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACC AAG CT – 3′ |
| cel-miR-39 fwd | 5′ – CCT CAC CGG GTG TAA ATC AG – 3′ |
| cel-miR-238 RT | 5′ – GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT CTG AA – 3′ |
| cel-miR-238 fwd | 5′ – CTT TGT ACT CCG ATG CCA TTC – 3′ |
| snoRNA202 RT (mmu) | 5′ – GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACC ATC AG – 3′ |
| snoRNA202 (mmu) fwd | 5′ – CCG TAC TTT TGA ACC CTT TTC – 3′ |
| RNU48 RT (hsa) | 5′ – GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACG GTC AG – 3′ |
| RNU48 (hsa) fwd | 5′ – CCA TGA GTG TGT CGC TGA TG – 3′ |
| universal reverse primer | 5′ – GAG GTA TTC GCA CTG GAT AC – 3′ |
| Gene expression primer | |
| β-actin fwd (hsa, mmu) | 5′ – TCA AGA TCA TTG CTC CTC CTG AG – 3′ |
| β-actin rev (hsa, mmu) | 5′ – TCC TGC TTG CTG ATC CAC ATC – 3′ |
| BMPR2 fwd (hsa) | 5′ – AGC CCA ACA GTC AAT CCA ATG – 3′ |
| BMPR2 rev (hsa) | 5′ – GGT TGC GTT CAT TCT GCA TAG – 3′ |
| CDKN1A fwd (hsa) | 5′ – AGC ATG ACA GAT TTC TAC CAC TC – 3′ |
| CDKN1A rev (hsa) | 5′ – GGC TTC CTC TTG GAG AAG ATC – 3′ |
| CDKN1B fwd (hsa) | 5′ – ACC TGC AAC CGA CGA TTC TTC – 3′ |
| CDKN1B rev (hsa) | 5′ – CAT TTG GGG AAC CGT CTG AAA C – 3′ |
| CDKN1C fwd (hsa) | 5′ – CCT CTG ATC TCC GAT TTC TTC – 3′ |
| CDKN1C rev (hsa) | 5′ – ACT TCT CAG GCG CTG ATC TC – 3′ |
| CDKN2A fwd (hsa) | 5′ – CTC AGA CAT CCC CGA TTG AAA G – 3′ |
| CDKN2A rev (hsa) | 5′ – CTT CGG TGA CTG ATG ATC TAA G – 3′ |
| CDKN2B fwd (hsa) | 5′ – TCA CCA TGA AGC GAA ACA CAG – 3′ |
| CDKN2B rev (hsa) | 5′ – GAA AAC CCT GAA AAG CAA ACG AC – 3′ |
| CDKN2C fwd (hsa) | 5′ – CGC TGC AGG TTA TGA AAC TTG – 3′ |
| CDKN2C rev (hsa) | 5′ – GGG ATT AGC ACC TCT AAG TAG – 3′ |
| CDKN2D fwd (hsa) | 5′ – CGC TGC AGG TCA TGA TGT TTG – 3′ |
| CDKN2D rev (hsa) | 5′ – GTC ATG GAC TGG ACT GGT AC – 3′ |
Obtained qPCR data of miRNAs derived from murine lung tissue and cultured human cells were normalized to the endogenous control snoRNA202 and RNU48, respectively. Differential miRNA expression was calculated with the Δct method.15 Specific PCR amplification was confirmed by performing dissociation curve analysis. Moreover, correct amplification of cel-miR-39, cel-miR-238, and miR-125a was confirmed by using DNA sequencing reaction (Microsynth).
Transient transfection of primary cultured cells
For manipulation of endogenous levels of miR-125a, HPASMC, and HPAEC were transfected with anti-miR-125a or scrambled negative control (both 25 nM) using Lipofectamine 2000 (Life Technologies). Methylated oligonucleotides (anti-miR-125a: 5′—UCA CAG GUU AAA GGG UCU CAG GGA—3′; and scrambled negative control: 5′—GAC CGU UCA CUA UUA CGA GUC AA—3′) were synthesized at Microsynth. Following an incubation period of 72 h cells were harvested and gene expression analysis was performed.
