Abstract
Particulate air pollution (PAP) exposure is associated with increased morbidity and mortality, particularly in patients with renal disease. However, there are only a few studies on the interaction between PAP and renal injury, and none on agents that may ameliorate it. We studied the interaction between cisplatin (CP) nephrotoxicity and a single exposure to diesel exhaust particle (DEP) in rats 24 h before sacrifice, and assessed the effect of co-treatment with the active ingredient in Nigella Sativa seed oil, thymoquinone (TQ) thereon. Rats were injected intraperitoneally with CP (6 mg/kg) and four days later, they were exposed intratracheally to DEP (0.5 mg/kg), and were sacrificed 24 h later. Oral TQ (20 mg/kg) was given daily throughout the experimental period. CP alone caused several physiological, biochemical, and histopathological changes that included reduced growth and creatinine clearance, and raised plasma neutrophil gelatinase-associated lipocalin (NGAL), interleukin 6 (IL-6) and C-reactive protein (CRP), creatinine and urea concentrations, and urinary N-acetyl-b-D-glucosaminidase (NAG) activities. It adversely affected several indices of oxidative damage in the kidneys, and induced renal tubular necrosis. Most of these actions were significantly potentiated in rats given both CP and DEP. TQ significantly abrogated many of the effects of CP and DEP, given alone and in combination. These results provide experimental evidence that subjects with renal diseases can be at higher risk from PAP, and that TQ, pending further pharmacological and toxicological studies, can be considered a useful agent in patients with renal diseases and exposed to PAP.
Keywords: Cisplatin, diesel exhaust particle, nephrotoxicity, thymoquinone, rats
Introduction
Cisplatin (cis-diamminedichloroplatinum II, CP), first discovered in 1965, is still one of the most effective and commonly used chemotherapeutic agents in the treatment of variety of solid tumors including those of the head, neck, testis, ovary, breast, and penis,1,2 mainly because of its high efficacy and low cost. The drug's efficacy increases with dose, but high doses are associated with many severe adverse effects, particularly nephrotoxicity which is often the main dose-limiting factor in therapy with CP.3–5 Despite many studies, the mechanism underlying the side effects induced by CP is not fully understood, but is hypothesized to be multi-factorial in nature.5–9 These possible mechanisms include the generation of reactive oxygen species (ROS) and inflammation. The former could interfere with the antioxidant defense system and result in oxidative damage in different tissues, and reaction with thiols in protein and glutathione, which could cause cell dysfunction.10,11 The latter (inflammation) could be provoked by damage to the epithelial cells of the kidneys and may amplify renal injury and dysfunction in vivo.12 Mediated by glutathione-S-transferase, CP is known to be transformed in vivo into a more toxic compound in the kidneys.13
Various strategies and drugs have been attempted to ameliorate or prevent CP nephrotoxicity via simultaneous supplementation of preventive agents. These include antioxidants, modulators of nitric oxide, diuretics, cytoprotective, and anti-apoptotic agents and others.11,14–17 However, none of these have been tested clinically in humans.
Particulate air pollution (PAP), especially with particle diameter less than 2.5 mm such as diesel exhaust particle (DEP) is established to induce pulmonary and extrapulmonary morbidity and mortality on vital systems that include the cardiovascular, respiratory and renal systems, especially in at-risk populations such as the elderly and those with diabetes or hypertension.18,19
Acute renal failure (ARF, or acute kidney injury) is becoming increasingly prevalent in both the developed and the developing world, ensuing in severe morbidity and mortality,20 and is on the rise due to ageing of the population.21 About 20% of ARF cases among hospitalized patients are caused by CP nephrotoxicity, and more than a third of the patients develop renal injury within 10 days following a single dose of CP.22
The number of subjects affected by PAP is extremely large, and as a result, estimates of its public health burden are substantial.23 A meta-analysis of 110 peer-reviewed time series studies have shown that exposure to air pollution can cause several health adverse conditions, and significantly increases the risk of morbidity and mortality.24 Lung and kidney functions are closely related in both healthy and diseased subjects. Several studies have reported consistent association between ARF and pulmonary dysfunction.25,26 Lung injury is established to aggravate ARF and vice versa.27 When ARF and acute lung injury are combined, the mortality rate exceeds 80%.28 Experimentally, ARF has been reported to induce caspase-dependent pulmonary apoptosis.29 Patients with various renal diseases are particularly vulnerable to the adverse effects of PAP.30
Thymoquinone (TQ; 2-isopropyl-5-methyl-1, 4-benzoquinone) is the main active component of Nigella sativa seed oil. TQ is known to possess strong antioxidant and anti-inflammatory properties.31–33 As TQ has been previously found to be beneficial in several experimental models in which oxidative and inflammatory tissue injuries are the basis of toxicity,19,25,34,35 and as it is present in the oil of the commonly available black seed, a safe natural product, it was therefore of interest to test here if this compound has a possible ameliorative action against the combined toxicity of CP and DEP. As far as we are aware, this has not been reported before.
Materials and methods
Animals
Forty-eight male Wistar rats, aged 7–8 weeks and initially weighing about 200 g, were obtained from the Small Animal House of Sultan Qaboos University (SQU) and provided with standard laboratory diet and water ad libitum. They were randomly divided into eight equal groups and housed in plastic cages, at a temperature of 23 ± 2℃, relative humidity of 50–60% and a 12-h dark–light cycle. An acclimatization period of four days was allowed for the rats before any experimentation. The rats were weighed at the beginning of the experiment and just before sacrifice. Rats were cared for under a protocol approved by the Animal Research Ethics Committee of our institution, and according to the NIH Guide for the Care and Use of Laboratory Animals, NIH publication no. 85-23, 1985.
Particles
DEP (SRM 2975) was obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, MD), and was suspended in sterile normal saline (NaCl 0.9%) containing Tween 80 (0.01%). To prevent aggregation of its particles, it was sonicated for 15 min and vortexed before dilution and intratracheal (i.t.) administration. Control rats were given the vehicle Tween 80 (0.01%). The size of the DEP used in this study has been analyzed before by transmission electron microscopy.18
Treatments
A summary of the treatment of the eight groups is given in Table 1. The groups were treated as follows:
Table 1.
