Abstract
Many studies have shown that natural dietary agents, in combination with chemical agents, can improve the therapeutic response of cancers to chemotherapy and reduce the associated side-effects. In the present study, we investigated the therapeutic potential and mechanisms of anticancer effects for the combination of 5-fluorouracil (5-FU) and resveratrol (Res). In these studies, we employed the cancer cell lines TE-1 and A431 and an animal model of skin cancer. The presented results provide the first evidence that Res can enhance the anti-tumor potency of 5-FU by inducing S-phase arrest. The combination of Res and 5-FU demonstrates synergistic efficacy, causing tumor regression in a two-stage model of mouse skin carcinogenesis induced by DMBA and TPA. There was clear evidence of Res augmenting the growth inhibitory effect of 5-FU on the TE-1 and A431 cancer cells in vitro. In the in vivo studies, the tumor regression rate in the combination group increased significantly after four weeks of treatment (P < 0.01). The combination of 5-FU and Res significantly increased the percentage of apoptotic cells and the level of activated caspase-3, cleaved PARP and p53 proteins as well as increased the Bax/Bcl-2 ratio. In conclusion, the 5-FU/Res combination enabled a more effective inhibition of cell growth and the induction of apoptosis in cancer cells than 5-FU alone. The results of this study suggest that chemotherapy using natural dietary agents with chemical agents represents a superior cancer treatment option.
Keywords: Resveratrol, 5-fluorouracil, synergistic effect, S-phase arrest, two-stage mouse skin carcinogenesis
Introduction
Cancer is a major public health problem in many parts of the world, and is a leading cause of death across the world.1 Esophageal cancer is the sixth most common cause of cancer-related death, and it is one of the most common cancers in China.2 Non-melanoma skin cancers are the most common skin cancers and account for approximately one-third of all cancers.3 There are more new cases annually than the total incidence of all other cancer types.4 The current modalities of cancer treatment include surgery, chemotherapy, radiotherapy, gene therapy and palliative care, which usually come with adverse reactions. In recent years, chemotherapy with multiple drugs or compounds at a lower dosage (combination shows additive or synergistic therapeutic effects) has frequently been used to improve treatment efficacy and reduce adverse reactions.
The cycle-specific antimetabolite 5-fluorouracil (5-FU) has been used clinically for more than 60 years and has played an important role in the treatment of a large spectrum of solid tumors, including gastrointestinal cancers, breast cancer, skin cancer, and bladder cancer.5,6 The compound 5-FU works by causing G1/S cell cycle arrest, which induces apoptosis in cancer cells by inhibiting essential biosynthetic processes. Although 5-FU is a potent drug, its serious adverse reactions and limited ability to prevent tumor recurrence cannot be ignored. The most serious adverse effect associated with high doses of 5-FU is toxicity.7 Novel anti-tumor agents or drug combinations need to be developed to increase the anti-tumor effects and reduce the adverse effects of chemotherapeutics. Efforts have been made to increase the anti-tumor effects and decrease the cytotoxicity of 5-FU, including using 5-FU in combination with several second-line agents and certain natural phytochemicals such as paclitaxel, mitomycin, L-canavanine, and geraniol.8–11
Resveratrol (Res) is a naturally occurring phytoalexin found in grapes, berries, peanuts, and some herbs as a response to stress, injury, or fungal infection. Evidence from numerous studies has confirmed the ability of Res to protect against many diseases, including cancer.12–14 Res modulates multiple pathways involved in the cell cycle, apoptosis, and inflammation through diverse mechanisms. Many in vitro and in vivo studies have provided a rational basis for the use of Res in human cancer chemoprevention.15 Studies have confirmed that Res combined with other chemotherapeutic drugs can be more effective at treating drug-refractory cancer cells.16
In this study, the human esophageal cancer cell line TE-1 and the human epidermal cancer cell line A431 were used to determine the anti-tumor effects of Res and 5-FU on cell growth, the cell cycle, and apoptosis. Two-stage mouse skin carcinogenesis was used to investigate the synergistic effect of these two agents in vivo. We also assessed the potential mechanisms of Res and 5-FU interactions.
