Abstract
Hypoxic pulmonary hypertension (HPH), which is characterized by pulmonary arteriolar remodeling and right ventricular hypertrophy, is still a life-threatening disease with the current treatment strategies. The underlying molecular mechanisms of HPH remain unclear. Our previously published study showed that Wnt5a, one of the ligands in the Wnt family, was critically involved in the inhibition of hypoxia-induced pulmonary arterial smooth muscle cell proliferation by downregulation of β-catenin/cyclin D1 in vitro. In this study, we investigated the possible functions and mechanisms of Wnt5a in HPH in vivo. Recombinant mouse Wnt5a (rmWnt5a) or phosphate buffered saline (PBS) was administered to male C57/BL6 mice weekly from the first day to the end of the two or four weeks after exposed to hypoxia (10% O2). Hypoxia-induced pulmonary hypertension was associated with a marked increase in β-catenin/cyclin D1 expression in lungs. Right ventricular systolic pressure and right ventricular hypertrophy index were reduced in animals treated with rmWnt5a compared with PBS. Histology showed less pulmonary vascular remodeling and right ventricular hypertrophy in the group treated with rmWnt5a than with PBS. Treatment with rmWnt5a resulted in a concomitant reduction in β-catenin/cyclin D1 levels in lungs. These data demonstrate that Wnt5a exerts its beneficial effects on HPH by regulating pulmonary vascular remodeling and right ventricular hypertrophy in a manner that is associated with reduction in β-catenin/cyclin D1 signaling. A therapy targeting the β-catenin/cyclin D1 signaling pathway might be a potential strategy for HPH treatment.
Keywords: Hypoxic pulmonary hypertension, pulmonary arteriolar remodeling, right ventricular hypertrophy, Wnt5a
Introduction
Hypoxic pulmonary hypertension (HPH) is a fatal disease characterized by acute pulmonary arteriolar vasoconstriction and chronic pulmonary arteriolar remodeling.1 These processes lead to increasing pulmonary vascular resistance, pulmonary arterial pressure, subsequent hypertrophy and failure of the right ventricle (RV), and even death.2 Several improvements in understanding the molecular pathology of HPH have been achieved over the past decade.3 However, the underlying molecular mechanisms are still incompletely understood. Therefore, to elucidate the signaling pathways involved in the HPH is a key goal for developing novel effective therapeutics.
The Wnts are a family of lipid-modified secreted glycoproteins, comprise 19 ligands that play crucial roles in development, cell fate specification, polarity, migration, differentiation, and proliferation.4,5 The Wnt signaling pathways have been classified into canonical signaling pathway and non-canonical signaling pathway. In canonical Wnt signaling pathway which is most studied and best understood, Wnt ligands interact with the Frizzled receptors and then lead to β-catenin accumulation in cytoplasm and translocation into nucleus, where β-catenin binds to transcription factors and activates the transcription of downstream target genes like cyclin D1.5,6 However, the non-canonical Wnt signaling pathway does not require β-catenin as a co-transcription factor.7
Wnt5a is a representative and specific inducer of the non-canonical Wnt signaling pathway.8–10 One function of the non-canonical pathway is its inhibition of the canonical Wnt signaling pathway.11 β-catenin stabilization has been reported to play an important role in the regulation of vascular remodeling via cyclin D1.12–14 However, a possible contribution of Wnt5a on β-catenin/cyclin D1 in pulmonary vascular remodeling remains uncertain. As shown in our previously published study, Wnt5a inhibits hypoxia-induced pulmonary arterial smooth muscle cell (PASMC) proliferation by the downregulation of β-catenin/cyclin D1.14 Therefore, the aim of our study is to elucidate the effects of Wnt5a on HPH and its potential mechanisms in vivo. We hypothesized that Wnt5a may alter pulmonary vascular and right ventricular response to hypoxia and that this inhibition may act through the suppression of β-catenin/cyclin D1.
Materials and methods
Ethical approval
All animal manipulations were conducted in accordance with the Regulations for the Management of Laboratory Animals published by the Ministry of Science and Technology of the People’s Republic of China and approved by the Institutional Animal Care and Use Committee of Capital Medical University.
Materials
Recombinant mouse Wnt5a (rmWnt5a) was purchased from R&D Systems (Minneapolis, MN, USA). Tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibodies, mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) were from Zhong Shan-Golden Bridge Biological Technology Company (Beijing, China). Anti-β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and anti-α-smooth muscle actin (anti-α-SMA) antibodies were from Sigma-Aldrich (St. Louis, MO, USA). Anti-von Willebrand factor (vWF) and anticyclin D1 antibodies were from Abcam (Cambridge, UK). Anti-Wnt5a and anti-β-catenin (for total protein) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). RIPA buffer was from Applygen Technologies (Beijing, China). Protease and phosphatase inhibitors were from Roche Applied Science (Basel, Switzerland). Bovine serum albumin was from MP Biomedicals (Santa Ana, CA, USA). IRDye™800-conjugated goat anti-rabbit IgG secondary antibody was from Odyssey (Lincoln, NE, USA). Antiproliferating cell nuclear antigen (anti-PCNA) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animal models
Eight-week old male C57BL/6 mice weighing 20–25 g were purchased from Vital River Laboratory Animal Technology Company (Beijing, China). The animals were housed in a 12 h–12 h light–dark cycle with free access to water and standard chow diet. Animals were allowed to acclimate for three days before exposed to normobaric hypoxia (10% O2).2,15,16 Then mice were randomized to receive weekly rmWnt5a injections at 75 ng per mouse17,18 in a final volume of 150 µL or equal volume of phosphate buffered saline (PBS) via the tail vein from the first day to the end of the two or four weeks after exposed to hypoxia.
Hemodynamic and right ventricular hypertrophy (RVH) measurements
Mice were anesthetized with 2% sodium pentobarbital (50 mg/kg i.p.). Right ventricular systolic pressure (RVSP) which was used as an indicator of mean pulmonary arterial pressure was measured using closed-chest cardiac puncture.19 Then mice were sacrificed by cervical dislocation and the lungs and hearts were collected. The weights of RV and left ventricle plus interventricular septum (LV+S) were measured separately to evaluate the extent of RVHI. The index of RVH was calculated by the formula: RVHI(%) = RV/(LV+S) × 100. The diameter of individual cardiomyocytes in histological sections of the left and right ventricular walls was measured using Nikon microscope digital camera system and its image analysis program (Nikon, Tokyo, Japan).