Treatments with intratracheal instillation of Saline (Sa) or diesel exhaust particles (DEP) with or without cisplatin (CP) administration (given intraperitoneally) in rats
| Groups/treatment | CO | TQ | Saline | CP | Sa | DEP |
|---|---|---|---|---|---|---|
| Control | + | − | + | − | + | − |
| CP | + | − | − | + | + | − |
| DEP | + | − | − | − | − | + |
| CP + DEP | + | − | − | + | + | + |
| TQ | + | + | + | − | − | − |
| CP + TQ | + | + | − | + | + | − |
| DEP + TQ | + | + | − | − | − | + |
| CP + DEP + TQ | + | + | − | + | + | + |
Note: Thymoquinone, TQ (20 mg/kg) in corn oil (CO) was given orally continuously for 11 days, and CP (6 mg/kg) was injected intraperitoneally on the sixth day of treatment. DEP was given by instillation on the 11th day. All rats were killed on the 12th day.
Group 1
Corn oil (control, 0.5 mL/rat) given orally for 11 consecutive days, and on day 7 also given a single intraperitoneal injection of saline (0.5 mL per rat), and also subjected to a single i.t. administration of Tween 80 (0.01%) (as a vehicle of DEP) 24 h before sacrifice.
Group 2
Corn oil (control, 0.5 mL/rat) given orally for 11 consecutive days, and on day 7 also given a single intraperitoneal injection of CP (6 mg/kg), at a concentration of 1 mg/mL, and also subjected to a single i.t. administration of Tween 80 (0.01%) 24 h before sacrifice.
Group 3
Corn oil (control, 0.5 mL/rat) given orally for 11 consecutive days, and on day 7 also given a single intraperitoneal injection of saline (0.5 mL per rat), and also subjected to a single i.t. administration of DEP (0.5 mg/kg) 24 h before sacrifice.
Group 4
Corn oil (control, 0.5 mL/rat) given orally for 11 consecutive days, and on day 7 also given a single intraperitoneal injection of CP (6 mg/kg), at a concentration of 1 mg/mL, and also normal saline (control, 0.5 mL/rat) given orally for seven consecutive days, and subjected to a single i.t. administration of DEP (0.5 mg/kg).
Groups 5, 6, 7, and 8
These groups were treated in an identical manner to groups 1, 2, 3, and 4, respectively, except that all rats in groups 5–8 were also given TQ (20 mg/kg) orally by gavage from day 1 until 24 h before sacrifice.
One day before the rats were sacrificed, urine of each rat was collected over a 24-h period, and its volume measured. After 24-h of DEP or vehicle administration, rats were anesthetized with a combination of ketamine (60 mg/kg) and xylazine (5 mg/kg) given intraperitoneally. Blood was collected from the inferior vena cava in heparinized tubes and centrifuged at 900 g for 15 min at 5℃, to separate plasma. The plasma harvested was stored frozen at −80℃ to await biochemical analyses, and the rats were killed by an overdose of anesthesia. The kidneys and lungs were removed from the rats, washed with ice-cold saline, blotted with a piece of filter paper and weighed. A small piece from the left kidney and lung were fixed in 10% buffered formalin for subsequent histopathological processing, and the rest of the renal tissue was immediately deep frozen at −80℃ for measuring platinum concentration. The right kidney was also stored immediately deep frozen at −80℃ and was thawed (within less than a week) and homogenized in ice-cold saline to produce 10% (w/v) tissue homogenate for the biochemical assays.
Biochemical methods
Plasma creatinine and urea and urinary creatinine concentrations, as well as plasma L-c-glutamyltransferase (GGT), aspartate transaminase (AST), alanine transaminase (ALT) activities, and sodium (Na), potassium (K), and chloride (Cl) concentrations were measured by an automated analyzer, as described before.19,36 N-acetyl-D-glucosaminidase (NAG) activity in urine was measured using kits purchased from Diazyme, General Atomics (San Diego, CA, USA). Plasma neutrophil gelatinase-associated lipocalin (NGAL) was measured with a sandwich enzyme linked immunosorbent assay (BioPorto Diagnostics, Hellerup, Denmark).
Interleukin-6 (IL-6) and C-reactive protein (CRP) were measured by ELISA kits from R & D systems (Minneapolis, MN, USA). Kidney reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) and total antioxidant capacity (TAC) were measured as described before.16
Histopathology
The formalin-fixed lungs and kidneys were dehydrated in increasing concentrations of ethanol, cleared with xylene and embedded in paraffin. Five-micrometer sections were prepared from the tissue blocks and stained with hematoxylin and eosin. The microscopic examination of the tissue sections was carried out in a blinded fashion by a histopathologist who was unaware of the treatment each donor animal had received.
Measurement of renal platinum concentration
The CP concentration (as platinum, Pt) in renal tissue was measured and validated by inductively coupled plasma atomic emission spectrometry (Perkin Elmer, Shelton, CT, USA) as described previously.37
Drugs and chemicals
CP used was from Pharma GES (Unterach, Austria) and platinum standard solution and thymoquinone were from Sigma (St. Louis, MO, USA). The rest of the chemicals were of the highest purity grade available.
Statistical analysis
All values are presented as means ± S.E.M. The data were analyzed by analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparison test. Analysis of the histology scores was by a two-sided Mann–Whitney test. A value of p < 0.05 was selected as the criterion for statistical significance. All statistical analyses were performed with GraphPad Prism version 4.03 (GraphPad Software Inc, San Diego, CA, USA).
Results
To induce ARF, rats were treated with a single CP injection at a dose of 6 mg/kg and this resulted in ARF, similar to previously reported studies.16,26
As shown in Table 2, rats given saline or TQ alone gained an increase in their body weight of about 16.5% and 18.6%, respectively (p < 0.05), while those receiving CP only lost 15.3%, and those given DEP only gained about 8.7% (p < 0.05, when compared to control). However, rats treated with CP together with TQ lost 11.5% of their bodyweight, while rats treated with DEP together with TQ gained only 0.6% of their body weight. When the three agents (CP, DEP, and TQ) were given concomitantly, the treated rats lost 12.0% of their body weight. The kidney weights were not significantly changed in rats treated with CP, DEP, or TQ (given either singly or all together) when compared to saline-treated rats. However, treatment with CP + DEP significantly increased relative kidney weight (p < 0.05).
