Abstract
Acute respiratory distress syndrome (ARDS) is a serious clinical problem that has a 30–50% mortality rate. Budesonide has been used to reduce lung injury. This study aims to investigate the effects of nebulized budesonide on endotoxin-induced ARDS in a rabbit model. Twenty-four rabbits were randomized into three groups. Rabbits in the control and budesonide groups were injected with endotoxin. Thereafter, budesonide or saline was instilled, ventilated for four hours, and recovered spontaneous respiratory. Peak pressure, compliance, and PaO2/FiO2 were monitored for 4 h. After seven days, PaO2/FiO2 ratios were measured. Wet-to-dry weight ratios, total protein, neutrophil elastase, white blood cells, and percentage of neutrophils in BALF were evaluated. TNF-α, IL-1β, IL-8, and IL-10 in BALF were detected. Lung histopathologic injury and seven-day survival rate of the three groups were recorded. Peak pressure was downregulated, but compliance and PaO2/FiO2 were upregulated by budesonide. PaO2/FiO2 ratios significantly increased due to budesonide. Wet-to-dry weight ratios, total protein, neutrophil elastase, white blood cells and percentage of neutrophils in BALF decreased in the budesonide group. TNF-α, IL-1β, and IL-8 levels decreased in BALF, while IL-10 levels increased in the budesonide group. Lung injuries were reduced and survival rate was upregulated by budesonide. Budesonide effectively ameliorated respiratory function, attenuated endotoxin-induced lung injury, and improved the seven-day survival rate.
Keywords: Budesonide, endotoxin, lung injury
Introduction
Acute respiratory distress syndrome (ARDS) is a serious clinical problem that has a 30–50% mortality rate,1 and currently has no effective treatment.2 In clinical practice, sepsis is the highest risk factor for ARDS progression with a 40% morbidity rate.3,4 ARDS is characterized by abnormalities in ventilation perfusion ratio, decrease in compliance, lung edema, severe hypoxemia, increase in lung permeability, and impaired gas exchange.5,6 These features are caused by the infiltration of neutrophils, protease activation, increased endothelial and epithelial permeability, and secretion of cytokines in lungs due to nuclear factor-κB (NF-κB) activation.7–10 Therefore, effective control of inflammation could be the best treatment for ARDS.
Glucocorticoids have been used to treat ARDS via the inhibition of NF-κB and inflammation.11–14 However, systemic use of glucocorticoids may cause immunosuppression15 and spread infection. Compared with systemic administration, inhaled glucocorticoids provide more effective local anti-inflammation and less systemic immunosupression. Moreover, compared with severe lung injury and high mortality associated with ARDS, the possibility of less immunusuppression of inhaled glucocorticoids is acceptable, if it can ameliorate lung injury and severe inflammation. In addition, severe lung injuries could be clinically treated with antibiotics, decreasing the dosage and reducing the complications of glucocorticoids. Budesonide can attenuate lung injuries induced by chlorine gas, surfactant-depletion, aspiration or LPS in short-term experiments.16–19 However, long-term outcome of budesonide applications in endotoxin-induced lung injuries remains unclear. Indeed, long-term application of glucocorticoids to treat ARDS remains controversial. In this study, we established an ARDS rabbit model via intravenous endotoxin injection, and investigated whether budesonide applications could provide positive outcomes and safety for ARDS in clinical therapy.
Methods
Animals
Twenty-four male New Zealand white rabbits (2.0–2.5 kg) were anesthetized with ketamine (35 mg/kg, i.m.) and xylazin hydrochloride (0.3 mg/kg, i.m). Intubation was performed using a 3.5 mm cuffed endotracheal tube through the glottis. Anesthesia was maintained with infusion of ketamine at 30 mg/kg·h and vecuronium bromide (0.05 mg/kg·h). Under local anesthesia, catheters were inserted into the left ear vein for infusion of saline or endotoxin, and into the right femoral artery for blood pressure monitoring and arterial blood analysis. Rabbits were mechanically ventilated with 40% oxygen for 20 min. Tidal volume was 10 ml/kg and respiratory rate was controlled to maintain an arterial carbon dioxide tension (PaCO2) of 35–45 mm Hg. Positive end-expiratory pressure was 3 cm H2O. Rabbits were placed on a heating pad to keep body temperature between 36.5℃ and 37.5℃ at the esophagus. After 20 minutes of ventilation, blood gas was analyzed and peripheral blood was collected. The 24 rabbits were then randomized into three groups: sham group, control group, and budesonide group. Rabbits in the sham group were injected with saline. Rabbits in the control and budesonide groups were injected with endotoxin (500 µg/kg, Escherichia coli endoxin, 0111:B4, Sigma, Saint Louis, Missouri, USA) via ear vein over 30 min to induce lung injury. After saline or endotoxin injection, rabbits in the sham and control groups received 5 ml saline for instillation, while rabbits in the budesonide group received budesonide (0.5 mg/kg, AstraZeneca Pharmaceuticals, Baldock, Herfordshire, UK)11 in 5 ml saline, which were instilled via the trachea. After instilling saline or budesonide, all rabbits were ventilated for 4 h. Arterial blood gas analysis and peripheral blood were obtained for further analysis before injecting saline or endotoxin (T0), and at 1 (T1), 2 (T2) 4 (T3) h after saline or budesonide instillation.