Quantitative real time-PCR (qPCR) analysis
Total RNA of cultured cells or murine lung tissue samples was purified using the miRNeasy kit (Qiagen). Isolated RNA was reverse transcribed by using random hexamers and MultiScribe reverse transcriptase (both from Life Technologies). Quantification of specific gene transcripts was performed by SYBR Green qPCR (Applied Biosystem StepOnePlus system, Life Technologies). Sequences of primers used in this study are shown in the Table 2. Specific amplification was verified by performing melt curve analysis. Obtained expression levels of genes of interest were normalized to the expression of β-actin. Differential gene expression was calculated with the threshold cycle (ct) method.15
Western blotting
For protein extraction, harvested cells were lysed with sample loading buffer (62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5 mM β-mercaptoethanol, bromphenolblue). Whole-cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred to nitrocellulose membrane (Whatman, GE Healthcare Life Sciences, Little Chalfont, UK). Membranes were incubated with the following primary antibodies: anti – BMPR2 (mouse antibody, ab78422, Abcam plc, Cambridge, UK), anti-CDKN1A (rabbit antibody, #2947 Cell Signaling Technologies, Danvers, MA, USA), anti-CDKN2A (rabbit antibody, ab108349, Abcam plc, Cambridge, UK) and anti-β-actin (mouse antibody, #A2228, Sigma-Aldrich, Buchs SG, Switzerland). Bands were detected with species-specific secondary antibodies coupled to horseradish peroxidase (Jackson ImmunoResearch Europe Ltd, Suffolk, UK). Calculation of the expression of proteins was performed using Adobe Photoshop CS5.1 software (Adobe Systems Incorporated, San Jose, CA, USA) via pixel quantification of the electronic image.
Proliferation assay
To assess the proliferation rate, HPASMC and HPAEC were seeded in 96-well plates and were transfected with 25 nM of anti-miRNAs (scrambled negative control or anti-miR-125a) using Lipofectamine 2000 (Life Technologies). After 48 h, 5-bromo-2-deoxy-uridine (BrdU) was added to each well and incubated for 24 h. Incorporation of BrdU was detected using the colorimetric BrdU assay from Roche (Roche Diagnostics, Mannheim, Germany) and spectrophotometrically measured at a test wavelength of 450 nm (reference wavelength: 620 nm). The measured absorbance directly correlates with the proliferation and the number of cells.
Statistics
For statistical analysis GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA) was used. Values are presented as mean ± standard deviation. Parametric or non-parametric distribution of data was determined using the Kolmogorov–Smirnov test. Data comparison was performed using independent Student's t-test as well as Pearson's correlation for parametric samples and Mann–Whitney test for non-parametric samples. For statistical analysis of multiple groups the 1way analysis of variance with Bonferroni post hoc test was used. A p value < 0.05 was considered significant (*p < 0.05, **p < 0.01, ***p < 0.001). In all statistical analyses, two sided tests were applied. The n number indicates independent experiments. Each experiment was measured in duplicates.
Results
Hypoxia induces miR-125a in vivo and in vitro
A computational target screen was performed (TargetScan, www.targetscan.org) and identified several miRNAs that potentially interact with the 3′ untranslated region (3′UTR) of BMPR2 (data not shown). Since a strong interaction was predicted for miR-125a, screening for the expression of this miRNA was performed in lung tissue samples of animals, in which PH was induced by hypoxia and in human pulmonary vascular cells.