Lung morphometric analysis
The left lungs embedded in paraffin were serially sectioned at a thickness of 4 µm for immunofluorescence. Pulmonary vessels with external diameters smaller than 100 µm were selected. Double-labeled immunofluorescence with anti-vWF (1:200 dilution) and anti-α-SMA (1:200 dilution) primary antibodies was used to quantify the muscularization of pulmonary vessels. vWF staining was performed to aid to identify vessels. α-SMA staining was used to differentiate the muscularized vessels. Pulmonary vessels were categorized as fully muscularized (>75% α-SMA positive circumference), partially muscularized (25–75% α-SMA positive circumference), and non-muscularized (<25% α-SMA positive circumference). Muscularization of pulmonary vessels was expressed as a percentage of each category of vessels to the total number of vessels examined.20 Medial thickness was counted as follows: percent medial thickness (%MT) = (circumferenceext/π-circumferenceint/π)/(circumferenceext/π) × 100. The circumferenceext and circumferenceint were demarcated by the external and internal elastic lamina. The data were divided into four groups based on the external diameters: 0–25, 26–50, 51–75, and 76–100 µm. Images of pulmonary vessels were captured with Nikon microscope digital camera system and circumferences were measured with its image analysis program.
Immunofluorescence and light microscopic analysis
Paraffin-embedded lung sections were deparaffinized in xylene and rehydrated through descending alcohol concentrations. Antigen retrieval was performed in citrate buffer using a microwave. Sections were incubated with 0.3% Triton X-100 for 10 min and then blocked with 10% goat serum for 1 h. They were then incubated with anti-Wnt5a (1:50 dilution), anti-β-catenin (1:50 dilution), or anticyclin D1 (1:25 dilution), in the presence of anti-α-SMA (1:200 dilution) overnight at 4℃. Samples were visualized with FITC-conjugated goat anti-mouse secondary antibody and TRITC-conjugated goat anti-rabbit secondary antibody (1:200 dilution) for 1 h at room temperature. Nuclei were counterstained with DAPI. Routine histochemical staining with H&E was used on heart sections to detect the diameter of individual cardiomyocytes of left and right ventricular walls. The sections were analyzed using Nikon microscope digital camera system. Images of β-catenin were captured by a confocal laser scanning microscope (FV1000, Olympus, Tokyo, Japan).
Western blot analysis
Lung homogenates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in 5% non-fat dry milk for 1 h, followed by incubation in anti-PCNA (1:100 dilution), anti-Wnt5a (1:50 dilution), anti-β-catenin (1:1000 dilution), anticyclin D1 (1:50 dilution), and anti-β-actin (1:6000 dilution) or GAPDH (1:6000 dilution) antibodies overnight at 4℃. The protein bands were visualized using the LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR, Lincoln, NE, USA). The value of the relative density of each target protein band was normalized to the density of the corresponding β-actin or GAPDH band.
Statistical analysis
Data were expressed as mean ± SEM. Unpaired Student’s t-test was used for comparisons between two groups. One-way ANOVA with the Newman–Keuls was used to evaluate differences between more than two groups. P < 0.05 was considered statistically significant.
Results
Hypoxia-induced pulmonary hypertension was accompanied with upregulation of Wnt5a and β-catenin/cyclin D1 in lungs
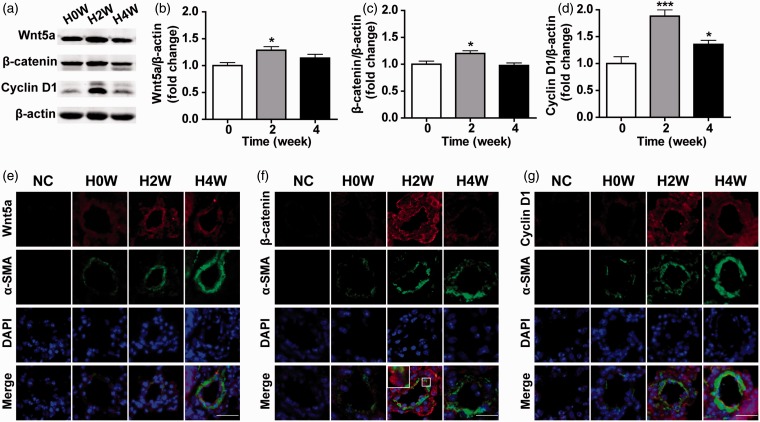
To induce the hypoxia-induced pulmonary hypertension animal model, mice were exposed to normobaric hypoxia for two and four weeks. Hypoxia significantly upregulated Wnt5a and β-catenin/cyclin D1 protein levels in lung tissue homogenates after two weeks (Figure 1(a) to (d), P < 0.05; n = 6). This agreed with the results of immunofluorescence, which showed that hypoxia induced Wnt5a and β-catenin/cyclin D1 upregulation in hypertensive pulmonary arterioles (Figure 1(e) to (g)). Nuclear translocation of β-catenin, which suggests its activation, can be observed as shown in the boxed area (Figure 1(f)). These results indicate that hypoxia-induced pulmonary hypertension is associated with Wnt5a and β-catenin/cyclin D1 upregulation.
Figure 1.
Hypoxia-induced pulmonary hypertension was associated with the upregulation of Wnt5a and β-catenin/cyclin D1 in lungs. (a) The Western blot analysis revealed the protein expression of Wnt5a, β-catenin, and cyclin D1 in lungs after exposed to hypoxia for zero, two, and four weeks. Statistical analysis of the expression levels of (b) Wnt5a/β-actin, (c) β-catenin/β-actin, and (d) cyclin D1/β-actin. Results are expressed as means ± SEM. (n = 6 mice for each group, *P < 0.05, ***P < 0.001, compared to 0 week.) Representative double-labeled immunofluorescence staining for the detection of (e) Wnt5a and α-SMA (f) β-catenin and α-SMA (g) cyclin D1 and α-SMA in the pulmonary arterioles after zero, two, and four weeks of hypoxia exposure. The nuclear translocation of β-catenin is shown in the boxed area. NC = negative control. (White scale bar = 25 µm; error bars indicate SEM.) (A color version of this figure is available in the online journal.)