After 4 h of ventilation, all the rabbits were allowed to spontaneously recover their breath from anesthesia, and then extubated. Survival in each group was assessed every 24 h for seven days. During the seven days, incisions of all rabbits were locally anesthetized with lidocaine for pain relief. Moribund animals were defined as bradycardia with a heart rate lower than 40 beats per minute, severely lethargic, and unresponsive to painful stimulation.
Moribund and survivor rabbits (after seven days) were euthanized by thiamylal overdose. Then, lungs were collected for histological examination or bronchoalveolar lavage fluid (BALF) was performed. Arterial blood analysis was performed and peripheral blood was collected for analysis.
Airway pressure and dynamic compliance
Peak pressure and dynamic compliance were monitored with Datex/Ohmeda S/5 (Datex Instrumentation, Helsinki, Finland).
Arterial blood gas analysis
Arterial blood gases were analyzed at all time-points during ventilation, and seven days with Bayer Rapidlab 348 (Bayer Diognostics, Germany). PaO2/FiO2 ratio was calculated.
Pulmonary alveolocapillary permeability
At day 7, part of the right lobe was weighed and dried to a constant weight for 48 h at 60℃. Wet/dry weight (W/D) ratio was calculated.
Local and systemic inflammation
After euthanasia, BALF was harvested from the left lung by infusing 4℃ of saline (15 ml/kg) containing (EDTA)-2Na, and withdrawal for five times. A cytocentrifuged spin preparation (CF-RD, Sakura, Tokyo, Japan) of BALF was stained for cell differentiation. BALF was centrifuged at 1000 g for 15 min at 4℃. After centrifugation, lavage fluids were immediately stored at −80℃. White blood cells and percentage of neutrophils in BALF were counted using a cell counter. Concentration of neutrophils elastase was analyzed. Then, TNF-α, IL-1β, IL-8, and IL-10 levels in peripheral blood and BALF were analyzed (Sigma-Aldrich, St. Louis, MO, USA). The sensitivity of these kits was: TNF-α, 3.12 pg/ml; IL-1β, 2.8 pg/ml; IL-8, 6.1 pg/ml; and IL-10, 2.9 pg/ml.
Histopathologic analysis of lung tissue
HE stain was performed to assess histopathologic injury. The right lower lobes were fixed with 10% formalin. Lungs were embedded in paraffin, and 4 -µm sections were stained with hematoxylin and eosin. Two independent pathologists, who were unaware of the experimental groupings, analyzed and scored the lung injuries under light microscopy (0, minimum damage; 1, mild damage; 2, moderate damage; 3, severe damage; and 4, maximum damage) according to the assessment of alveolar congestion, edema, neutrophil infiltration in airspace or vessel wall, hemorrhage, alveolar wall thickness and hyaline membrane formation.
Statistical analysis
All normally distributed data were presented as mean and SD, which were analyzed using SPSS version 11.0 statistical software (SPSS, Chicago, IL, USA). Normally distributed data were analyzed using an unpaired t-test for a single time-point, non-normally distributed data were analyzed using the Mann–Whitney rank sum test, and histologic data were analyzed with the Wilcoxon U-test. Survival curves were derived using the Kaplan–Meier method, and differences were evaluated by log-rank tests. P < 0.05 was considered statistically significant.