Materials and methods
Chemicals and cells
Resveratrol (>99% purity), 5-FU, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies against B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein (Bax), caspase-3, cleaved caspase-3, poly ADP ribose polymerase (PARP), cleaved PARP, and horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). The antibodies against wild-type p53 and β-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). TE-1 and A431 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The 10% homemade 5-FU ointment contained 10% 5-FU in a vanishing cream base consisting of white petrolatum, stearyl alcohol, propylene glycol, polysorbate 60 and parabens (methyl and propyl).
Animals and treatment
Swiss albino mice (12–14 g body weight) were obtained from the Laboratory Animal Center of Hebei Province in China. The mice were kept in well ventilated polypropylene cages (10 animals per cage) in an air-conditioned room maintained at 25℃ and fed with standard laboratory diet and water ad libitum. The dorsal skin of each mouse was shaved two days prior to treatment. The topical use of 7, 12-dimethylbenz[a]anthracene (DMBA) in acetone (150 µg/mouse) served as a tumor initiator. One week after initiation, papilloma formation was promoted by the application of 12-O-tetradecanoylphorbol-13-acetate (TPA, 5 nmol) in acetone (200 µL) to the skin twice weekly for 20 weeks.17
After 20 weeks of TPA treatment, the mice were screened for the cumulative number of tumors (only those having a diameter >1 mm were considered positive). All of the mice that were tested positive were divided into four experimental groups and treated with different drugs for four weeks (n = 10 per group, the number of tumors in each group was approximately equal). The mice were treated as follows: Group I (control group) was given ointment lacking 5-FU and Res; Group II was given a topical 10% homemade 5-FU ointment; Group III was given 50 µmol of Res topically; and Group IV was given 10% 5-FU ointment and 50 µmol of Res topically. Papillomas were recorded weekly during the experimental period. At the end of study period (24 weeks), the mice were euthanized, and the dorsal skin (with or without tumors) was excised, cleaned, snap frozen in liquid nitrogen, and stored at −80℃ for further use.
Cell growth inhibition assay
Cells were seeded (2 × 104 cells per well) in 96-well microtiter plates and grown at 37℃ in a 5% CO2 incubator. After a 24-h incubation, the cells were treated with various concentrations of 5-FU (1--1000 µmol/L) and Res (1--500 µmol/L) alone or in combination. The cells were incubated for approximately 24 to 72 h. Next, growth-inhibitory effects were determined with an MTT assay.18 Stock solutions of 5-FU and Res were made with distilled water and dimethyl sulfoxide (DMSO), respectively, and stored at −20℃ until use. The final concentration of DMSO was less than 0.1% (v/v) in the culture medium and the same concentration of DMSO was present in the control group.
Clonogenic assay
Cells (2000 cells per well) were cultured in 6-well plates for 48 h at 37℃ in 10% fetal bovine serum (FBS) medium. Next, the cells were treated with 5-FU and/or Res for 24 h, after which the drugs were washed out and fresh medium was added. Fourteen days after drug withdrawal, colonies were fixed and stained with 1% fresh crystal violet. Visible colonies containing 50 or more cells were recorded.
Cell cycle analysis
Cell cycle distribution was measured using a flow cytometric DNA analysis after the cells were treated with 5-FU and/or Res. Cells were harvested, washed twice with cold phosphate-buffered saline (PBS), and fixed with pre-cooled 70% ethanol at 4℃. After fixation, the cells were permeabilized with 0.1% Triton X-100 for 5 min and centrifuged at 1600–2000 r/min. The pellets were resuspended i 50 µg/mL PI with 100 µg/mL RNase and incubated for 30 min in the dark at 4℃. The samples were acquired and analyzed with a flow cytometer and CellQuest 3.3 software.19
Analysis of early apoptosis
Apoptosis can be measured using flow cytometry to quantify the levels of detectable phosphatidylserine on the outer membranes of apoptotic cells.20 Once the cells reached 70% confluence, they were incubated for 24 h with 5-Fu and/or Res. Apoptotic cells were identified using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Beijing 4 A Biotech, China) according to the manufacturer’s instructions. Flow cytometry was performed immediately after supravital staining. Data were obtained and analyzed with a Beckman Coulter Epics XL Flow Cytometer and CellQuest 3.3 software.
Observation of nuclear morphology
Cells were grown on coverslips in petri dishes and treated with 5-FU and/or Res. After treatment, the cells were washed with 4′, 6-diamidino-2-phenylindole (DAPI)-methanol (working solution, 1 µg/mL) and stained with DAPI-methanol for 15 min at 37℃. Nuclear morphology was observed via a fluorescence microscope (Olympus IX-70, Japan).