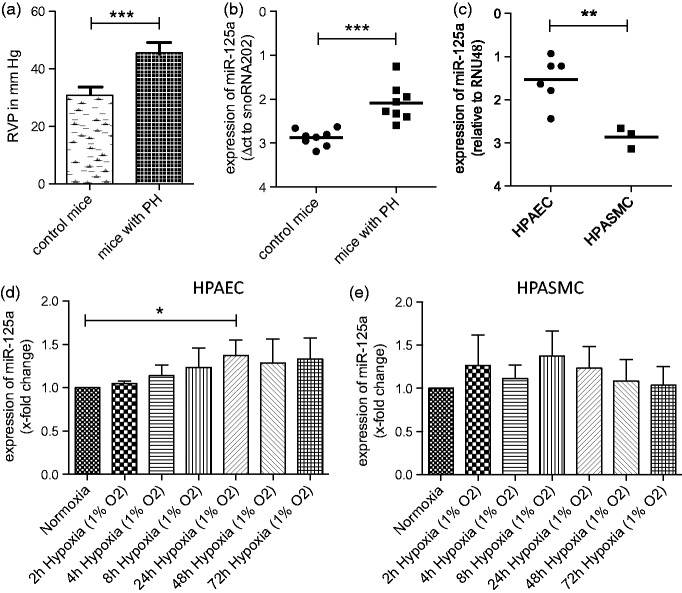
As shown in Figure 1(a), animals exposed to hypoxia (10% oxygen for five weeks) developed significantly higher RVP as their normoxic controls (45.45 ± 3.63 mm Hg vs. 30.83 ± 2.56 mm Hg, p < 0.001). Expression levels of miR-125a were analyzed in lung tissue and were found to be significantly upregulated (by 73%) in mice with PH as compared to control mice (in ct values normalized to snoRNA202. Higher ct values indicate lower abundance of the miRNA of interest: 2.09 ± 0.42 vs. 2.88 ± 0.19, p < 0.001, Figure 1(b)). Next, we assessed the effect of oxygen deprivation on distinct pulmonary vascular cells by using HPAEC and HPASMC. Baseline expression levels of miR-125a were found to be 2.5-fold higher in HPAEC than in HPASCM (in ct values normalized to RNU48: 1.54 ± 0.54 vs. 2.86 ± 0.25, p < 0.01, Figure 1(c)). Hypoxic conditions further induced the levels of miR-125a in HPAEC significantly (by 1.4-fold after 24 h of hypoxia, Figure 1(d)). Conversely, levels of miR-125a in HPASMC were unchanged by hypoxic conditions (Figure 1(e)).
Figure 1.
Hypoxia induces miR-125a in vivo and in vitro. (a) Animals exposed to hypoxia (10% oxygen for five weeks) developed significantly higher right ventricular pressure as their normoxic controls (n = 8 each). (b) Expression levels of miR-125a in lung tissue were found to be significantly upregulated in mice with PH as compared to control mice as analyzed by qPCR. (c) Baseline expression levels of miR-125a were found to be higher in HPAEC than in HPASCM as shown by qPCR analysis. (d) Hypoxia (1% oxygen) increased the levels of miR-125a in HPAEC significantly (n = 6), whereas (e) the levels of miR-125a in HPASMC were unchanged by hypoxic conditions (n = 3). Statistical analysis by unpaired Student's t-test and one way analysis of variance with Bonferroni post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001)
The expression of BMPR2 is regulated by miR-125a in human endothelial cells
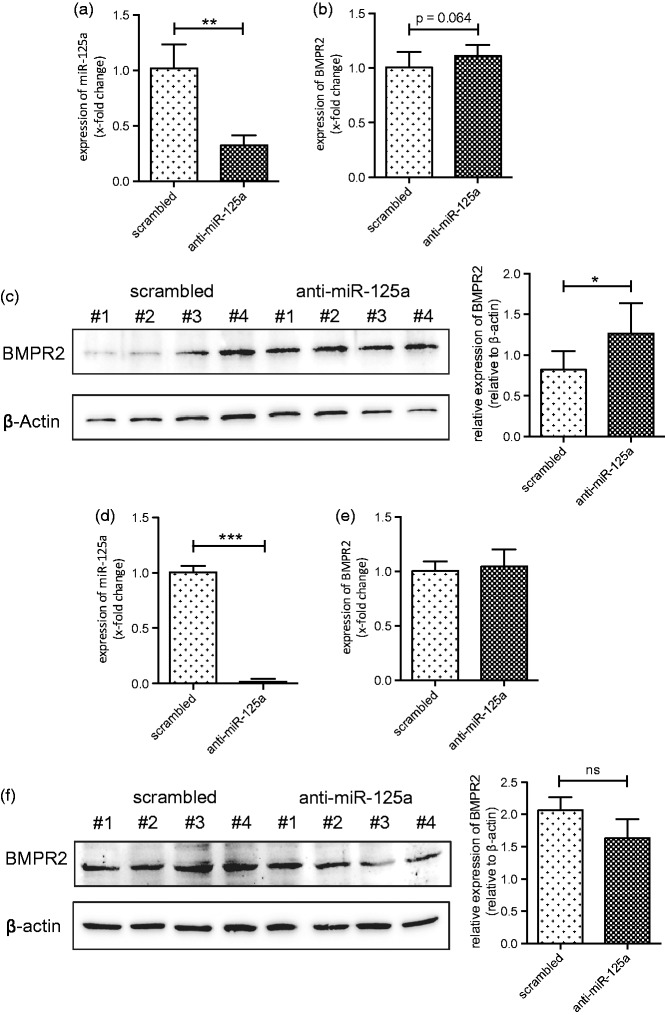
miR-125a is predicted to bind to and regulate the expression of BMPR2 (according to miRNA prediction software TargetScan). To confirm this interaction, the endogenous expression of miR-125a in HPAEC and HPASMC was silenced by transfection of anti-miR-125a oligonucleotides and expression levels of BMPR2 were analyzed. As measured by qPCR, transfection of HPAEC with anti-miR-125a significantly reduced the expression of miR-125a when compared to scrambled transfected cells (by 67.8 ± 9.5%, p < 0.01, Figure 2(a)). Silencing of miR-125a increased the mRNA levels of BMPR2 without reaching statistical significance in HPAEC (Figure 2(b)). Of interest, protein levels of BMPR2 were found significantly upregulated when the expression of miR-125a was antagonized (from 0.82 ± 0.23 to 1.27 ± 0.37, p = 0.031, normalized to β-actin expression, Figure 2(c)) indicating a posttranscriptional mechanism of action.