Beneficial effects of Wnt5a on hypoxia-induced pulmonary hypertension
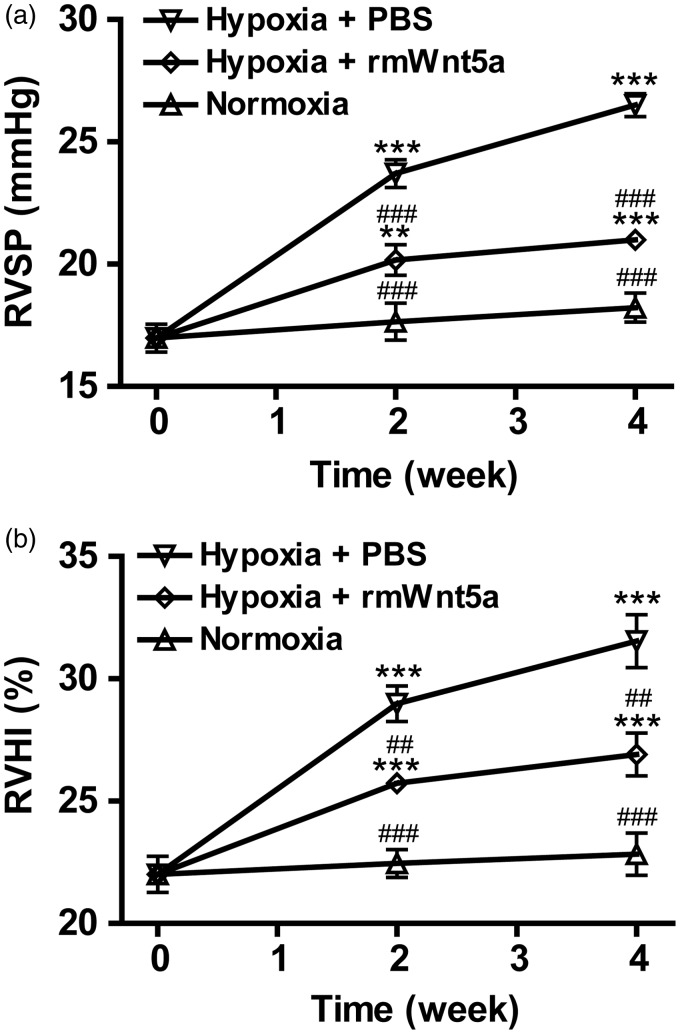
To understand the effects of Wnt5a on hypoxia-induced pulmonary hypertension, mice were exposed to normobaric hypoxia while receiving weekly injections of rmWnt5a or PBS. As shown in Figure 2(a), PBS-treated mice developed pulmonary hypertension characterized by significantly elevated RVSP in response to chronic hypoxia exposure as compared to normoxic group (normoxic mice without any injection) (Figure 2(a), P < 0.001; n = 8). The RVSP increased from 16.98 ± 0.57 to 23.70 ± 0.56 mmHg (Figure 2(a), P < 0.001; n = 8) and 26.50 ± 0.46 mmHg (Figure 2(a), P < 0.001; n = 8) after two and four weeks’ hypoxia exposure. However, compared with PBS-treated mice, the RVSP of rmWnt5a-treated mice was markedly reduced to 20.17 ± 0.63 mmHg (Figure 2(a), P < 0.001; n = 8) after two weeks and 20.99 ± 0.33 mmHg (Figure 2(a), P < 0.001; n = 8) after four weeks. As shown in Figure 2(b), RVHI in hypoxic PBS-treated mice was also demonstrated by the marked elevation in the index of RVHI as compared with normoxic group (Figure 2(b), P < 0.001; n = 8). RVHI grew from 22.01 ± 0.74 to 28.98 ± 0.72% (Figure 2(b), P < 0.001; n = 8) and 31.54 ± 1.08% (Figure 2(b), P < 0.001; n = 8) after exposed to hypoxia for two and four weeks. A prominent reduction in RVHI was observed in those animals treated with rmWnt5a. The RVHI of rmWnt5a-injected mice decreased to 25.73 ± 0.33% (Figure 2(b), P < 0.01; n = 8) after two weeks and 27.91 ± 0.88% (Figure 2(b), P < 0.01; n = 8) after four weeks. The results show that chronic hypoxia exposure effectively induces pulmonary hypertension in mice which can be distinctly attenuated by rmWnt5a.
Figure 2.
Beneficial effects of Wnt5a on hypoxia-induced pulmonary hypertension. Pretreatment with rmWnt5a attenuated hypoxia-induced pulmonary hypertension as determined by (a) right ventricular systolic pressure (RVSP) and (b) the index of right ventricular hypertrophy (RVHI) after zero, two, and four weeks of hypoxia exposure. Data are represented as means ± SEM. (n = 8 mice for each group; **P < 0.01, ***P < 0.001, compared to 0 week; ##P < 0.01, ###P < 0.001, compared to PBS-treated group at corresponding time points; error bars indicate SEM.)