Western blotting analysis
Cytosolic extracts were prepared from TE-1 and A431 cells and mice skin homogenates using a lysis buffer with a freshly added protease inhibitor cocktail. After incubation for 1 h, cells were centrifuged at 12,000 r/min for 30 min at 4℃. The protein in the supernatant was quantified using a BCA Protein Assay Kit. Samples were electrophoresed on 10%, 12%, or 15% sodium dodecyl sulfate (SDS)–polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes. A mouse monoclonal β-actin antibody and rabbit polyclonal antibodies against human p53, Bcl-2, Bax, caspase-3, cleaved caspase-3, PARP, and cleaved PARP were used to probe the separate membranes. Next, the appropriate secondary antibodies were applied. β-actin was used as the internal control. Specific protein bands were detected with the DAB visualization method.
Evaluation of combined effect and statistical analysis
The interaction between 5-FU and Res was calculated and assessed using a combination index (CI)
where DX1 and DX2 are the concentrations of 5-FU and Res, respectively, that resulted in a cell growth inhibition of x%, and D1 and D2 are the concentrations of 5-FU and Res, respectively, that inhibited cell growth by x% when they were used in combination. A CI < 1, CI = 1, or CI > 1 indicates synergistic, additive, or antagonistic effects, respectively.21 Data were analyzed with Calcusyn software (Biosoft, UK).
All of the experiments were repeated at least three times. Significant differences between two groups were determined with a one-way analysis of variance (ANOVA) followed by a Dunnett’s test or Tukey’s test. A P < 0.05 was considered as statistically significant.
Results
The inhibitory effects of 5-FU and Res on cell growth alone and in combination
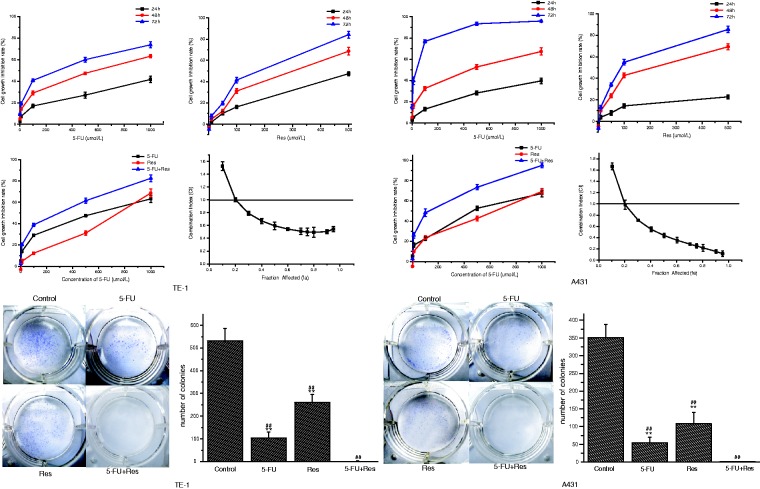
Using 5-FU inhibited cell growth in a concentration- and time-dependent manner. Figure 1(a) shows the effects of 5-FU and/or Res on cell growth. Higher concentrations of Res inhibited cell growth. Lower concentrations (less than 10 µmol/L) of Res produced a small promotion of growth in TE-1 and A431 cells. Similar results in bovine aortic endothelial cells were previously reported.22,23 These differential effects indicate that Res exerts concentration-dependent biphasic actions on cell growth. The combination of 5-FU and Res enhanced the growth-inhibition effect, and the IC50 in cancer cells (cells were treated for 48 h) decreased from 480.8 µmol/L to 151.9 µmol/L (TE-1, 5-FU), from 248.6 µmol/L to 69.4 µmol/L (TE-1, Res), from 322.9 µmol/L to 72.0 µmol/L (A431, 5-FU), and from 174.6 µmol/L to 37.6 µmol/L (A431, Res). This result indicates that the treatment combination of 5-FU and Res had a synergistic anti-tumor effect. The growth inhibition rates were analyzed using the Chou and Talalay method.24 The CI values were <1 (Figure 1a), indicating that the combination of 5-FU and Res had a synergistic inhibitory effect on the growth of TE-1 and A431 cells across the broad range of fraction affected.