Table 2.
The effect of treatment of rats with saline (CON), cisplatin (CP), diesel exhaust particle (DEP), thymoquinone (TQ) singly or in combination, on biological characteristics
| CON | CP | DEP | CP + DEP | TQ | CP + TQ | DEP + TQ | CP + DEP + TQ | |
|---|---|---|---|---|---|---|---|---|
| Initial Body wt. (g) | 274.0 ± 7.6 | 279.3 ± 3.7 | 275.0 ± 6.8 | 279.2 ± 8.3 | 279.2 ± 5.2 | 274.1 ± 5.9 | 274.7 ± 13.5 | 276.7 ± 8.2 |
| Final Body wt. (g) | 290.2 ± 7.1 | 264.6 ± 5.5 | 283.8 ± 6.6 | 240.3 ± 11.8*,‡ | 297.6 ± 6.6§§ | 262.6 ± 9.5 | 275.3 ± 15.8 | 264.3 ± 6.5 |
| Change in body wt. (%) | 6.2 ± 1.9 | −5.5 ± 0.7** | 3.4 ± 2.8† | −11.8 ± 2.1***,‡‡‡ | 6.6 ± 1.3††,§§§ | −4.3 ± 1.7**,¶¶ | 0.3 ± 2.7§§ | −4.4 ± 0.7**,¶¶ |
| Kidney wt. (g) | 2.0 ± 0.1 | 2.5 ± 0.1 | 2.0 ± 0.1 | 2.7 ± 0.2**,‡‡ | 2.1 ± 0.1§ | 2.4 ± 0.1 | 2.0 ± 0.1§§ | 2.2 ± 0.1 |
| Kidney wt. (% final body wt.) | 0.7 ± 0.03 | 0.9 ± 0.05 | 0.7 ± 0.03 | 1.1 ± 0.06***,‡‡‡ | 0.7 ± 0.06§§§ | 0.9 ± 0.03 | 0.7 ± 0.01§§§ | 0.8 ± 0.06§§§ |
| Water intake (ml) | 21.3 ± 3.6 | 28.5 ± 3.3 | 22.3 ± 1.5 | 35.2 ± 2.3*,‡ | 19.0 ± 1.9§§ | 33.3 ± 3.6¶ | 19.7 ± 2.5§§,$ | 28.3 ± 2.9 |
| Urine output (ml) | 7.6 ± 0.8 | 19.5 ± 1.1*** | 11.8 ± 1.6†† | 20.6 ± 1.5***,‡‡‡ | 6.2 ± 0.7†††,‡,§§§ | 18.3 ± 1.9***,‡,¶¶¶ | 5.6 ± 0.6***,†,‡‡‡,§§§,$$$ | 13.3 ± 0.9*,†,§§,¶¶,## |
| Food intake (g) | 16.1 ± 1.4 | 15.2 ± 1.1 | 11.0 ± 1.4 | 9.5 ± 1.0**,† | 12.4 ± 1.7 | 12.2 ± 1.0 | 11.4 ± 0.7 | 12.2 ± 1.0 |
| Feces wt. (g) | 5.6 ± 0.4 | 5.2 ± 0.5 | 3.9 ± 1.0 | 3.2 ± 0.8 | 3.9 ± 0.6 | 5.1 ± 0.5 | 5.0 ± 0.4 | 4.5 ± 0.7 |
Note: Values in the table are means ± SEM (n = 6). TQ (20 mg/kg) in corn oil was given orally continuously for 11 days, and CP (6 mg/kg) was injected intraperitoneally on the 6th day of treatment. DEP was given by inhalation on the 11th day. All rats were killed on the 12th day.
p < 0.05, **p < 0.001, ***p < 0.0001 vs. CONTROL.
†p < 0.05, ††p < 0.001, †††p < 0.0001 vs. CP.
‡p < 0.05, ‡‡p < 0.001, ‡‡‡p < 0.0001 vs. DEP.
§p < 0.05, §§p < 0.001, §§§p < 0.0001 vs. CP + DEP.
¶p < 0.05, ¶¶p < 0.001, ¶¶¶p < 0.0001 vs. TQ.
$p < 0.05, $$p < 0.001, $$$p < 0.0001 vs. CP + TQ.
p < 0.05, ##p < 0.001, ###p < 0.0001 vs. DEP + TQ.
Water intake was insignificantly increased by treatment of CP or CP + TQ (by about 34% and 37%, respectively). Urine output was significantly increased in rats treated with CP, CP + DEP, and CP + TQ (p < 0.05). Rats treated with CP + DEP and TQ urinated significantly less than rats treated with CP + DEP (p < 0.05). Neither food intake, nor fecal outputs were significantly affected by any of the agents used.
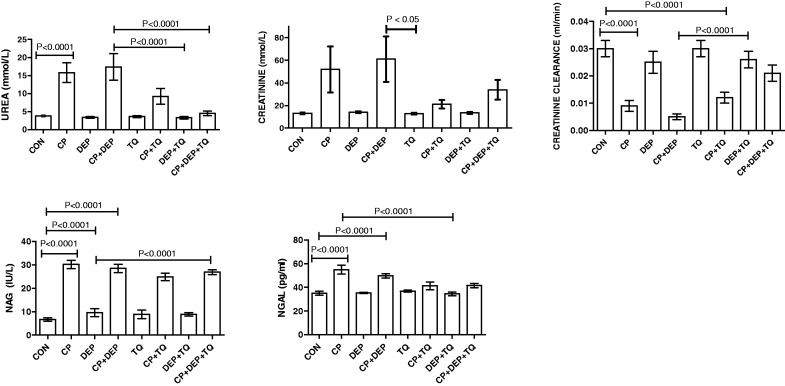
Figure 1 illustrates the effects of saline, CP, DEP, TQ, each one alone or in combination, on some kidney function tests in plasma and urine. CP alone induced significant rises in the concentrations of urea, NGAL, and in NAG activity, and a significant reduction in creatinine clearance (p < 0.001). Treatment of DEP alone did not significantly affect any of the indices measured. Treatment of rats with CP + DEP did not significantly affect the actions of CP mentioned above. In CP and CP + DEP-treated rats, however, the concentration of urea were significantly higher than the corresponding values obtained from control rat (p < 0.05) or rats treated with CP + DEP + TQ. The values for the other renal function tests in plasma and urine were insignificantly abated in rats treated with CP + DEP + TQ, when compared with values obtained from rats treated with CP alone, or CP + DEP. Creatinine clearance was significantly diminished in groups treated with CP, CP + DEP, and CP + TQ when compared (p < 0.05), with that in saline-treated control (p < 0.0001).