The antiremodeling effects of Wnt5a on hypoxia-induced pulmonary arteriolar remodeling
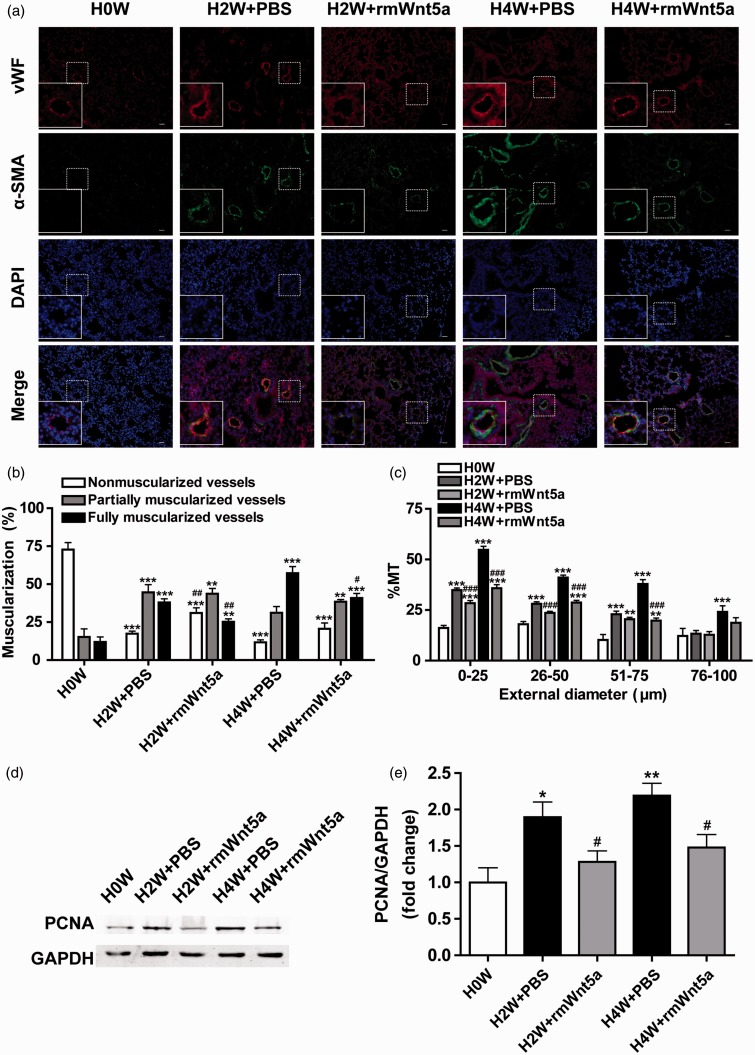
Abnormal muscularization and progressive medial thickening are major characteristics of pulmonary arteriolar remodeling in HPH.21 To investigate the effects of Wnt5a on hypoxia-induced remodeling of pulmonary arterioles, immunofluorescence of lungs from rmWnt5a-treated mice and PBS-treated mice was stained with vWF (endothelial cell marker) and α-SMA (smooth muscle cell marker) for morphometric analysis. Representative immunofluorescence exhibited that muscularization and MT of pulmonary vessels were evidently increased in PBS-treated mice under hypoxia. However, these changes appeared to be ameliorated in rmWnt5a-treated mice (Figure 3(a)).
Figure 3.
The antiremodeling effects of Wnt5a on hypoxia-induced pulmonary arteriolar. (a) Representative double-labeled immunofluorescence staining with antibodies specific for vWF and α-SMA in the pulmonary arterioles after zero, two, and four weeks of hypoxia exposure with or without rmWnt5a administration. (White scale bar = 25 µm.) (b) Percentage of non-muscularized vessels, partially muscularized vessels, and fully muscularized vessels in PBS- and rmWnt5a-treated mice. (c) Quantitative analysis of the percent medial thickness (%MT) in PBS- and rmWnt5a-treated mice. Pulmonary arterioles were separated into four groups according to the external diameter: 0–25 , 26–50, 51–75, and 76–100 µm. (d) The Western blot analysis revealed the protein expression of PCNA in lungs after exposed to hypoxia for zero, two, and four weeks in PBS- and rmWnt5a-treated mice. (e) Quantitative analysis of the proliferating rates of cells in the mouse lung determined by PCNA/GAPDH. (n = 5 mice for each group; *P < 0.05, **P < 0.01, ***P < 0.001, compared to 0 week; #P < 0.05, ##P < 0.01, ###P < 0.001, compared to PBS-treated group at corresponding time points; error bars indicate SEM.) (A color version of this figure is available in the online journal.)
Quantitative analysis of the muscularization of peripheral pulmonary vessels demonstrated a substantially growing proportion of full muscularization from 11.96 ± 3.27 to 37.96 ± 2.40% (Figure 3(b), P < 0.001; n = 8) and 57.19 ± 4.41% (Figure 3(b), P < 0.001; n = 8), and a decreasing proportion of non-muscularization from 72.82 ± 4.59 to 17.38 ± 1.65% (Figure 3(b), P < 0.001; n = 8) and 11.69 ± 1.57% (Figure 3(b), P < 0.001; n = 8) in PBS-treated group after two and four weeks’ hypoxia exposure. In contrast, rmWnt5a significantly reduced the percentage of fully muscularized vessels to 25.24 ± 1.90% (Figure 3(b), P < 0.01; n = 8) and 40.86 ± 3.01% (Figure 3(b), P < 0.05; n = 8), and it raised the percentage of non-muscularized vessels to 31.06 ± 3.43% (Figure 3(b), P < 0.01; n = 8) and 20.66 ± 3.83% (Figure 3(b), n = 8) compared with the PBS-treated group at the end of two and four weeks.
The %MT was used to evaluate medial thickening of pulmonary arterioles, which were divided into four orders according to their external diameters. Compared with PBS-treated group, %MT was significantly decreased in rmWnt5a-treated group with external diameters smaller than 75 µm. %MT of pulmonary arterioles in rmWnt5a-treated mice decreased by 18.50 and 34.62% (Figure 3(c), P < 0.001; n = 8) (external diameter 0–25 µm), 15.98 and 29.97% (Figure 3(c), P < 0.001; n = 8) (external diameter 26–50 µm), 10.74 and 47.65% (Figure 3(c), P < 0.001; n = 8) (external diameter 51–75 µm) after exposed to hypoxia for two and four weeks. These data suggest that simultaneous rmWnt5a administration can ameliorate hypoxia-induced pulmonary arteriolar progressive remodeling.
To affirm Wnt5a’s effect on inhibiting the proliferation of cells in the mouse lung we performed western blot analysis to detect the protein level of PCNA, a cell proliferation indicator. The results showed that compared to the normoxic control, the PCNA level was 1.89-fold (Figure 3(d) and (e), P < 0.05; n = 5) and 2.19-fold (Figure 3(d) and (e), P < 0.01; n = 5) higher in PBS-treated mice after two and four weeks’ exposure to hypoxia. However, in the lung tissues of hypoxic mice injected with rmWnt5a, the PCNA protein level was down to 0.68 - and 0.67-fold compared to the PBS-treated mice, which indicates that rmWnt5a inhibited the hypoxia-induced cell proliferation (Figure 3(d) and (e), P < 0.05; n = 5).