Figure 1.
Effects of 5-FU (70 µmol/L) and/or resveratrol (Res, 50 µmol/L) on cell growth and proliferation of TE-1 and A431. (a) Growth inhibition effect of drugs on cancer cells and combination index (CI) of the two drugs. Cells were treated with various concentrations of 5-FU and/or Res for 24-72 h. Cells were treated with 5-FU + resveratrol for 48 h, and the CI values were determined using the Chou-Talalay method. When the fa (fraction affected, a fa of 0.20 is a growth inhibition of 20%) is higher than 0.20, CI < 1 indicates a synergistic effect. (b) Photos of colony formation of TE-1 and A431 treated with 5-FU and/or Res and the number of colonies. The colony-forming cells were stained by hematoxylin. Data were presented as means ± SD of five independent experiments. ##P < 0.01 vs control, **P < 0.01 vs 5-FU + Res group. (A color version of this figure is available in the online journal.)
The long-term effects of 5-FU and Res on cell proliferation alone and in combination
A clonogenic assay was used for study the long-term anti-proliferative effects of 5-Fu and/or Res on the cells. Colony formation by TE-1 cells was significantly suppressed from 535 ± 97 to 105 ± 49 and 262 ± 63, respectively, for 5-FU and Res treatments alone. The colony formation of A431 cells was significantly suppressed from 352 ± 49 to 54 ± 13 and 108 ± 28, respectively, for 5-FU and Res treatments alone. Few colonies were formed by cells treated with the combination of 5-FU and Res (Figure 1b).
The effects of 5-FU and Res on the cell cycle alone and in combination
To evaluate whether the inhibition of cell proliferation was due to alterations in cell-cycle regulation, we assessed the effects of 5-FU and Res on cell cycle progression alone and in combination using flow cytometry. The results revealed that cells treated with Res for 48 h accumulated in the S-phase (>70% in TE-1, >50% in A431) and showed a reduction in the ratio of G0/G1-phase cells compared with the control group (P < 0.05) (Figure 2). Similar findings were observed in cells treated for 24 h and 72 h (data not shown). The appearance of a large S-phase peak was associated with a reduction in the G1 peak; however, the Res-induced G1-phase arrest of cells reported by Ahmad et al was not observed.25,26 The percentage of G1-phase cells increased in cells treated with 5-FU alone or 5-FU in combination with Res (Figure 2b).
Figure 2.
FACS analysis of cell cycle of TE-1 and A431 treated with 5-FU (70 µmol/L) and/or Res (50 µmol/L) for 48 h. (A) Representative images of the cell-cycle distribution of cancer cells in different groups. (B) The statistic analysis for cell-cycle distribution. *P < 0.05, **P < 0.01 vs control, ##P < 0.01vs 5-FU + Res group. (A color version of this figure is available in the online journal.)
Apoptosis induced by 5-FU and Res alone and in combination
Cells treated with 5-FU and/or Res showed significant morphological changes indicative of apoptosis such as cell shrinkage, separation from neighboring cells and nuclear condensation; furthermore, these changes were more evident with combination therapy (figures shown in the Supplementary file). Next, the different stages of apoptosis induced by 5-FU and/or Res were evaluated using Annexin V/propidium iodide (PI) staining and nuclear staining. The result showed that either 5-FU or Res alone caused an increase in the percentage of early apoptotic cells compared with the control group (Figure 3a). In the TE-1 cell line, the early apoptotic rate was 4.0 ± 0.8%, 11.0 ± 1.2%, and 7.9 ± 0.8% in the control, 5-FU, and Res groups, respectively (P < 0.01). The early apoptotic rate of cells in the combination group was 23.0 ± 3.1%, which was significantly higher (P < 0.01) than the 5-FU group and Res group. We found similar results in the A431 cell line. The early apoptotic rates results were 5.2 ± 0.6% (control group), 18.6 ± 2.0% (5-FU group), 9.4 ± 0.9% (Res group), and 35.0 ± 2.8% (combination group).
Figure 3.