Figure 1.
Plasma urea, creatinine and NGAL concentrations, and urine NAG activity, as well as creatinine clearance in rats treated with saline (control, CON), cisplatin (CP) 6 mg/kg, diesel exhaust particles (DEP) 0.5 mg/kg, CP + DEP, thymoquinone (TQ) 20 mg/kg, CP + TQ, DEP + TQ, and CP + DEP + TQ. Each column represents mean ± SE (n = 6). Statistical analysis by Tukey–Kramer multiple comparison test
As shown in Table 3, CP significantly increased the plasma activities of ALT and AST (p < 0.05) and insignificantly increased the activity of GGT. These actions were potentiated by concomitant DEP treatment. Adding TQ to either CP or DEP alone or in combination abrogated these effects.
Table 3.
The effect of treatment of rats with cisplatin (CP),diesel exhaust particle(DEP), thymoquinone (TQ) given singly or in combination, on some biochemical parameters in plasma
| Groups | ALT (IU/L) | AST (IU/L) | GGT (IU/L) |
|---|---|---|---|
| Control | 27.3 ± 1.5 | 54.2 ± 1.7 | 3.6 ± 0.4 |
| CP | 47.3 ± 1.6** | 76.2 ± 8.9* | 4.8 ± 0.7 |
| DEP | 38.0 ± 2.4 | 70.6 ± 4.9 | 3.5 ± 0.4 |
| CP + DEP | 52.6 ± 6.5*** | 77.5 ± 8.8*** | 8.3 ± 2.4*,‡ |
| TQ | 25.2 ± 2.0†††,§§§ | 62.2 ± 2.7†,§§§ | 3.6 ± 0.4§ |
| CP + TQ | 38.3 ± 4.5 | 65.0 ± 3.7§ | 4.2 ± 0.5 |
| DEP + TQ | 29.1 ± 2.3†,§§§ | 58.2 ± 3.1§§ | 3.3 ± 0.6§ |
| CP + DEP + TQ | 38.6 ± 3.0 | 75.8 ± 15.5§§ | 5.0 ± 0.1 |
Note: Values in the table are mean ± SEM (n = 6). TQ (20 mg/kg) in corn oil (CO) was given orally continuously for 11 days, and CP (6 mg/kg) was injected intraperitoneally on the 6th day of treatment. DEP was given by inhalation on the 11th day. All rats were killed on the 12th day to collect blood.
Values with different superscript are statistically different
p < 0.05, **p < 0.001, ***p < 0.0001 vs CONTROL.
†p < 0.05, ††p < 0.001, †††p < 0.0001 vs CP.
p < 0.05 vs DEP.
p < 0.05, §§p < 0.001, §§§p < 0.0001 vs CP + DEP.
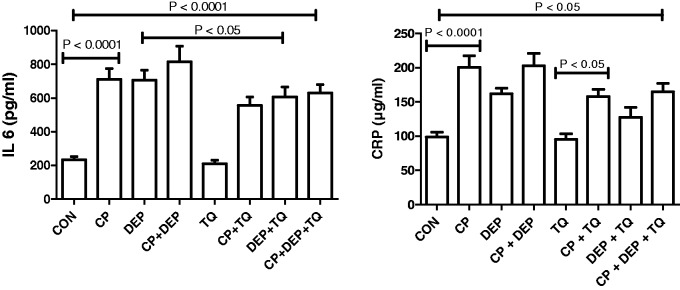
Treatment with CP alone significantly increased the concentrations of the two pro-inflammatory cytokines IL-6 and CRP (p < 0.0001). DEP alone significantly increased the concentration of IL-6 but not CRP. Combination of CP + DEP did not significantly increased the concentrations of the two pro-inflammatory cytokines -IL-6 and CRP beyond that caused by CP alone. Adding TQ to CP and DEP treatments insignificantly reduced the rise in the concentrations of the two pro-inflammatory cytokines (Figure 2).
Figure 2.
Plasma interleukin-6 (IL-6) and C-reactive protein (CRP) in rats treated with saline (control, CON), cisplatin (CP) 6 mg/kg, diesel exhaust particles (DEP) 0.5 mg/kg, CP + DEP, thymoquinone (TQ) 20 mg/kg, CP + TQ, DEP + TQ and CP + DEP + TQ. Each column represents mean ± SE (n = 6). Statistical analysis by Tukey–Kramer multiple comparison test
As shown in Table 4 treatment with CP, alone or together with DEP, caused significant decreases in GSH, SOD, CAT, and TAC (p < 0.05). Treatment with TQ in rats treated with CP and DEP caused insignificant improvement in the values of these antioxidant indices.
Table 4.