Results
Budesonide improved respiratory function
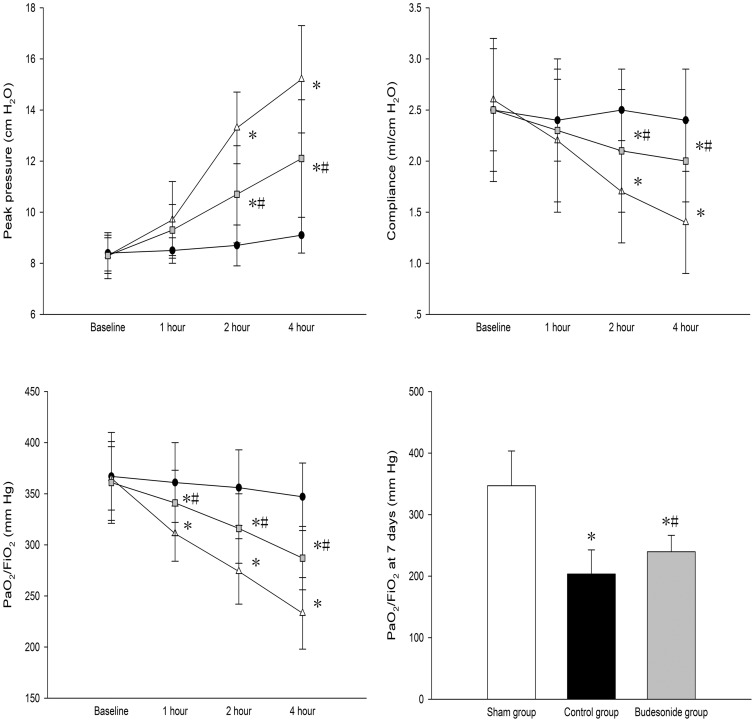
Compared to the sham group, after injecting endotoxin in both control and budesonide groups, peak pressure was significantly greater, while dynamic compliance and PaO2/FiO2 ratio were significantly lower within 4 h of ventilation. Peak pressure was significantly lower, while dynamic compliance and PaO2/FiO2 ratio were significantly higher in the budesonide group, compared to the control group. After seven days, PaO2/FiO2 ratio in both control and budesonide groups were significantly lower than the sham group. PaO2/FiO2 ratio in the budesonide group was higher than the control group after seven days (347.52 ± 56.74 vs. 203.31 ± 39.27 vs. 240.75 ± 26.36; P < 0.001, P < 0.001, P = 0.045) (Figure 1).
Figure 1.
Comparison of peak pressure, dynamic compliance, and PaO2/FiO2 among different groups is shown. *P < 0.05, compared with the sham group; #P < 0.05, compared with the control group. ( , Group S;
, Group S;  , Group C;
, Group C;  , Group B)
, Group B)
Budesonide improved alveolocapillary permeability
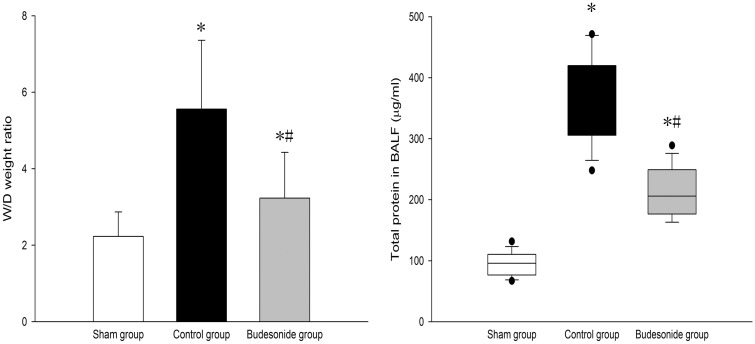
W/D weight ratio and total protein in BALF significantly increased in both control and budesonide groups, compared to the sham group, but were significantly lower in the budesonide group than in the control group; (2.23 ± 0.64 vs. 5.56 ± 1.81 vs. 3.33 ± 1.22; P < 0.001, P = 0.04, P = 0.01), (95.87 ± 19.52 vs. 369.83 ± 70.09 vs. 212.78 ± 39.87; P < 0.001, P < 0.001, P < 0.001) (Figure 2).
Figure 2.
Comparison of W/D weight ratio and total protein in BALF among the three groups is shown. *P < 0.05, compared with the sham group; #P < 0.05, compared with the control group. ( , Group S;
, Group S;  , Group C;
, Group C;  , Group B)
, Group B)
Budesonide inhibited inflammation
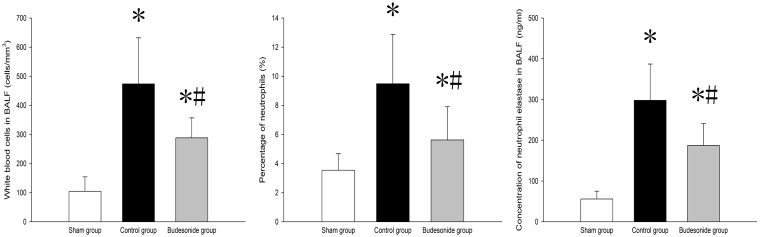
White cell count and percentage of neutrophils in BALF were higher in both control and budesonide groups than in the sham group, but were significantly lower in the budesonide group compared to the control group; (104.63 ± 49.92 vs. 473.56 ± 158.36 vs. 288.43 ± 68.61; P < 0.001, P < 0.001, P < 0.001), (3.54 ± 1.14 vs. 9.48 ± 3.39 vs. 5.62 ± 2.28; P < 0.001, P = 0.03, P = 0.01) (Figure 3). In addition, neutrophil elastase concentrations were significantly higher in both control and budesonide groups, compared to the sham group, but were lower in the budesonide group than in the control group (56.23 ± 19.45 vs. 298.78 ± 89.56 vs. 187.82 ± 54.93; P < 0.001, P < 0.001, P < 0.001) (Figure 3).