Effects of 5-FU (70 µmol/L) and/or Res (50 µmol/L) on apoptosis of cancer cells and mouse skin tumors. (a) The apoptotic status determined by Annexin V/PI staining. Flow cytometric analysis clearly differentiates among PI-positive (necrotic) cells (B1), annexin V and PI double-positive (late apoptotic) cells (B2), negative (viable) cells (B3), and annexin V-positive (early apoptotic) cells (B4). (b) Nuclear morphologic changes shown by DAPI staining assay (magnification, ×200. The apoptotic cells were indicated by arrows). (c) Expression levels of caspase-3/cleaved caspse-3 and PARP/cleaved PARP proteins. The β-actin was used as an internal control for different proteins. The bar chart showed quantitative analysis of the protein expression normalized to β-actin. **P < 0.01 vs control, #P < 0.05, ##P < 0.01 vs 5-FU + Res group. (A color version of this figure is available in the online journal.)
The apoptosis induced by 5-FU and/or Res was confirmed by the morphological changes detected using DAPI staining and fluorescence microscopy. DAPI staining is a classical way to identify the morphology of apoptotic cells. Our results showed that untreated cells displayed a normal nuclear size, while morphological changes such as pyknosis, chromatin condensation, and apoptotic body formation were found in cells incubated continuously with 5-FU and/or Res for 48 h. An increase in fragmented nuclei and typical apoptotic morphological changes were observed in cells treated with 5-FU and Res in combination compared with cells treated with 5-FU or Res alone (Figure 3b).
Western blotting results
To determine whether treatment-induced apoptosis was associated with altered expression of apoptosis-regulating proteins, cells were treated for 48 h with 5-FU and/or Res, and then subjected to Western blotting. Treatment with 5-FU or Res alone caused a marked down-regulation of Bcl-2 protein expression, and the combination was even more effective at lowering the level of Bcl-2. The expression of the pro-apoptotic proteins Bax and p53 increased significantly in all of the treated groups, especially in the combination group. In addition, the ratio of Bax/Bcl-2 was significantly enhanced with the 5-FU and Res combination treatment (Figure 4(a) and (b)). The protein expression of PARP and caspase-3 was down-regulated, and cleaved caspase-3 and PARP were up-regulated, especially in the combination group (Figure 3c). These findings suggest that the apoptosis induced by 5-FU and Res may be associated with the enhanced protein expression of pro-apoptotic Bax and p53 and reduced expression of anti-apoptotic Bcl-2. In addition, active caspase-3 was generated by 5-FU or Res alone; however, the combination treatment was more effective. Similar protein expression results were found in the animal model (Figure 4c).
Figure 4.
Effects of 5-FU (70 µmol/L) and/or Res (50 µmol/L) on expression levels of Bcl-2, Bax, p53 proteins, and the ratio of Bax/Bcl-2 in cancer cells and mouse skin tumors. (a) Expression levels of proteins and the ratio of Bax/Bcl-2 in TE-1. (b) Expression levels of proteins and the ratio of Bax/Bcl-2 in A431. (c) Expression levels of proteins and the ratio of Bax/Bcl-2 in mouse skin tumors. The β-actin was used as internal control for different proteins. The bar chart showed the quantitative analysis of the protein expression normalized to β-actin. **P < 0.01 vs control, #P < 0.05, ##P < 0.01 vs 5-FU + Res group
The effects of 5-FU and Res on the cumulative number of skin papillomas alone and in combination
The synergistic inhibitory effect of Res and 5-FU was observed in a reduction in the cumulative number of tumors (CNT). After 4 weeks of treatment, the CNT was 56, 21, 34, and 10 in Groups I (carcinogen control group), II, III, and IV, respectively. The CNT in all of the drug-treated groups was significantly lower (P < 0.01) compared with Group I (Figure 5). The combination of 5-FU and Res further reduced the CNT compared with treatment with either of the agents alone.
Figure 5.
Changes in cumulative number of tumors (CNT) of DMBA and TPA-induced mouse skin tumors treated by 5-FU and Res. The tumor-bearing mice were divided into four experimental groups and treated with different drugs for four weeks (n = 10 per group, the number of tumors in each group was approximately equal). **P < 0.01 vs control, ##P < 0.01 vs 5-FU + Res group
Discussion
Cancer chemotherapy using natural dietary agents in combination with chemical agents has garnered a great deal of attention from researchers because of its effectiveness and improved safety. The potent chemotherapeutic agent 5-FU is often used in combination therapies for the treatment of many types of cancers.27–29 Res possesses anti-tumor effects that may play a role in the famous French Paradox phenomenon.30 Res has been shown to suppress the survival rate of a wide variety of tumor cells.31 In this study, we report the first evidence that Res enhances the anti-tumor potency of 5-FU on TE-1 and A431 cells in vitro. Furthermore, the combined treatment with Res and 5-FU synergistically suppressed mouse skin tumors more efficiently than treatment with either drug alone. Furthermore, we found that Res remarkably increased the accumulation of S-phase cells and accelerated cell apoptosis, which might play a key role in the synergism of the two agents.