The effect of treatment of rats with cisplatin (CP), diesel exhaust particle (DEP), thymoquinone (TQ) singly or in combination on the renal concentration of some oxidative stress parameters
| GROUP | GSH (nmol/mg protein) | SOD (μmol/min/mg/protein) | CAT (μmol/min/mg/protein) | TAC (nmol/mg protein) |
|---|---|---|---|---|
| CONTROL (C) | 35.5 ± 1.6 | 49.4 ± 1.9 | 64.0 ± 3.4 | 70.3 ± 5.4 |
| CP | 11.7 ± 1.0*** | 26.0 ± 1.7** | 36.1 ± 2.2*** | 37.8 ± 2.4*** |
| DEP | 31.4 ± 2.2††† | 45.2 ± 1.1† | 51.9 ± 3.3† | 54.9 ± 1.7*,† |
| CP + DEP | 12.3 ± 1.2***,‡‡‡,§§§ | 27.4 ± 5.9**,‡ | 35.6 ± 2.7***,‡‡ | 36.2 ± 2.8*** |
| TQ | 32.2 ± 1.5††† | 47.4 ± 4.9††,§ | 55.1 ± 4.9††,§ | 61.1 ± 2.6†,§ |
| CP + TQ | 16.1 ± 1.6***,‡‡‡,¶¶¶ | 28.2 ± 0.5**,$ | 38.3 ± 3.9***,¶ | 42.2 ± 3.0***,¶ |
| DEP + TQ | 27.0 ± 1.9*,†††,§§§,$$$ | 46.2 ± 3.3† | 52.9 ± 3.3†,§ | 55.6 ± 3.5*,†,§ |
| CP + DEP + TQ | 17.2 ± 1.4***,‡‡‡,¶¶¶,$$,### | 28.2 ± 6.0$ | 37.5 ± 2.2***,¶,# | 41.0 ± 2.9***,¶,# |
Values in the table are mean ± SEM (n = 6). TQ (20mg/kg) in corn oil (CO) was given orally continuously for 11 days, and CP (6mg/Kg) was injected intraperitoneally on the 6th day of the treatment. DEP was given by inhalation on the 11th day. All the rats were killed on the 12th day and the kidneys removed to measure the above indices of oxidative stress.
GSH = reduced glutathione; SOD = superoxide dismutase; CAT = catalase; and TAC = total antioxidant capacity.
p < 0.05, **p < 0.001, ***p < 0.0001 vs CONTROL.
†p < 0.05, ††p < 0.001, †††p < 0.0001 vs CP.
‡p < 0.05, ‡‡p < 0.001, ‡‡‡p < 0.0001 vs DEP.
§p < 0.05, §§p < 0.001, §§§p < 0.0001 vs CP + DEP.
¶p < 0.05, ¶¶p < 0.001, ¶¶¶p < 0.0001 vs TQ.
$p < 0.05, $$p < 0.001, $$$p < 0.0001 vs CP + TQ.
p < 0.05, ##p < 0.001, ###p < 0.0001 vs DEP + TQ.
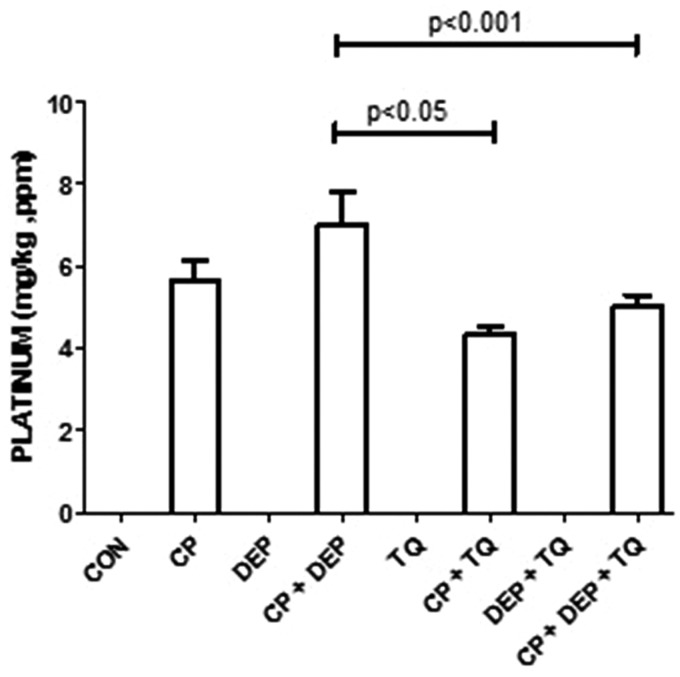
As shown in Figure 3, the concentration of Pt in the renal tissue of rats given CP alone (5.65 ± 0.5 ppm) was insignificantly higher than that in rats treated with CP + TQ (4.34 ± 0.2 ppm). However, the concentration of Pt in the renal tissue of rats given CP + DEP (7.00 ± 0.80 ppm) was significantly higher than in rats given CP + DEP + TQ (5.00 ± 0.30 ppm).
Figure 3.
Platinum concentration in rats treated with saline (control, CON), cisplatin (CP) 6 mg/kg, diesel exhaust particles (DEP) 0.5 mg/kg, CP + DEP, thymoquinone (TQ) 20 mg/kg, CP + TQ, DEP + TQ and CP + DEP + TQ. Each column represents mean ± SE (n = 6). Statistical analysis by Tukey–Kramer multiple comparison test
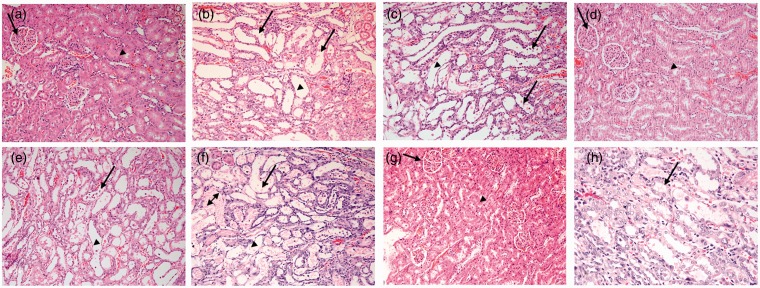
The effects of the agents used in this work on the histopathological appearance of the kidneys are shown in Figure 4. The renal section of the rats treated with saline (A), DEP (D), TQ (E) and TQ+DEP (G) showed normal glomeruli and tubules (A). Sections from the CP-treated rats showed acute tubular necrosis with tubular dilatation, interstitial edema and congestion (B). These changes were aggravated when CP and DEP were given together, with more foci of acute tubular necrosis and dilatation with some apoptotic cells in the tubules, interstitial edema and congestion (C). TQ treatment lessened the combined effect of CP + DEP (H).
Figure 4.