Figure 3.
White blood cell count, percentage of neutrophils and neutrophil elastase levels in BALF among different groups is shown. *P < 0.05, compared with the sham group; #P < 0.05, compared with the control group. ( , Group S;
, Group S;  , Group C;
, Group C;  , Group B)
, Group B)
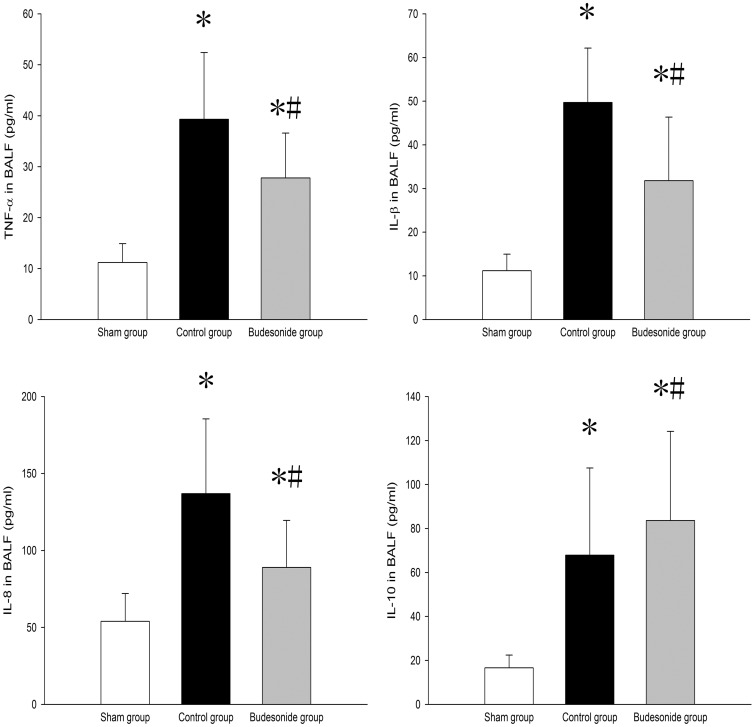
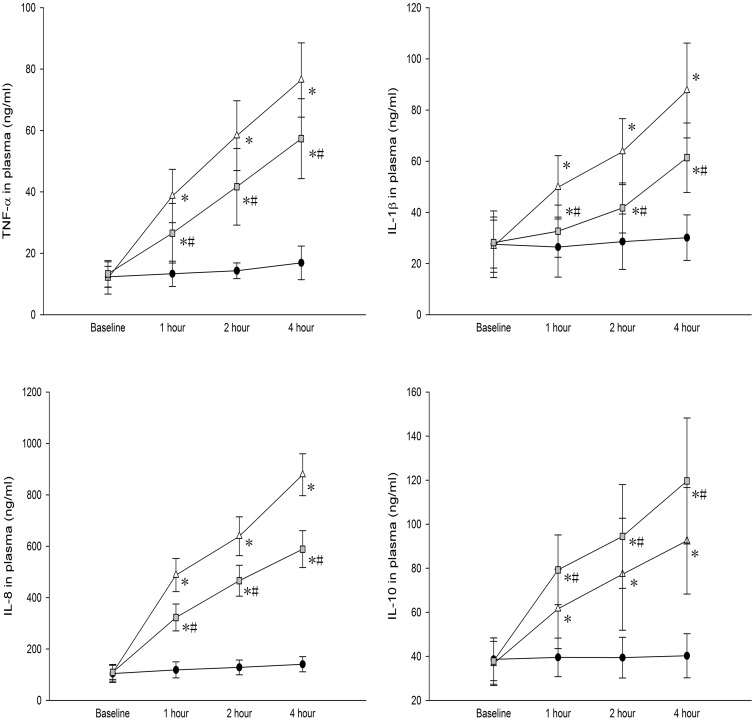
TNF-α, IL-1β, IL-8, and IL-10 levels were significantly higher in BALF and peripheral blood of both control and budesonide groups than in the sham group. Compared to the control group, TNF-α, IL-1β, and IL-8 levels in BALF were significantly lower; but IL-10 levels were significantly higher in the budesonide group (Figures 4 and 5).