Figure 2.
miR-125a targets BMPR2 in human pulmonary artery endothelial cells. (a) Addition of anti-miRs directed against miR-125a significantly decreased the levels of miR-125a in cultured HPAEC (n = 6). (b) As shown by qPCR analysis inhibition of miR-125a slightly upregulated the mRNA levels of BMPR2 in HPAEC when compared to scrambled transfected cells (n = 6). (c) Protein levels of BMPR2 were found significantly increased in anti-miR-125a transfected HPAEC (n = 6). A representative Western blot is shown. (d) Transfection of HPASMC with anti-miR-125a significantly repressed the levels of miR-125a (n = 6), but (e) did not change the expression of BMPR2 on the mRNA (n = 6) as well as (f) on the protein level (n = 4). A representative Western blot is provided. Statistical analysis by unpaired Student's t-test (*p < 0.05, **p < 0.01, ***p < 0.001)
In HPASMC, the expression of miR-125a was strongly reduced upon transfection of anti-miR oligonucleotides as measured by qPCR (by 98.3 ± 2.4% p < 0.001, Figure 2(d)). In contrast to HPAEC, treatment of HPASMC with anti-miR-125a failed to modulate the expression levels of BMPR2 on the mRNA (Figure 2(e)) as well as on the protein level (Figure 2(f)).
These data demonstrate that BMPR2 is targeted by miR-125a in HPAEC but not in HPASMC.
miR-125a regulates the proliferation of HPAEC
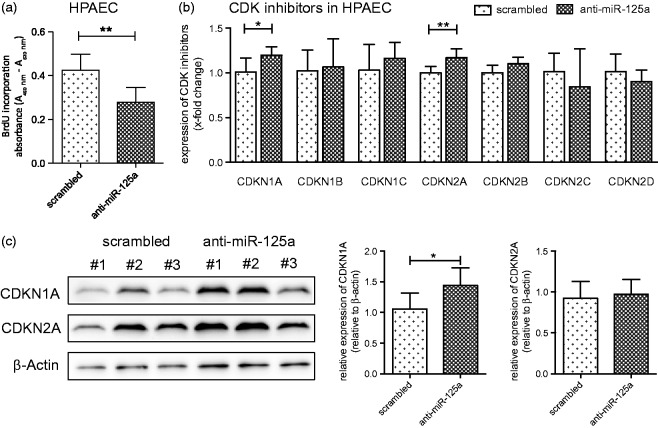
Our group recently demonstrated that an upregulation of BMPR2 in vascular cells inhibited cell proliferation probably by induction of cyclin-dependent kinase inhibitor 1A (CDKN1A).12 To test whether miR-125a modulates cell proliferation in a similar way, HPAEC and HPASMC were transfected with anti-miR-125a oligonucleotides and BrdU incorporation assays were performed. Moreover, the mRNA levels of all relevant CDK inhibitors (CDKN1A/-1B/-1C/-2A/-2B/-2C and -2D) were measured by qPCR. As presented in Figure 3(a), silencing of miR-125a significantly decreased the proliferation rate of HPAEC when compared to negative control cells (in absorbance units measured at a test wavelength of 450 nm: from 0.42 ± 0.07 to 0.28 ± 0.07, p < 0.01). Of interest, levels of the most abundantly expressed CDK inhibitors, CDKN1A and CDKN2A, were found significantly increased when miR-125a was silenced (Figure 3(b)). These data, at least for CDKN1A, were confirmed on protein level by Western blotting and densitometric analysis (Figure 3(c)).