Representative light microscopy sections of renal tissue of rats treated with: saline (control, CON) (a), cisplatin (CP) 6 mg/kg (b), diesel exhaust particles (DEP) 0.5 mg/kg (c), thymoquinone (TQ) 20 mg/kg (d), CP + DEP (e), CP + TQ (f), DEP + TQ (g) and CP + DEP + TQ (H).The kidney architecture was not affected in groups (a), (d), and (g). The renal glomeruli (arrows) and renal tubules (arrow heads) indicate normal appearance. The micrographs in groups (B), (C), (E), and (F) showed acute tubular necrosis with eosinophilic material within the lumen (double headed arrow), shedding of epithelial cells within lumen (arrows) and flattened epithelial cells of the tubules with dilated lumens (arrow heads). (A color version of this figure is available in the online journal.)
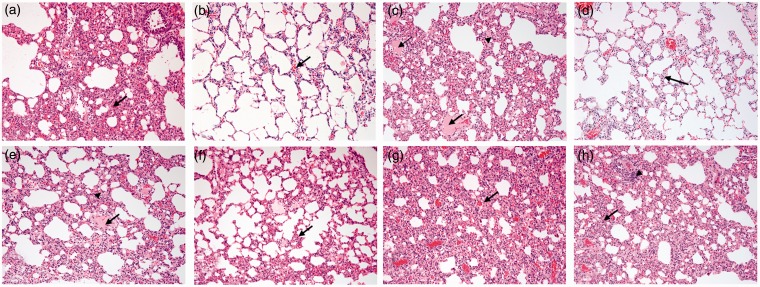
The effects of the agents used in this work on the histopathological appearance of the lungs are shown in Figure 5. Lung sections from rats treated with saline (A), CP (B), and TQ (G) showed only mild congestion or no apparent damage. Section in the lung from DEP-treated rats showed increased interstitial cellularity with widening of the inter-alveolar spaces with congested inter-alveolar capillaries and foci of intra-alveolar edema (C). TQ treatment did not seem to affect the combined action of CP + DEP (H).
Figure 5.
Representative light microscopy sections of lung tissues of rats given: saline (control, CON) (a), cisplatin (CP) 6 mg/kg (b), diesel exhaust particles (DEP) 0.5 mg/kg (c), thymoquinone (TQ) 20 mg/kg (d), CP + DEP (e), CP + TQ (f), DEP + TQ (g) and CP + DEP + TQ (h). The lung tissue showed only mild congestion (arrows) in groups (a), (b), (f), and (g), and increased interstitial cellularity with widening of the inter-alveolar spaces with congested inter-alveolar capillaries (arrow heads) and foci of intra-alveolar edema (arrows) in the groups (c), (e), and (h). (A color version of this figure is available in the online journal.)
Discussion
We have previously shown, and for the first time, that the air pollutant DEP (deposited in the lungs) aggravates experimental ARF induced by CP.19 In this study, we presented further experimental evidence that exposure of rats to DEP can potentiate CP nephrotoxicity, and that treatment with TQ can mitigate some of the effects of CP and DEP, when each is given alone or in combination.
Treatment with CP caused a significant loss in body weight in rats (amounting to about 5% of their initial body weight, compared to a gain in their control counterparts of about 17%). The causes of this action could not be related to anorexia, as the feed intake of these rats was not significantly reduced but may be due to the cytotoxic effect of CP, or to renal tubular injury influencing the reabsorption of water and causing dehydration and/or due to gastrointestinal disturbance and inflammation.12,15 Renal damage was also marked by elevated relative kidney weight which has been ascribed to the increase in glomerular volume and other cellular changes, and retention of water and solutes in renal tissues or changes induced by CP in renal blood flow.38,39
CP treatment in this work induced, as has consistently been shown before, nephrotoxicity which was evident by several biochemical alterations. Plasma creatinine and urea levels were elevated because of decreased glomerular filtration, or secondarily, due to increased generation of free radicals, which is known to induce mesangial cells contraction, changing the filtration surface area and/or modifying the ultrafiltration factors that can all lead to decreased glomerular filtration rate.6 CP treatment also caused polyuria, probably due to reduced glomerular filtration or to disturbance in the water permeability regulation in the collecting ducts that is associated with a decreased expression of renal aquaporins in the upper medulla.40
In the present study, we showed that a single exposure to DEP exacerbates some signs of CP-induced ARF, an action which was similar to the effect of repeated DEP exposure, albeit with severer tubular necrosis in the renal tissues of rats treated with CP + DEP compared with those from rats treated with CP + saline.41 This seems to suggest that a single exposure to DEP is still a risk factor in subjects with ARF, as it is a risk in healthy, hypertensive, or diabetic animals.18
In this work, and as shown in Table 3, the plasma activities of ALT, AST, and GGT (all markers of tissue damage) have been measured 24 h after the end of the experiments. TQ treatment did not significantly affect the activity of any of these enzymes, confirming the safety of this compound. Treatment with either CP or DEP alone significantly increased the activities of the three enzymes. Earlier, we have histologically shown that DEP exposure causes lung inflammation,41 and this may be the cause of the elevation of the activities of these plasma enzymes. CP treatment, because of hepatic and nephrotoxicity also increased the activities of these enzymes. However, treatment with a combination of CP and DEP potentiated GGT activity but not the two other enzymes. TQ completely prevented the rise of the three enzymes measured seen after DEP, and significantly abated the elevations of the three enzymes measured seen after CP. TQ, however, was less effective in antagonizing the rises in the activities of the three enzymes in rats treated with both CP and DEP.
CP nephrotoxicity and DEP exposure are both known to be associated with increased production of inflammatory mediators, and this has been shown in the current study by significant elevations in the pro-inflammatory cytokines CRP and IL-6. TQ anti-inflammatory action was evident in significant reduction in the concentration of both cytokines in plasma.