Figure 4.
TNF-α, IL-1β, IL-8, and IL-10 levels in BALF among different groups are shown. *P < 0.05, compared with the sham group; #P < 0.05, compared with the control group. ( , Group S;
, Group S;  , Group C;
, Group C;  , Group B)
, Group B)
Figure 5.
TNF-α, IL-1β, IL-8, and IL-10 levels in serum of the different groups are shown. *P < 0.05, compared with the sham group; #P < 0.05, compared with the control group. ( , Group S;
, Group S;  , Group C;
, Group C;  , Group B)
, Group B)
Budesonide attenuated lung injury
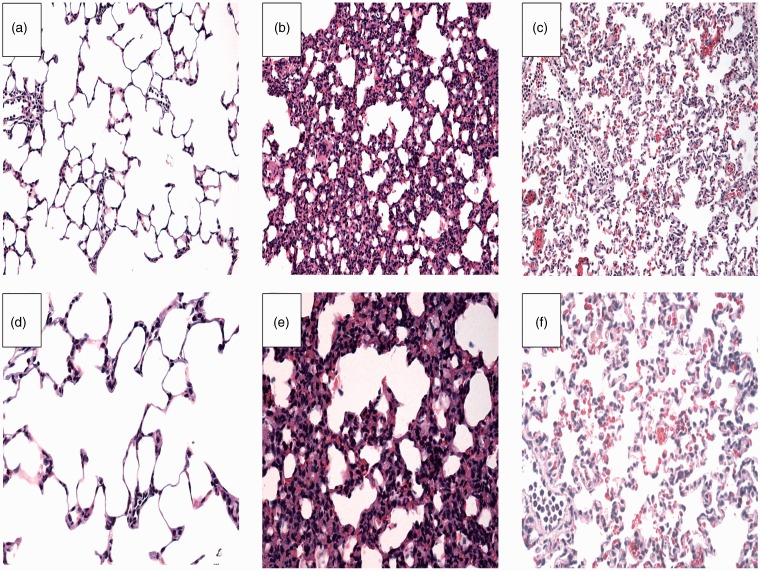
We observed typical ARDS pathologic aspects using light microscopy in the saline group such as severe edema, alveolar wall thickening, hyaline membrane formation, hemorrhage, and neutrophil infiltration in lung parenchyma. These lung tissue damages were notably reduced in the budesonide group (Figure 6). Lung injury scores in the control, saline and budesonide groups were 1 (range: 1 to 2), 3 (range: 2 to 4) and 2 (range: 2–3), respectively. Lung injury was significantly reduced in the budesonide group compared to the saline group (P < 0.05).
Figure 6.
Histopathological analysis of lung tissues among different groups is shown. (A, D) sham group, (B, E) control group, and (C, F) budisonide group. A–C: × 200; D–F: × 400. Lungs in the saline group showed thickened alveolar wall, edema and less alveolar space, and obvious inflammatory cell infiltration. Budesonide significantly decreased endotoxin-induced histopathological injury. (A color version of this figure is available in the online journal.)
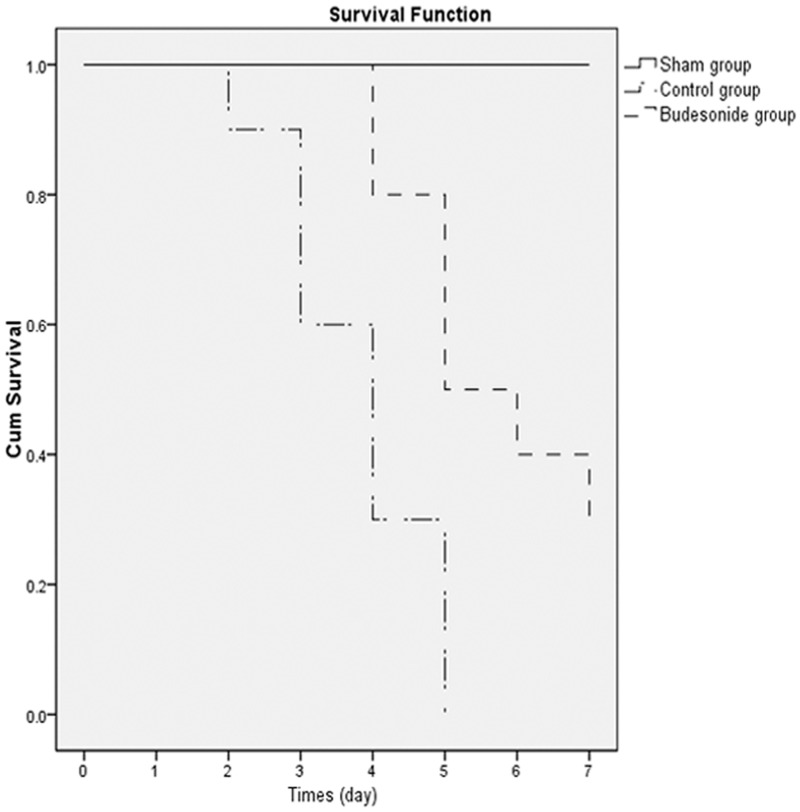
Budesonide improved rabbit survival with endotoxin-induced ARDS
To investigate the long-term therapeutic effect of budesonide on ARDS induced by intravenous injection of endotoxin, we analyzed the survival of rabbits with endotoxin-induced ARDS. Survival rate of rabbits treated with budesonide was 30%, compared to 0% for rabbits that received saline (P < 0.001) (Figure 7).