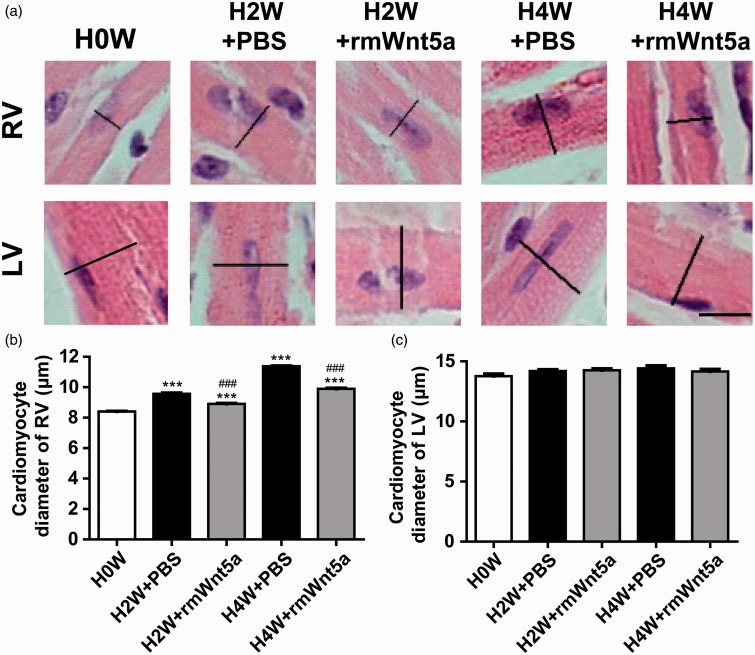
Wnt5a alleviated hypoxia-triggered hypertrophy of individual cardiomyocytes from RV
The presence of RVH is a hallmark of the end-stage pulmonary hypertension.22 To determine whether the administration of rmWnt5a could also protect against the development of hypoxia-triggered right heart hypertrophy in mice, H&E staining of individual cardiomyocytes was performed on the histological section of the LV and RV (Figure 4(a)). The RVH, expressed as the diameter of right ventricular cardiomyocytes, was prominently elevated by 13.80 and 35.29% (Figure 4(b), P < 0.001; n = 8) after hypoxia exposure for two and four weeks in the PBS-treated animals. Treatment with rmWnt5a markedly reduced right ventricular cardiomyocytes diameter by 6.87 and 12.99% (Figure 4(b), P < 0.001; n = 8). Nevertheless, neither hypoxia exposure nor rmWnt5a injections affected the diameter of left ventricular cardiomyocytes (Figure 4(c), P > 0.05; n = 8). These date demonstrate that rmWnt5a can effectively protect against the hypoxia-triggered hypertrophy of individual cardiomyocytes in the RV.
Figure 4.
Wnt5a alleviated right ventricular hypertrophy in hypoxia-induced pulmonary hypertension. (a) H&E stained paraffin-embedded sections of cardiomyocytes from left and right ventricle (black scale bar = 10 µm). (b) Quantitative analysis of the diameter of individual cardiomyocytes in right ventricular wall. (c) Quantitative analysis of the diameter of individual cardiomyocytes in left ventricular wall. (n = 8 mice for each group; ***P < 0.001, compared to 0 week; ###P < 0.001, compared to PBS-treated group at corresponding time points; error bars indicate SEM.) (A color version of this figure is available in the online journal.)
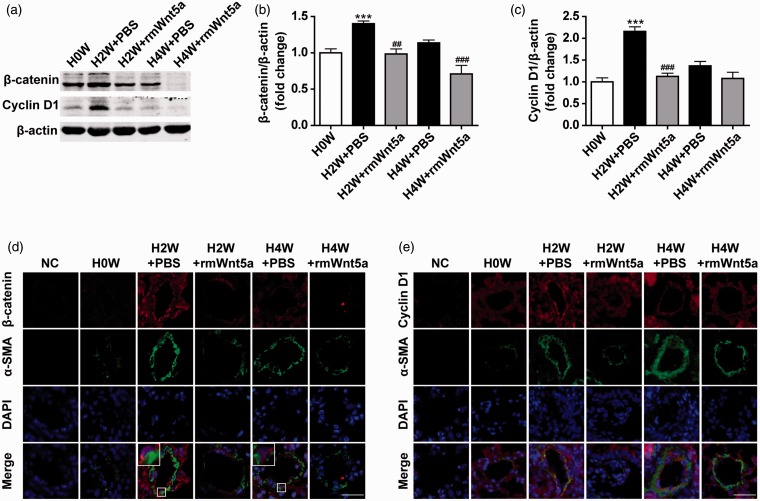
Wnt5a downregulated β-catenin/cyclin D1 in HPH mouse lungs
To elucidate whether Wnt5a’s ability to attenuate HPH was associated with suppression of β-catenin/cyclin D1, lung homogenates were detected for β-catenin and cyclin D1 expression (Figure 5(a)). β-catenin and cyclin D1 protein levels were 1.40-fold (Figure 5(a) and (b), P < 0.001; n = 8) and 2.16-fold (Figure 5(a) and (c), P < 0.001; n = 8) higher in PBS-treated mice after two weeks exposed to hypoxia. Nuclear translocation of β-catenin, which indicates its activation, can be observed as shown in the boxed areas (Figure 5(d)). Treatment with rmWnt5a diminished β-catenin and cyclin D1 protein levels to 0.70-fold (Figure 5(a) and (b), P < 0.01; n = 8) and 0.52-fold (Figure 5(a) and (c), P < 0.001; n = 8) compared to the PBS-treated mice. The immunofluorescent staining displayed the same results as western blot analysis (Figure 5(d) and (e)). These dates reveal that rmWnt5a downregulates the expression of β-catenin/cyclin D1 in HPH.