In the present study, we showed that a single exposure to DEP exacerbate some signs of CP-induced ARF, an action which was similar to the effect of repeated DEP exposure, albeit with severer tubular necrosis in the renal tissues of rats treated with CP + DEP compared with those from rats treated with CP + saline.41 This seems to suggest that a single exposure to DEP is still a risk factor in subjects with ARF, as it is a risk in healthy, hypertensive, or diabetic animals.18
In this work, and as shown in Table 3, the plasma activities of ALT, AST, and GGT (all markers of tissue damage) have been measured 24 after the end of the experiments. TQ treatment did not significantly affect the activity of any of these enzymes, confirming the safety of this compound. Treatment with either CP or DEP alone significantly increased the activities of the three enzymes. Earlier, we have histologically shown that DEP exposure causes lung inflammation,41 and this may be the cause of the elevation of the activities of these plasma enzymes. CP treatment, because of hepatic and nephrotoxicity also increased the activities of these enzymes. However, treatment with a combination of CP and DEP potentiated GGT activity but not the two other enzymes. TQ completely prevented the rise of the three enzymes measured seen after DEP, and significantly abated the elevations of the three enzymes measured seen after CP. TQ, however, was less effective in antagonizing the rises in the activities of the three enzymes in rats treated with both CP and DEP.
TQ is the bioactive constituent of the volatile oil of black seed (Nigella sativa) with relatively strong anti-inflammatory, anti-oxidant, and anti-tumor activity.32 As the toxicity of both CP and DEP involve inflammation and generation of free radicals, it was logical to test whether TQ would ameliorate the single and combined toxicity of CP and DEP. TQ treatment was effective in mitigating some of the their toxic effects.
The functional alterations induced by CP treatment, was broadly in close agreement with the histopathological data. The effect of TQ on lung function in the rats treated with CP, DEP or their combination has not been investigated here. However, it was shown histopathologically that TQ was effective in lessening the effect of CP and CP + DEP on the kidneys, with no apparent effect on lung histology.
The Pt renal concentration in CP-treated rats was slightly and insignificantly decreased by TQ treatment. However, the concentration of Pt in the renal tissue of rats given CP + DEP was significantly higher than in rats given CP + DEP + TQ. We have not studied here the mechanism(s) by which TQ caused this small reduction in Pt renal concentration in the latter group, its possible therapeutic implications, if any, or whether the TQ-induced amelioration in the signs of CP + DEP toxicity could be ascribed with certainty to that reduction in renal Pt accumulation. Accumulation of Pt in renal tubular cells is one of the reasons for CP nephrotoxicity.42 Future in vitro experiments using renal cells and in vivo experiments in animals with tumor xenografts could provide an explanation for the Pt reduction in renal tissues after TQ, and whether it would affect the anti-tumor action of CP.
The molecular mechanism by which TQ has produced its salutary action on the combined toxicity of CP and DEP is not fully known. However, it is known that an early response to CP treatment may include an elevated nuclear factor kappa-B (NF-κB) signaling, associated with increases in TNFα and other molecules (such as intercellular adhesive molecule-1).43 TQ has also been shown to cause its ameliorative action on CP toxicity via NF-κB,44 and this may be a plausible molecular mechanism for its beneficial action on the toxicity induced in this work by CP and DEP. At the cellular level TQ is known to possess strong antiapoptotic, anti-oxidant, and anti-inflammatory actions, and with such properties, it is conceivable that it will be successful in mitigating the actions of CP and DEP, both of which are known to increase apoptosis, systemic inflammation, and generation of free radicals.31–33,45
In conclusion, this work has shown that a single exposure to DEP potentiated the nephrotoxicity of CP. TQ abrogated the toxicities of CP and DEP, when given singly or in combination, probably through its antioxidant and anti-inflammatory actions.
ACKNOWLEDGMENTS
We thank SQU and UAEU for financial support of this work, and the staff in the Small Animal House of SQU for looking after the rats. This work was supported by a grant from the SQU–UAEU Joint Research Program to BH Ali (Oman) and A Nemmar (UAE).
Authors' contributions
Conceived and designed the experiments: BHA. Performed the experiments: MZ, ES, PM, MIW, JY, AN, MF. Analyzed the data: BHA, MZ, AN. Wrote the MS: BHA. Revised the MS: MZ, AN. All authors read and approved the submission.
References
- 1.Fury MG, Sherman E, Ho AL, Xiao H, Tsai F, Nwankwo O, Sima C, Heguy A, Katabi N, Haque S, Pfister DG. A phase 1 study of everolimus plus docetaxel plus cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer 2013; 119: 1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson S, Hall E, Harland SJ, Chester JD, Pickering L, Barber J, Elliott T, Thomson A, Burnett S, Cruickshank C, Carrington B, Waters R, Bahl A. Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br J Cancer 2013; 109: 2554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Máthé C, Bohács A, Duffek L, Lukácsovits J, Komlosi ZI, Szondy K, Horváth I, Müller V, Losonczy G. Cisplatin nephrotoxicity aggravated by cardiovascular disease and diabetes in lung cancer patients. Eur Respir J 2011; 3: 888–94. [DOI] [PubMed] [Google Scholar]
- 4.Stathopoulos GP. Cisplatin: Process and future. J BUON 2013; 18: 564–9. [PubMed] [Google Scholar]
- 5.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci 2007; 334: 115–24. [DOI] [PubMed] [Google Scholar]
- 6.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 2003; 10: 1663–82. [PubMed] [Google Scholar]
- 7.Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 2011; 41: 803–821. [DOI] [PubMed] [Google Scholar]
- 8.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2010; 2: 2490–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch Toxicol 2012; 86: 1233–50. [DOI] [PubMed] [Google Scholar]