Figure 7.
Effects of budesonide on rabbit survival with endotoxin stimulation are shown. Survival was observed for seven days after the endotoxin challenge. Results were expressed as percent of survival (n = 10). Survival rate was estimated by the Kaplan-Meier method. Budesonide significantly delayed endotoxin-induced rabbit death
Discussion
In this study, we found that budesonide not only significantly reduced short-term lung injury including improved ventilation function, increased PaO2/FiO2 and reduced inflammation, but also improved the seven-day outcome such as decrease of alveolocapillary permeability and edema, histological injury, and survival in endotoxin-induced lung injury.
Endotoxin is derived from the cell wall of gram-negative bacteria and has been implicated as a major factor in the pathogenesis of septic shock caused by gram-negative bacteria.20 Endotoxin is known to induce infiltration of inflammatory cells and overproduction of cytokines, severe hypoxemia, decrease of compliance, accumulation and activation of neutrophil, increase of endothelial and epithelial permeability, and parenchymal injury.21 In experimental animals, endotoxin challenge causes pathophysiologic changes that are similar to those observed in human patients with septic shock.5,6 Endotoxin-induced ARDS could result in severe inflammation response, and lead to lung edema and injury.5,6 Effective control of inflammation could be the best treatment for ARDS. However, there are no effective drugs that could reduce endotoxin-induced lung injuries. Although glucocorticoids have been used for treating ARDS via inhibition of NF-κB and inflammation,11–14 systemic immunosuppression15 of glucocorticoids may allow infection to spread. Compared with other glucocorticoids, inhaled budesonide not only provides more effective local anti-inflammation, but also less systemic immunosuppression. More importantly, no evidence associated between budesonide, increased frequency or severity of infectious diseases,22,23 and safety of long-term budesonide applications has been accepted. In this study, we investigated the effects of budesonide on both short-term and long-term outcomes of ARDS to evaluate whether budesonide could be applied in patients with ARDS.
We found that inhaled budesonide significantly reduced peak pressure, and increased compliance and PaO2/FiO2 ratio, indicating that airway responsiveness was inhibited by budesonide. Indeed, budesonide reduces airway responsiveness in asthma patients,24 and it also improves airway pressure, compliance, and PaO2/FiO2 ratio in chlorine gas- or meconium aspiration-induced ARDS models.16,17 In addition, improved respiratory function may be associated with alveolocapillary permeability improvement. In this study, budesonide significantly reduced protein contents in BALF and lung edema. The protective effects of budesonide on alveolocapillary permeability might be due to local anti-inflammatory action.25
In endotoxin-induced ARDS, neutrophil elastase plays an important role in mediating neutrophil extravasation, tissue damage, and endothelial cell injury.26 Elastase inhibition could provide protection against lung injury.27 In this study, severe lung tissue injury increased neutrophil infiltration, and increased neutrophil elastase levels were observed in BALF after endotoxin injection. Compared to the control group, budesonide dramatically ameliorated hyaline membrane formation and hemorrhage areas, and significantly reduced lung injury scores. In addition, budisonide also significantly decreased white blood cell count, as well as the percentage of neutrophils and neutrophils elastase in BALF. Collectively, these data indicate that budesonide significantly inhibited the infiltration and activation of neutrophils.