Figure 5.
Wnt5a downregulated β-catenin/cyclin D1 in hypoxic pulmonary hypertension in mouse lungs. (a) The Western blot analysis revealed the protein expression of β-catenin and cyclin D1 in lungs after exposed to hypoxia for zero, two, and four weeks in PBS- and rmWnt5a-treated mice. Statistical analysis of the expression levels of (b) β-catenin/β-actin and (c) cyclin D1/β-actin are represented. Results are expressed as means ± SEM. (n = 8 mice for each group, ***P < 0.001, compared to 0 week; ##P < 0.01, ###P < 0.001, compared to PBS-treated group at corresponding time points.) Representative double-labeled immunofluorescence staining for the detection of (d) β-catenin and α-SMA (e) cyclin D1 and α-SMA in the pulmonary arterioles after zero, two, and four weeks of hypoxia exposure in PBS- and rmWnt5a-treated mice. The boxed areas display the nuclear translocation of β-catenin. NC = negative control. (White scale bar = 25 µm; error bars indicate SEM.) (A color version of this figure is available in the online journal.)
Discussion
HPH is a pathophysiologic status caused by many sorts of pulmonary diseases.23,24 Although recent evidence suggests that current treatment strategies (prostacyclin analogs, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors) have improved the symptoms of patients with pulmonary hypertension, the mortality rates still remain high.25–27 In this study, we have demonstrated that Wnt5a ameliorates pulmonary vascular remodeling and RVH. Furthermore, we have revealed that the beneficial effects of Wnt5a on HPH are associated with the downregulation in β-catenin/cyclin D1 signaling. Targeting β-catenin/cyclin D1 signaling pathway might be a promising therapeutic strategy for HPH.
Wnt members are involved in vascular cell survival, proliferation, migration, and morphogenesis.28,29 The Wnt signaling pathways, which are initiated by the Wnt proteins, include canonical Wnt signaling and non-canonical ones. So far, the canonical Wnt/β-catenin pathway has been studied extensively, while the functions of non-canonical Wnt pathways remain to be further explored. It is generally considered that non-canonical Wnt pathways consist of planar cell polarity pathway (Wnt/PCP pathway or Wnt/JNK pathway in vertebrates) and Wnt/Ca2+ pathway. Wnt/PCP pathway is believed to be involved in the regulation of cell polarity, cytoskeletal organization, and gastrulation movements.30,31 Laumanns found out that Wnt/PCP pathway may participate in the regulation of vascular remodeling in idiopathic pulmonary arterial hypertension (IPAH).32 Wnt/Ca2+ pathway can induce the release of intracellular calcium and regulates the activation of protein kinase C and calcium/calmodulin-regulated kinase (CamKII). Despite the different downstream target genes and the fact that non-canonical pathways can antagonize the canonical pathway, they all signal through the combination of Wnt ligands and Fzd receptors.30,31,33–35
Though not yet investigated systematically, Wnt5a, -7 a, -10 b, -11, and -13 have been shown to be expressed in vascular cells.36 Recently, Wnt11 was reported to be upregulated in pulmonary arteries from IPAH patients compared with healthy donors.32 A growing number of evidence has suggested that Wnt5a may be involved in the pathogenesis of pulmonary hypertension. It has been shown to promote or inhibit endothelial cell proliferation.37,38 Our previously published study demonstrated that Wnt5a inhibits hypoxia-induced PASMC proliferation.14 Wnt5a also presents as a key regulator of fibroblast proliferation and resistance to apoptosis.39 Elevated Wnt5a expression has been proven to be important for cancer progression of lung, stomach, skin, and prostate. However, Wnt5a inhibits tumor cell proliferation in other tumor models, including hematopoietic tissues, brain, breast, thyroid, and uroepithelial cancers.40–42 These findings suggest that dysregulation of Wnt5a expression is involved in cell proliferation, although its exact role is still controversial. Meanwhile, pulmonary hypertension is characterized by the aberrant proliferation of vascular cells and the possible contribution of Wnt5a to the disease remains unclear. Thus in our study, in order to detect whether Wnt5a has any effect on HPH, mice were exposed to hypoxia and simultaneous administration with rmWnt5a weekly. The results show that chronic hypoxia exposure can effectively induce pulmonary hypertension in mice. Treatment with rmWnt5a leads to a significant decrease of pulmonary arterial pressure, muscularization, and medial thickening.
In the study we have noticed that Wnt5a was increased by hypoxia at two weeks. The real meaning of the transient Wnt5a upregulation observed during hypoxia exposure (upregulated in two weeks and downregulated in four weeks) still cannot be clearly explained and should be further investigated. We inferred that it might be the outcome of a self-feedback mechanism. It is quite possible that for the protection of the organism itself, Wnt5a showed a compensatory upregulation to antagonize the damage caused by hypoxia exposure. And at later stage of hypoxia exposure, this protective mechanism gradually failed. Decompensation occurred for the severe consequences of hypoxia exposure and Wnt5a was finally downregulated. The experiments have demonstrated that the injections of rmWnt5a inhibited the development of HPH. Therefore, Wnt5a does have an inhibitory role in the disease.
There is also abundant evidence to support that β-catenin/cyclin D1, the downstream of canonical Wnt signaling plays a crucial role in pulmonary hypertension.43,44 In the present study, we confirmed that two weeks’ exposure to hypoxia enhanced β-catenin accumulation and the expression of the target gene cyclin D1 which indicates hypoxia activated Wnt/β-catenin signaling pathway. This was in accordance with previous reports.45–47 It has been shown that Wnt5a-mediated canonical and non-canonical pathways can have opposing effects on vascular cells and antagonize each other in order to finely balance vascular cell proliferation.37,38 We thus sought to determine whether Wnt5a ameliorated pulmonary arterial remodeling and RVH through downregulation of β-catenin/cyclin D1. We found that the expression of β-catenin/cyclin D1 in HPH was downregulated by rmWnt5a.