In the present study, the CI values indicated that the combination of 5-FU and Res had a synergistic inhibitory effect on the growth of cells. The IC50 value of the 5-FU and Res combination treatment was reduced by two-thirds compared with 5-FU treatment alone. These data suggest that combining 5-FU with Res enables a reduction in 5-FU while maintaining the desired effects. This synergistic effect between 5-FU and Res can also enable a reduction in the dose of 5-FU needed clinically and thereby decrease the toxicity associated with higher doses.
The clonogenic assay represents a more direct method to measure long-term growth inhibition compared with the MTT assay because it evaluates the ability of a cell to undergo several cycles of mitosis over a longer time period. In this study, the inhibitory effect on the clonogenic potential of cancer cells was much stronger for the 5-FU and Res combination treatment than for either drug alone. Furthermore, the clonogenic assay was used to assess the ability of cancer cells to maintain reproductive potential after treatment.32 The reproductive potential of cancer cells is a characteristic that facilitates tumor recurrence in patients. The result of the clonogenic assay suggests that 5-FU combined with Res may have the added ability to exert a long-term inhibition on the proliferation of cancer cells and thus reduce tumor recurrence in cancer patients. This finding is vital for the clinical application of 5-FU and Res as a combined treatment for cancer chemotherapy.
Although the aforementioned results showed that a combination of 5-FU and Res synergistically inhibited cell growth, the mechanisms by which these drugs exert this effect remain unknown. Different drugs may have different molecular targets in cancer cells; thus, if two or more drugs act on different targets, their combination may result in a higher potency for repressing tumor growth. In terms of the mechanism, the anti-tumor effects of Res are related to the induction of apoptosis and changes in the distribution of cells in the cell cycle. As a pyrimidine antimetabolite, 5-FU has a structure similar to uracil, which is needed for DNA synthesis. As a substitute, 5-FU is transformed inside the cell into several active metabolites that are then incorporated into DNA and RNA. This effect ultimately reduces cell growth by inhibiting the cell’s ability to synthesize DNA; thus, 5-FU is an S-phase-specific drug.33 In our study, Res led to S-phase arrest, but the cell cycle distribution was only slightly altered when the cells were treated with 5-FU alone or in a combination of the two drugs. This result suggests that the anti-tumor activity of Res is based on both the induction of apoptosis and cell-cycle arrest, and the anti-tumor activities of 5-FU and the combination treatment were less involved with changes in cell-cycle progression. We hypothesize that the synergistic interaction between Res and 5-FU is partially due to Res enhancing the sensitivity of tumor cells to 5-FU by increasing the number of S-phase cells.
Cancer treatment responses can be predicted by the induction of tumor cell apoptosis. We demonstrated a significant increase in apoptosis in vitro with the combination of 5-FU and Res compared with either treatment alone. Western blotting of mouse skin proteins showed an increase in the protein expression of cleaved caspase-3, cleaved-PARP, p53, and Bax, and an increase in the Bax/Bcl-2 ratio after 4 weeks of topical treatment with 5-FU and Res alone or in combination.
Compared with tumor xenograft experiments, mouse multistage carcinogenesis has been found to be similar to the development of skin cancer in humans. Previous research focused on the chemoprevention effects of drugs at the initiation or promotion stages of carcinogenesis.17,34 In our study, mice were treated with 5-FU and/or Res after skin papilloma formation to detect the therapeutic effect of drugs. The reduction in CNT and changes in protein expression showed obvious synergetic effects of 5-FU and Res. Surprisingly, no skin damage, such as swelling or scabbing, was found in the combination group compared with the 5-FU group. This unexpected finding suggests that Res might reduce the side-effects of 5-FU on the skin and thus lessen pain during treatment. Studies in other types of tumor models need to be further investigated. The half-life of 5-FU and Res in rats is less than 30 min and 8–14 min, respectively.35–37 This half-life similarity suggests that the two drugs are well suited for use together, and we believe that the clinical efficacy of 5-FU combined with Res for treating cancer will be consistent with our experimental results.