A complex network of inflammatory cytokines, chemokines, and anti-inflammatory cytokines plays a major role in mediating, amplifying, and perpetuating lung injury during ARDS. During ARDS, TNF-α and IL-1β are key cytokines involved in inflammatory processes of ARDS.2,28 IL-1β stimulates the production of a variety of chemotactic cytokines and directly causes injury to endothelial and epithelial cells.29 TNF-α is not only an important pro-inflammatory cytokine, but also promotes apoptosis in ARDS.29,30 Furthermore, IL-8 is the most important chemokine of neutrophils, and promotes adherence, activation, and release of lysosomal enzymes of neutrophils during ARDS. In this study, budesonide significantly inhibited TNF-α, IL-1β, and IL-8 production in BALF; decreased inflammation; and reduced endotoxin-induced lung injury, which was consistent with previous reports associated with budesonide applications for ARDS or lung inflammation after lung ventilation.16–18,25 Budesonide also inhibits T cell-mediated epithelial inflammation, as well as inflammatory neutrophil recruitment and alveolar macrophage infiltration.24,31,32 In addition to inhibiting pro-inflammatory factors, budesonide amelioration on ARDS also includes the promotion of budesonide on anti-inflammatory factors.25 IL-10 can mediate anti-inflammatory responses through suppressing TNF-α and other pro-inflammatory cytokines.33 Budesonide increases IL-10 levels, but decreases pro-inflammatory factors in serum.34 Together, these data indicate that budesonide modulated immune cell response by adjusting pro-inflammatory cytokines and anti-inflammatory cytokines, and extended the immunoregulatory role of budesonide in different injury models.
Budesonide could reduce inflammation-induced lung injury.16–19 However, Liu et al. have shown that a high dosage of dexamethasone was ineffective in reducing phosgene-induced lung injury.35 This may be due to the fact that phosgene-induced lung injury is non-inflammatory and cardiogenic, and the effect of corticosteroids in lung injury depends on its anti-inflammatory property. Moreover, among various lung injury factors including endotoxin, meconium, bleomycin, and chlorine, endotoxin is mostly used to model the consequences of bacterial sepsis, and it could mimic human ARDS caused by sepsis with high reproducibility.36 ARDS were caused by meconium, bleomycin, and chlorine in only few patients, compared with endotoxin. In addition, the physiopathology of ARDS that was caused by these factors could not mimic human ARDS.36
In conclusion, in this study, we demonstrated that budesonide could improve respiratory function, ameliorate lung injury and epithelial permeability, decrease edema, and inhibit local inflammation in an endotoxin-induced ARDS rabbit model. Therefore, budesonide inhalation is a potential approach for ARDS therapy in clinical practice. Results of this study may provide a new clinical therapeutic strategy for ARDS caused by lung infection.
ACKNOWLEDGEMENTS
This study was funded by the Department of Health of Heilongjiang Province (2014-351). We acknowledge Prof. Guang-xiao Cui (Department of Anesthesia, the 2nd Affiliated Hospital of Harbin Medical University) for his assistance in the study.
Ethics approval
This study was approved by the Experimental Animal Ethics Committee of Harbin Medical University. In caring for the animals, investigators adhered to the Care and Use of Laboratory Animals prepared by the Experimental Animal Ethics Committee of Harbin Medical University.
Authors’ contributions
WG and N-YJ carried out the immunoassays. WG and N-YJ participated in the design of the study, performed the statistical analyses, and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003; 167: 1027–35. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004; 202: 145–56. [DOI] [PubMed] [Google Scholar]
- 3.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995; 151: 293–301. [DOI] [PubMed] [Google Scholar]
- 4.Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med 2009; 180: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 5.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31: S195–9. [DOI] [PubMed] [Google Scholar]
- 6.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Moll Physiol 2005; 289: L807–15. [DOI] [PubMed] [Google Scholar]
- 7.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 2006; 176: 4995–5005. [DOI] [PubMed] [Google Scholar]
- 8.Park GY, Christman JW. Nuclear factor kappa B is a promising therapeutic target in inflammatory lung disease. Curr Drug Target 2006; 7: 661–8. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med 1996; 24: 1285–92. [DOI] [PubMed] [Google Scholar]
- 10.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol 2000; 12: 85–98. [DOI] [PubMed] [Google Scholar]
- 11.Rocco PR, Souza AB, Faffe DS, Passaro CP, Santos FB, Negri EM, Lima JG, Contador RS, Capelozzi VL, Zin WA. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med 2003; 168: 677–84. [DOI] [PubMed] [Google Scholar]