The reason why Wnt5a can activate both canonical and non-canonical pathways is that, as some researchers suggest, it is probably decided by several certain Wnts-binding receptors on the surface of the cells that Wnt5a enters.33,34 When binding to some specific receptors on the surface of the cells like Ror2, Wnt5a activates non-canonical pathway and possibly downregulates β-catenin/cyclin D1 and ultimately, canonical Wnt/β-catenin pathway may be inhibited. However, Wnt5a can also activate canonical Wnt pathway by binding to some other certain receptors like Fzd4 or Fzd7.33,34 Under the circumstances in our study, Wnt5a might activate the non-canonical pathway which antagonizes the canonical pathway and then downregulates β-catenin/cyclin D1.
Another interesting finding we have noticed is that the increase in cyclin D1 was considerably greater than that in β-catenin, which implies other pathways of activation of cyclin D1. According to many reports, cyclin D1 is the downstream target gene of not only canonical Wnt pathway but also many other signaling pathways. Some reports mentioned that cyclin D1 is a downstream key target gene of mTOR signaling pathway48 which can be activated by hypoxia.49 Besides, cyclin D1 is also downstream of NF-κB50 which can be activated by hypoxia as well.51 Both pathways can upregulate cyclin D1 when activated. In a word, in this study, the upregulation of cyclin D1 might be the result of the activation of many different signaling pathways altogether.
The presence of RVH is a prominent feature of the end-stage pulmonary hypertension.22 The Wnt signaling pathway was identified as a crucial mediator of cardiomyocyte hypertrophy. There has been reports implying that inhibition of canonical Wnt signaling attenuates LV remodeling.52 Recent data suggest that Wnt5a is required for the activation of protein synthesis and cardiomyocyte hypertrophy.53 In our study, hypoxia induced an elevation in diameters of right ventricular cardiomyocytes, while cardiomyocytes of the LV were not affected by hypoxia. Hypoxia-induced hypertrophy of RV and right ventricular cardiomyocytes were mitigated by rmWnt5a, which is opposite to the findings in Hagenmueller and his co-workers’ study.53 This is because, due to the difference of research conditions and specific diseases, Wnt5a can play quite different roles. According to some points of view, the reason why Wnt5a can act so differently is that it combines with diverse receptors on the surface of cytomembrane under certain circumstances.35
There are some limitations in our study. First, only the alterations of canonical β-catenin/cyclin D1 pathways were observed after rmWnt5a treatment. The possible involvement of non-canonical Wnt pathways is yet to be investigated. Second, although we have confirmed that Wnt5a has preventative effects on HPH, that whether it can reverse established HPH remains unclear. Since the relevance and translational value of the study would be higher with this issue solved, we also consider studying the therapeutic effects of Wnt5a on established HPH in further investigations.
In summary, the results of the present study have demonstrated for the first time that HPH is associated with the upregulation of the β-catenin/cyclin D1 pathway. RmWnt5a administration can improve pulmonary hemodynamics, pulmonary vascular remodeling, and RVH in vivo through suppression of β-catenin/cyclin D1. These findings may have significant clinical application value to HPH and point to novel targets for its treatments.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81070042, 81370152, and 81228001) and Beijing Natural Science Foundation (7142027).
Authors’ Contribution
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; YJ and WW conducted the experiments; YJ, WW, and SC performed statistical analyses; and YJ, WW, TY, JW, and JL materially participated in data interpretation and manuscript preparation. All authors approved the final version of the manuscript for submission. YJ and WW contributed equally to this work.
REFERENCES
- 1.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Cir Res 2005; 97: 95–8. [DOI] [PubMed] [Google Scholar]
- 2.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2011; 184: 1171–82. [DOI] [PubMed] [Google Scholar]
- 3.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res 2006; 7: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 2010; 107: 943–52. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–80. [DOI] [PubMed] [Google Scholar]
- 7.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 8.He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 2008; 135: 3871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 2010; 42: 21–31. [DOI] [PubMed] [Google Scholar]
- 10.Hung TH, Hsu SC, Cheng CY, Choo KB, Tseng CP, Chen TC, Lan YW, Huang TT, Lai HC, Chen CM, Chong KY. Wnt5A regulates ABCB1 expression in multidrug-resistant cancer cells through activation of the non-canonical PKA/β-catenin pathway. Oncotarget 2014; 5: 12273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 2003; 162: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slater SC, Koutsouki E, Jackson CL, Bush RC, Angelini GD, Newby AC, George SJ. R-cadherin:beta-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 2004; 24: 1204–10. [DOI] [PubMed] [Google Scholar]
- 13.Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res 2006; 99: 1329–37. [DOI] [PubMed] [Google Scholar]
- 14.Yu XM, Wang L, Li JF, Liu J, Li J, Wang W, Wang J, Wang C. Wnt5a inhibits hypoxia-induced pulmonary arterial smooth muscle cell proliferation by downregulation of beta-catenin. Am J Physiol Lung Cell Mol Physiol 2013; 304: L103–11. [DOI] [PubMed] [Google Scholar]
- 15.Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 2013; 127: 115–25. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y, Christou H, Liu L, Visner G, Mitsialis SA, Kourembanas S, Liu H. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med 2013; 188: 482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, Emeche CD, Hewitt KJ, Rosenthal DT, Leotlela PD, Wade MS, Yang SW, Brant L, Nickoloff BJ, Messina JL, Biragyn A, Hoek KS, Taub DD, Longo DL, Sondak VK, Hewitt SM, Weeraratna AT. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res 2008; 68: 10205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SE, Yoon JY, Jeong WJ, Jeon SH, Park Y, Yoon JB, Park YN, Kim H, Choi KY. H-Ras is degraded by Wnt/β-catenin signaling via β-TrCP-mediated polyubiquitylation. J Cell Sci 2009; 122: 842–8. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Liu J, Ma A, Miao R, Jin Y, Zhang H, Xu K, Wang C and Wang J. mTORC1 is involved in hypoxia-induced pulmonary hypertension through the activation of Notch3. J Cell Physiol 2014;229:2117–25. [DOI] [PubMed]