- 10.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 2009; 61: 223–42. [DOI] [PubMed] [Google Scholar]
- 11.Koyner JL, Sher Ali R, Murray PT. Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury?. Nephron Exp Nephrol 2008; 109: e109–17. [DOI] [PubMed] [Google Scholar]
- 12.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 2008; 73: 994–1007. [DOI] [PubMed] [Google Scholar]
- 13.Hanigan MH, Townsend DM, Cooper AJ. Metabolism of cisplatin to a nephrotoxin. Toxicology 2009; 257: 174–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: A review of some recent research. Food Chem Toxicol 2006; 44: 1173–83. [DOI] [PubMed] [Google Scholar]
- 15.Ali BH, Al-Moundhri M, Eldin MT, Nemmar A, Al-Siyabi S, Annamalai K. Amelioration of cisplatin-induced nephrotoxicity in rats by tetramethylpyrazine, a major constituent of the Chinese herb Ligusticum wallichi. Exp Biol Med (Maywood) 2008; 233: 891–6. [DOI] [PubMed] [Google Scholar]
- 16.Ali BH, Al-Salam S, Al Husseini IS, Al-Lawati I, Waly M, Yasin J, Fahim M, Nemmar A. Abrogation of cisplatin-induced nephrotoxicity by emodin in rats. Fundam Clin Pharmacol 2013; 27: 192–200. [DOI] [PubMed] [Google Scholar]
- 17.Al-Kharusi N, Babiker HA, Al-Salam S, Waly MI, Nemmar A, Al-Lawati I, Yasin J, Beegam S, Ali BH. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: A dose-dependent study. Eur Rev Med Pharmacol Sci 2013; 17: 299–310. [PubMed] [Google Scholar]
- 18.Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. Biomed Res Int 2013;2013:279371. [DOI] [PMC free article] [PubMed]
- 19.Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, Dhanasekaran S, Yasin J, Ali BH, Kazzam EE. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol 2011; 164: 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li PK, Burdmann EA, Mehta RL. World Kidney Day Steering Committee 2013. Acute kidney injury: global health alert. Transplantation 2013; 95: 653–7. [DOI] [PubMed] [Google Scholar]
- 21.Peng Q, Zhang L, Ai Y, Zhang L. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin Med J (Engl) 2014; 127: 1820–6. [PubMed] [Google Scholar]
- 22.Perazella MA. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 2012; 7: 1713–21. [DOI] [PubMed] [Google Scholar]
- 23.Burki TK. Twice as bad: New estimates for mortality from air pollution. Lancet Respir Med 2014; 2: 355. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014; 69: 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali BH. The effect of Nigella sativa oil on gentamicin nephrotoxicity in rats. Am J Chin Med 2004; 32: 49–55. [DOI] [PubMed] [Google Scholar]
- 26.Zang D, Shao Y, Li X. Ultrastructural pathology of rat lung injury induced by ischemic acute kidney injury. Ultrastruct Pathol 2013; 37: 433–9. [DOI] [PubMed] [Google Scholar]
- 27.Pierson DJ. Respiratory considerations in the patient with renal failure. Respir Care 2006; 51: 413–22. [PubMed] [Google Scholar]
- 28.Chien CC, King LS, Rabb H. Mechanisms underlying combined acute renal failure and acute lung injury in the intensive care unit. Contrib Nephrol 2004; 144: 53–62. [DOI] [PubMed] [Google Scholar]
- 29.Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 2009; 297: F125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laden F, Winkelmayer WC. Air pollution and coronary risk in kidney transplant recipients. Am J Kidney Dis 2011; 58: 506–7. [DOI] [PubMed] [Google Scholar]
- 31.Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct 2002; 20: 143–51. [DOI] [PubMed] [Google Scholar]
- 32.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2003; 17: 299–305. [DOI] [PubMed] [Google Scholar]
- 33.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem Pharmacol 2012; 83: 443–51. [DOI] [PubMed] [Google Scholar]
- 34.Aycan IÖ, Tüfek A, Tokgöz O, Evliyaoğlu O, Fırat U, Kavak GÖ, Turgut H, Yüksel MU. Thymoquinone treatment against acetaminophen-induced hepatotoxicity in rats. Int J Surg 2014; 12: 213–8. [DOI] [PubMed] [Google Scholar]
- 35.Bai T, Yang Y, Wu YL, Jiang S, Lee JJ, Lian LH, Nan JX. Thymoquinone alleviates thioacetamide-induced hepatic fibrosis and inflammation by activating LKB1-AMPK signaling pathway in mice. Int Immunopharmacol 2014; 19: 351–7. [DOI] [PubMed] [Google Scholar]
- 36.Ali BH, Al Salam S, Al Za'abi M, Al Balushi K, AlMahruqi AS, Beegam S, Al-Lawatia I, Waly MI, Nemmar A. Renoprotective effects of gamma-aminobutyric acid (GABA) on cisplatin-induced acute renal injury in rats. Basic Clin Pharmacol Toxicol 2015; 35: 29–37. [DOI] [PubMed] [Google Scholar]
- 37.Esteban-Fernández D, Verdaguer JM, Ramírez-Camacho R, Palacios MA, Gómez-Gómez MM. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J Anal Toxicol 2008; 32: 140–6. [DOI] [PubMed] [Google Scholar]
- 38.Abdelrahman AM, Al Salam S, AlMahruqi AS, Al husseni IS, Mansour MA, Ali BH. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J Appl Toxicol 2010; 30: 15–21. [DOI] [PubMed] [Google Scholar]
- 39.Ali BH, Abdelrahman AM, Al-Salam S, Sudhadevi M, AlMahruqi AS, Al-Husseni IS, Beegam S, Dhanasekaran S, Nemmar A, Al-Moundhri M. The effect of sildenafil on cisplatin nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 2011; 109: 300–8. [DOI] [PubMed] [Google Scholar]
- 40.Do Amaral CL, Francescato HD, Coimbra TM, Costa RS, Darin JD, Antunes LM, Bianchi Mde L. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol 2008; 82: 363–70. [DOI] [PubMed] [Google Scholar]
- 41.Nemmar A, Beegam S, Yuvaraju P, Yasin J, Fahim MA, Kazzam EE, Alhaddabi I, Ali BH. Potentiation of cisplatin-induced nephrotoxicity by repeated exposure to diesel exhaust particles: An experimental study in rats. Exp Biol Med (Maywood) 2014; 239: 1036–44. [DOI] [PubMed] [Google Scholar]
- 42.Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS One 2014; 9: e105070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Webb HK, Fukushima H, Micheli J, Markova S, Olson JL, Kroetz DL. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor κB signaling. J Pharmacol Exp Ther 2012; 341: 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Malki AL, Sayed AA. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement Altern Med 2014; 14: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galaly SR, Ahmed OM, Mahmoud AM. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J Physiol Pharmacol 2014; 65: 823–32. [PubMed] [Google Scholar]