- 12.Silva PL, Garcia CS, Maronas PA, Cagido VR, Negri EM, Damaceno-Rodrigues NR, Ventura GM, Bozza PT, Zin WA, Capelozzi VL, Pelosi P, Rocco PR. Early short-term versus prolonged low-dose methylprednisolone therapy in acute lung injury. Eur Respir J 2009; 33: 634–45. [DOI] [PubMed] [Google Scholar]
- 13.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol 1995; 15: 943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science (New York, NY) 1995; 270: 286–90. [DOI] [PubMed] [Google Scholar]
- 15.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ 2008; 336: 1006–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intens Care Med 2002; 28: 352–7. [DOI] [PubMed] [Google Scholar]
- 17.Mokra D, Mokry J, Drgova A, Petraskova M, Bulikova J, Calkovska A. Intratracheally administered corticosteroids improve lung function in meconium-instilled rabbits. J Physiol Pharmacol 2007; 58: 389–98. [PubMed] [Google Scholar]
- 18.Kohno M, Haramoto M, Nakajima O, Yang L, Hinotsu S, Yokohira M, Imaida K, Kawakami K. Antedrug budesonide by intrapulmonary treatment attenuates bleomycin-induced lung injury in rats with minimal systemic adverse effects. Biol Pharm Bull 2010; 33: 1206–11. [DOI] [PubMed] [Google Scholar]
- 19.Jansson AH, Eriksson C, Wang X. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation, inflammation and injury in rats. Vasc Pharmacol 2005; 43: 101–11. [DOI] [PubMed] [Google Scholar]
- 20.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Med 2001; 29: 1303–10. [DOI] [PubMed] [Google Scholar]
- 21.Villa P, Ghezzi P. Animal models of endotoxic shock. Meth Mol Med 2004; 98: 199–206. [DOI] [PubMed] [Google Scholar]
- 22.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, Ullman A, Lamm CJ, O'Byrne PM. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003; 361: 1071–6. [DOI] [PubMed] [Google Scholar]
- 23.Sheffer AL, Silverman M, Woolcock AJ, Diaz PV, Lindberg B, Lindmark B. Long-term safety of once-daily budesonide in patients with early-onset mild persistent asthma: results of the Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) study. Ann Allergy, Asthma Immunol: Official publication of the American College of Allergy, Asthma, & Immunology 2005; 94: 48–54. [DOI] [PubMed] [Google Scholar]
- 24.Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J 2000; 15: 682–6. [DOI] [PubMed] [Google Scholar]
- 25.Ju NY, Gao H, Huang W, Niu FF, Lan WX, Li F, Gao W. Therapeutic effect of inhaled budesonide (Pulmicort(R) Turbuhaler) on the inflammatory response to one-lung ventilation. Anaesthesia 2014; 69: 14–23. [DOI] [PubMed] [Google Scholar]
- 26.Fujishima S, Morisaki H, Ishizaka A, Kotake Y, Miyaki M, Yoh K, Sekine K, Sasaki J, Tasaka S, Hasegawa N, Kawai Y, Takeda J, Aikawa N. Neutrophil elastase and systemic inflammatory response syndrome in the initiation and development of acute lung injury among critically ill patients. Biomed Pharmacother 2008; 62: 333–8. [DOI] [PubMed] [Google Scholar]
- 27.Kawabata K, Hagio T, Matsumoto S, Nakao S, Orita S, Aze Y, Ohno H. Delayed neutrophil elastase inhibition prevents subsequent progression of acute lung injury induced by endotoxin inhalation in hamsters. Am J Respir Crit Care Med 2000; 161: 2013–8. [DOI] [PubMed] [Google Scholar]
- 28.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 2003; 14: 523–35. [DOI] [PubMed] [Google Scholar]
- 29.Dunican AL, Leuenroth SJ, Grutkoski P, Ayala A, Simms HH. TNFalpha-induced suppression of PMN apoptosis is mediated through interleukin-8 production. Shock 2000; 14: 284–8; discussion 8-9.. [DOI] [PubMed] [Google Scholar]
- 30.Guan J, Jin DD, Jin LJ, Lu Q. Apoptosis in organs of rats in early stage after polytrauma combined with shock. J Trauma 2002; 52: 104–11. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SJ, Wallin A, Della-Cioppa G, Sandstrom T, Holgate ST. Effects of budesonide and formoterol on NF-kappaB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med 2001; 164: 1047–52. [DOI] [PubMed] [Google Scholar]
- 32.Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Budesonide and formoterol inhibit ICAM-1 and VCAM-1 expression of human lung fibroblasts. Eur Respir J 2000; 15: 68–74. [DOI] [PubMed] [Google Scholar]
- 33.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling–regulation of the immune response in normal and critically ill states. Crit Care Med 2000; 28: N3–12. [DOI] [PubMed] [Google Scholar]
- 34.John M, Lim S, Seybold J, Jose P, Robichaud A, O'Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am J Respir Crit Care Med 1998; 157: 256–62. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Pauluhn J, Trubel H, Wang C. Single high-dose dexamethasone and sodium salicylate failed to attenuate phosgene-induced acute lung injury in rats. Toxicology 2014; 315: 17–23. [DOI] [PubMed] [Google Scholar]
- 36.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295: L379–99. [DOI] [PMC free article] [PubMed] [Google Scholar]