- 20.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol 2003; 285: H938–45. [DOI] [PubMed] [Google Scholar]
- 21.Faber JE, Szymeczek CL, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Zhang H. Alpha1-adrenoceptor-dependent vascular hypertrophy and remodeling in murine hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2007; 292: H2316–23. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 2008; 294: H801–9. [DOI] [PubMed] [Google Scholar]
- 23.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med (Berl) 2007; 85: 1317–24. [DOI] [PubMed] [Google Scholar]
- 24.Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013; 8: 97–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh ME, Hemnes AR, Robbins IM. Combination therapy in pulmonary arterial hypertension. Clin Chest Med 2013; 34: 841–55. [DOI] [PubMed] [Google Scholar]
- 26.Siehr SL, Ivy DD, Miller-Reed K, Ogawa M, Rosenthal DN, Feinstein JA. Children with pulmonary arterial hypertension and prostanoid therapy: long-term hemodynamics. J Heart Lung Transplant 2013; 32: 546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavasin MA, Demos-Davies KM, Schuetze KB, Blakeslee WW, Stratton MS, Tuder RM, McKinsey TA. Reversal of severe angioproliferative pulmonary arterial hypertension and right ventricular hypertrophy by combined phosphodiesterase-5 and endothelin receptor inhibition. J Transl Med 2014; 12: 314–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin AM, D’Amore PA. Wnt signaling in the vasculature. Angiogenesis 2002; 5: 1–9. [DOI] [PubMed] [Google Scholar]
- 29.Villar J, Cabrera-Benitez NE, Ramos-Nuez A, Flores C, Garcia-Hernandez S, Valladares F, López-Aguilar J, Blanch L, Slutsky AS. Early activation of pro-fibrotic WNT5A in sepsis-induced acute lung injury. Crit Care 2014; 18: 568–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Schans VA, Smits JF, Blankesteijn WM. The Wnt/frizzled pathway in cardiovascular development and disease: friend or foe? Eur J Pharmacol 2008; 585: 338–45. [DOI] [PubMed] [Google Scholar]
- 31.Lee PN, Pang K, Matus DQ, Martindale MQ. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Semin Cell Dev Biol 2006; 17: 157–67. [DOI] [PubMed] [Google Scholar]
- 32.Laumanns IP, Fink L, Wilhelm J, Wolff JC, Mitnacht-Kraus R, Graef-Hoechst S, Stein MM, Bohle RM, Klepetko W, Hoda MA, Schermuly RT, Grimminger F, Seeger W, Voswinckel R. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2009; 40: 683–91. [DOI] [PubMed] [Google Scholar]
- 33.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 2006; 4: e115–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, Arenas E, Schulte G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microgliaproinflammatory transformation. J Neuroinflammation 2012; 9: 111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q, Yin X, Widura S, Nelson J, Ruiz N, Camilli TC, Indig FE, Flaherty KT, Wargo JA, Frederick DT, Cooper ZA, Nair S, Amaravadi RK, Schuchter LM, Karakousis GC, Xu W, Xu X, Weeraratna AT. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov 2013; 3: 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masckauchan TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006; 21: 181–8. [DOI] [PubMed] [Google Scholar]
- 37.Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 2008; 365: 285–90. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin AM, Kitajewski J, D’Amore PA. Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 2007; 25: 25–32. [DOI] [PubMed] [Google Scholar]
- 39.Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, Kaminski N. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol 2009; 41: 583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying J, Li H, Chen YW, Srivastava G, Gao Z, Tao Q. WNT5A is epigenetically silenced in hematologic malignancies and inhibits leukemia cell growth as a tumor suppressor. Blood 2007; 110: 4130–2. [DOI] [PubMed] [Google Scholar]
- 41.Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis 2013; 34: 2629–38. [DOI] [PubMed] [Google Scholar]
- 42.Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res 2008; 14: 55–61. [DOI] [PubMed] [Google Scholar]
- 43.Alapati D, Rong M, Chen S, Hehre D, Hummler SC, Wu S. Inhibition of beta-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2014; 51: 104–13. [DOI] [PubMed] [Google Scholar]
- 44.Zeng DX, Xu GP, Lei W, Wang R, Wang CG, Huang JA. Suppression of cyclin D1 by plasmid-based short hairpin RNA ameliorated experimental pulmonary vascular remodeling. Microvasc Res 2013; 90: 144–9. [DOI] [PubMed] [Google Scholar]
- 45.Peng J, Lai ZG, Fang ZL, Xing S, Hui K, Hao C, Jin Q, Qi Z, Shen WJ, Dong QN, Bing ZH, Fu DL. Dimethyloxalylglycine prevents bone loss in ovariectomized C57BL/6J mice through enhanced angiogenesis and osteogenesis. PLoS One 2014;9:e112744. [DOI] [PMC free article] [PubMed]
- 46.Varela-Nallar L, Rojas-Abalos M, Abbott AC, Moya EA, Iturriaga R, Inestrosa NC. Chronic hypoxia induces the activation of the Wnt/beta-catenin signaling pathway and stimulates hippocampal neurogenesis in wild-type and APPswe-PS1DeltaE9 transgenic mice in vivo. Front Cell Neurosci 2014; 8: 17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gersten M, Zhou D, Azad P, Haddad GG, Subramaniam S. Wnt pathway activation increases hypoxia tolerance during development. PLoS One 2014; 9: e103292–e103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu Q, Han N, Yuan X, Nie X, Wu H, Chen Y, Guo M, Yu S, Wu K. DACH1 inhibits cyclin D1 expression, cellular proliferation and tumor growth of renal cancer cells. J Hematol Oncol 2014; 7: 73–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 2014; 129: 864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development 2006; 133: 1045–57. [DOI] [PubMed] [Google Scholar]
- 51.Angelo MF, Aguirre A, Avilés Reyes RX, Villarreal A, Lukin J, Melendez M, Vanasco V, Barker P, Alvarez S, Epstein A, Jerusalinsky D, Ramos AJ. The proinflammatory RAGE/NF-kB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS One 2014; 9: e107901–e107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res 2010; 107: 1198–208. [DOI] [PubMed] [Google Scholar]
- 53.Hagenmueller M, Riffel JH, Bernhold E, Fan J, Katus HA, Hardt SE. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett 2014; 588: 2230–7. [DOI] [PubMed] [Google Scholar]