Figure 3.
miR-125a regulates the expression of cyclin-dependent genes and modulates endothelial cell proliferation. (a) As assessed by BrdU incorporation assay, the antagonization of miR-125a significantly reduced the proliferation rate of HPAEC (n = 6). (b) The mRNA levels of all relevant CDK inhibitors were measured in HPAEC by qPCR showing a significant increase of the expression of CDKN1A and CDKN2A after treatment with anti-miR-125a when compared to scrambled transfected cells (n = 6). (c) A representative Western blot and densitometric analysis is provided. Statistical analysis by unpaired Student's t-test (*p < 0.05, **p < 0.01)
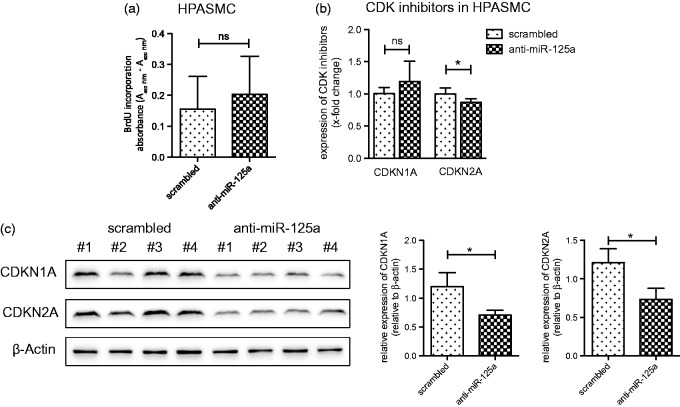
In HPASMC, transfection with anti-miR-125a did not change the proliferation rate (Figure 4(a)). Along that line, inhibition of miR-125a in HPASMC failed to increase the levels of CDKN1A whereas a significant decrease of mRNA levels of CDKN2A was observed (Figure 4(b)). Protein levels of CDKNA1 and CDKNA2 were significantly decreased after antagonizing miR-125a (Figure 4(c)).
Figure 4.
miR-125a has no effect on the proliferation of smooth muscle cells. (a) In HPASMC, the inhibition of miR-125a did not change the proliferation rate as demonstrated by BrdU incorporation assay (n = 9). (b) A significant decrease of the mRNA expression of CDKN2A was found in anti-miR-125a transfected HPASMC (n = 6). (c) A representative Western blot and densitometric analysis is provided. Statistical analysis by unpaired Student's t-test (**p < 0.01)
These data imply that inhibition of miR-125a represses proliferation of HPAEC via upregulation of CDKN1A and CDKN2A. In HPASMC, this mechanism was not observed, implying a differential regulation of miR-125a in these two cell types.
Hypoxia-induced expression of miR-125a correlates with downregulation of BMPR2 and CDKN1A in vivo
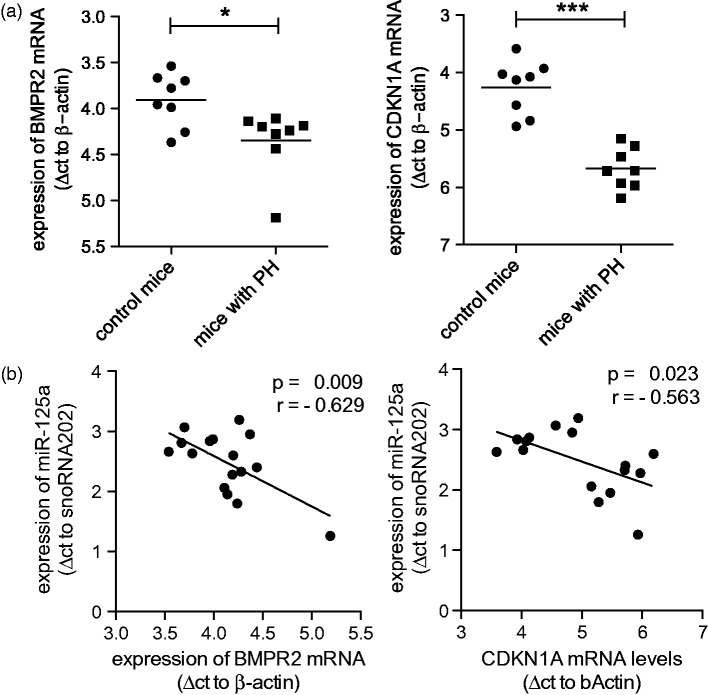
The regulation of BMPR2 and CDKN1A by miR-125a was further characterized in lung samples of the mouse model of hypoxia-induced PH (same samples as in Figure 1(b)). As presented in Figure 5(a), chronic exposure to hypoxia significantly reduced the mRNA expression of BMPR2 (in Δct values: control mice 3.91 ± 0.29 vs. mice with PH 4.35 ± 0.36, p = 0.028; indicating a reduction of 26%) and of CDKN1A (control mice 4.26 ± 0.47 vs. mice with PH 5.68 ± 0.36, p < 0.001; indicating a reduction of 62%). Since the levels of miR-125a were significantly increased in hypoxic lungs (shown in Figure 1(b)), a correlation analysis was performed (Figure 5(b)) showing a significant inverse correlation of miR-125a with BMPR2 (r = −0.629, p < 0.01) as well as with CDKN1A (r = −0.563, p = 0.023).
Figure 5.
Hypoxia-induced expression of miR-125a correlates with downregulation of BMPR2 and CDKN1A in vivo. (A) The expression of BMPR2 and CDKN1A was measured by qPCR in lung tissue samples obtained from mice that developed PH after chronic exposure to hypoxia (same samples as in Figure 1(b)). When compared to normoxic samples, a significant decrease of the mRNA expression of BMPR2 and CDKN1A was observed (n = 8). (b) A significant inverse correlation was found when the expression of miR-125a was compared to the levels of BMPR2 and CDKN1A in lungs of mice with PH (n = 8). Statistical analysis by unpaired Student's t-test, Pearson's correlation, and Mann–Whitney test (*p < 0.05, ***p < 0.001)
These data indicate that miR-125a regulates the expression of BMPR2 and CDKN1A in vivo. Thus miR-125a appears to have a functional role in pulmonary artery remodeling that involves regulation of BMPR2 and the tumor suppressor genes CDKN1A and CDKN2A in cells of the endothelial layer.
Circulating miR-125a in experimental and human precapillary PH
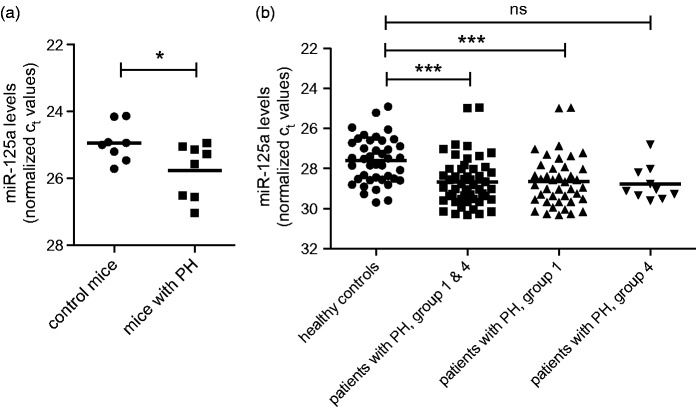
Plasma samples of mice with PH showed a significant reduction (by 43%) of the levels of circulating miR-125a as compared to control mice (25.76 ± 0.81 vs. 24.94 ± 0.56, p = 0.035, Figure 6(a)). These data were confirmed by analyzing the expression levels of miR-125a in patients (n = 51) in which the diagnosis of precapillary PH has been made by right heart catheterization (Figure 6(b)). Circulating levels of miR-125a were found to be significantly reduced (by 52%) in patients as compared to controls (28.68 ± 1.2 vs. 27.62 ± 1.15, p < 0.001). Lower expression levels of miR-125a were found in both subgroups of patients, i.e. in patients with PAH (28.65 ± 1.28 vs. 27.62 ± 1.15, p < 0.001, reduction of 51%) and in patients with PH due to chronic thromboembolic events (28.78 ± 0.87 vs. 27.62 ± 1.15, reduction of 55%) when compared to the expression level of control subjects.
Figure 6.
miR-125a in experimental and human pulmonary hypertension. (a) Plasma levels of circulating miR-125a were significantly reduced in mice with PH as compared to control mice as shown by qPCR analysis (n = 8 each). (b) Serum levels of miR-125a are significantly lower in patients with precapillary pulmonary hypertension (second from left, n = 51) as compared to healthy controls (left, n = 41). This trend is confirmed by analysis of clinical subgroups including 41 patients with pulmonary arterial hypertension (group 1, third from left) and in 10 patients with pulmonary hypertension due to chronic thromboembolic events (group 4, right). Statistical analysis by unpaired Student's t-test and 1way analysis of variance with Bonferroni post hoc test (*p < 0.05, ***p < 0.001)
Discussion
In this translational study we identified BMPR2 as a novel target of miR-125. Inhibition of miR-125a resulted in reduced proliferation of HPAEC in vitro, an effect probably mediated by direct interaction of miR-125a with BMPR2 and indirect involvement of the tumor suppressors CDKN1A and CDKN2A. These data are supported by findings from an experimental model of PH, in which hypoxic animals with PH showed higher expression levels of miR-125a in lung tissue compared to normoxic control mice. Of note, however, lower circulating levels of miR-125a were found in experimental PH. These results were confirmed in a small cohort of human samples showing that the levels of miR-125a are significantly lower in the serum of patients with precapillary PH as compared to healthy control subjects.
By computational screening we identified several miRNAs to interact with BMPR2, the most important mediator of vascular remodeling in PH. Functional in vitro experiments confirmed that inhibition of miR-125a by addition of anti-miRs increased the expression of BMPR2, whereas proliferation of HPAEC was found to be reduced implicating a role of miR-125a in vascular remodeling. Our data provide no experimental proof for a direct interaction between miR-125a and BMPR2 but confirm this correlation. Of note, the pro-proliferative effects of miR-125a were found to be associated with decreased expression of the tumor suppressors CDKN1A and CDKN2A, which represent major control factors in the regulation of cell cycle. CDKN1A and 2A were found upregulated following treatment with anti-miRs directed against miR-125a in HPAEC. Since both CDKN1A/2A have not been predicted as targets of miR-125a by a computational approach, we suggest that this upregulation is the consequence of an indirect effect of miR-125a probably mediated by downstream signaling of BMPR2. Such downstream effects on CDKN1A have already been implicated by stimulation experiments with BMP-2 in tumor cells16 and by a previous study in which functional levels of BMPR2 were restored in an in vivo model for PH by treatment with antagomirs.12
Depending on the type and context of different cells, the miR-125 family has been implicated in several mechanisms including inflammation, cell growth, differentiation, and metastasis (reviewed in Sun et al.17). Moreover, the miR-125 family has been associated with the signal transducer and activator of transcription (STAT)-3,18 which, directly10 or through the action of nuclear factor of activated T-cells-1 (NFAT-1),19 has also been implicated in the context of vascular remodeling in PH. In our experiments performed in pulmonary artery endothelial cells, inhibition of miR-125a decreased proliferation whereas no effects have been observed in vascular smooth muscle cells. Conversely, a recent study has described that antagonization of miR-125 has proliferative effects in human umbilical vein endothelial cells (HUVECs) and suggested that reduced levels of miR-125a might have a proangiogenic effect.20 These controversial findings are not completely explained, but paradoxical reactions of the pulmonary vasculature in response to hypoxia as compared to systemic vessels are a well-known phenomenon. Moreover, dysfunctional angiogenesis is an important feature in pulmonary arterial hypertension despite it is considered to be a proangiogenic disease.21
However, the differential effects of miR-125a on BMPR2 expression and proliferation of endothelial and smooth muscle cells are a novel observation. The mechanisms of these effects are not completely clear but might be explained by the differential baseline expression and a different behavior of miR-125a upon oxygen deprivation in these cells. Data from an experimental model confirmed that hypoxia activates the expression of miR-125a in lung tissue of mice, i.e. mice with PH showed significantly higher levels of miR-125a in lung tissue as control animals. Along the line of the in vitro data, the increased levels of miR-125a were associated with reduced expression of BMPR2 and CDKN1A and, as such, highlight the relevance of the identified pathway in vivo.
Finally, we assessed the levels of miR-125a in the circulation of both animals and patients. Of interest, we found lower circulating levels of miR-125a in mice with PH as compared to normoxic controls. This might be unexpected at a first glance. However, we suggest that the low circulating levels of miR-125a are the consequence of high intracellular activity of miR-125a. This intracellular activity, as predicted from computational studies and supported by our in vitro experiments, might reflect the interaction between miR-125a and BMPR2 in endothelial cells. Alternatively, the low circulating levels of miR-125a might be the consequence of the previously described dysregulated expression of DICER in patients with PH.22 Data from a small and heterogeneous collective of patients with pulmonary arterial hypertension confirmed the experimental findings by showing that the expression of miR-125a is most significantly downregulated in the serum of patients as compared to the levels of healthy controls. At the moment it remains unclear, however, whether decreased expression levels of miR-125a contribute to progression of the disease and, as such, are of prognostic value or, alternatively, whether low levels of miR-125a are a consequence of hypoxic conditions observed in patients with PH. Our data are further limited by the lack of data on pharmacological inhibition of miR-125a which precludes conclusions on the clinical relevance.
Conclusions
In conclusion, these data describe a novel pathway involving miR-125a, BMPR2, and CDKN1A, which regulates a proproliferative phenotype of pulmonary endothelial cells. This pathway appears to be specific for endothelial cells. This is of particular interest since reduced expression levels of circulating miR-125a are found in experimental PH and in patients with precapillary PH as compared to healthy controls.
ACKNOWLEDGEMENTS
Brock and Ulrich have received travel and meeting fees from Actelion Pharmaceutical Ltd, Switzerland. Huber has received travel and meeting fees from Actelion and Bayer AG, Germany. All other authors declare that they have no competing interests. This study has been supported by the Swiss National Science Foundation (SNF Grant 31003A_144212), the Zurich Lung Foundation, the EMDO Foundation and the Theodor and Ida Herzog-Egli Foundation.
Authors' contributions
LCH and MB participated in the design of the study. CL, LGvB, JV, and MB carried out the data acquisition. LCH, SU, MG, RS, MC, and MB participated in the writing of the article. All authors read and approved the final manuscript.
References
- 1.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–537. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54(1 Suppl): S43–54. [DOI] [PubMed] [Google Scholar]
- 3.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 2000; 26: 81–4. [DOI] [PubMed] [Google Scholar]
- 4.Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, 3rd, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(1 Suppl): S32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002; 105: 1672–8. [DOI] [PubMed] [Google Scholar]
- 6.Ishida H, Kogaki S, Takahashi K, Ozono K. Attenuation of bone morphogenetic protein receptor type 2 expression in the pulmonary arteries of patients with failed Fontan circulation. J Thorac Cardiovasc Surg 2012; 143: e24–6. [DOI] [PubMed] [Google Scholar]
- 7.Dalvi P, O'Brien-Ladner A, Dhillon NK. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: Implications for HIV-related pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2013; 33: 2585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006; 290: L450–8. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Banerjee S, Freitas Ad, Chui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 2012; 302(6): L521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009; 104: 1184–91. [DOI] [PubMed] [Google Scholar]
- 11.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 2012; 185: 409–19. [DOI] [PubMed] [Google Scholar]
- 12.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 2014; 35: 3203–11. . [DOI] [PubMed] [Google Scholar]
- 13.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010; 50: 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 2005; 33: e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–8. [DOI] [PubMed] [Google Scholar]
- 16.Chen A, Wang D, Liu X, He S, Yu Z, Wang J. Inhibitory effect of BMP-2 on the proliferation of breast cancer cells. Mol Med Rep 2012; 6: 615–20. [DOI] [PubMed] [Google Scholar]
- 17.Sun YM, Lin KY, Chen YQ. Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol 2013; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 2013; 145: 842–52 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svensson D, Gidlof O, Turczynska KM, Erlinge D, Albinsson S, Nilsson BO. Inhibition of MicroRNA-125a promotes human endothelial cell proliferation and viability through an antiapoptotic mechanism. J Vasc Res 2014; 51: 239–45. [DOI] [PubMed] [Google Scholar]
- 21.Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension: The angiogenesis paradox. Am J Respir Cell Mol Biol 2014; 51: 474–84. [DOI] [PubMed] [Google Scholar]
- 22.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 2010; 30: 716–23. [DOI] [PubMed] [Google Scholar]