Taken together, our data indicate that a combined treatment with 5-FU and Res showed a greater effect on inducing growth-inhibition, apoptosis and tumor regression in cancer cells. Res increased the number of S-phase cells that were susceptible to 5-FU, and this mechanism may explain the synergistic effect of the combination therapy. The results of this study suggest that chemotherapy using natural dietary agents with chemical agents represents a superior cancer treatment option.
ACKNOWLEDGEMENTS
This work was supported by Hebei Education Department (Z2011163), the natural science foundation of Hebei Province (H2013206147), and by National Natural Science Foundation of China (81201642) to Haixia Gao.
Authors’ contributions
JD and YJZ participated in the design, interpretation of the studies and analysis of the data. XC and YZ conducted the experiments, HZ performed statistical analyses. JD, YJZ, and HG materially participated in data interpretation and JD wrote the manuscript. All authors have read and approved the submission of the manuscript.
References
- 1.Institute for Health Metrics and Evaluation. The global burden of disease: generating evidence, guiding policy, Seattle: WA: IHME, 2013. [Google Scholar]
- 2.Wei WQ, Yang J, Zhang SW, Chen WQ, Qiao YL. Analysis of the esophageal cancer mortality in 2004 - 2005 and its trends during last 30 years in China. Chinese J Prevent Med 2010; 44: 398–402. [PubMed] [Google Scholar]
- 3.American Cancer Society. Global cancer facts & figures, 2nd ed Atlanta: American Cancer Society, 2011. [Google Scholar]
- 4.Jemal A, Siegel R, Xu JQ, Ward E. Cancer Statistics 2010, CA. Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 5.Hosono Y, Osada S, Nawa M, Takahashi T, Yamaguchi K, Kawaguchi Y, Yoshida K. Combination therapy of 5-fluorouracil with rapamycin for hormone receptor-negative human breast cancer. Anticancer Res 2010; 30: 2625–30. [PubMed] [Google Scholar]
- 6.Taguchi T. Clinical application of biochemical modulation in cancer chemotherapy: biochemical modulation for 5-FU. Oncology 1997; 54: 12–18. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy BJ. 5-fluorouracil toxicity: old or new? Cancer 1999; 86: 1099–100. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XD, Shu YQ, Liang J, Zhang FC, Ma XZ, Huang JJ, Chen L, Shi GM, Cao WG, Guo CY, Shen L, Jin ML. Combination chemotherapy with paclitaxel, cisplatin and fluorouracil for patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study. Chin J Cancer Res 2012; 24: 291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aphinives P, Bhudhisawasdi V, Sae-seow O, Uttaravichien T. 5-fluorouracil and mitomycin-C: effective, low-cost chemotherapy for colorectal cancer. J Med Assoc Thai 2006; 89: 1885–9. [PubMed] [Google Scholar]
- 10.Swaffar DS, Ang CY, Desai PB, Rosenthal GA, Thomas DA, Crooks PA, John WJ. Combination therapy with 5-fluorouracil and L-canavanine: in vitro and in vivo studies. Anticancer Drug 1995; 6: 586–93. [DOI] [PubMed] [Google Scholar]
- 11.Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, Raul F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett 2004; 215: 53–9. [DOI] [PubMed] [Google Scholar]
- 12.Falcao JM, Dias JA, Miranda AC, Leitao CN, Lacerda MM, da Motta LC. Red wine consumption and gastric cancer in Portugal: a case-control study. Eur J Cancer Prev 1994; 3: 269–76. [DOI] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–20. [DOI] [PubMed] [Google Scholar]
- 14.Clement MV, Hirpara JL, Chawdhury, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998; 92: 996–1002. [PubMed] [Google Scholar]
- 15.Hsieh TC, Wu JM. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors 2010; 36: 360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seve M, Chimienti F, Devergnas S, Aouffen M, Douki T, Chantegrel J, Cadet J, Favier A. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem 2005; 1: 629–33. [DOI] [PubMed] [Google Scholar]
- 17.George J, Singh M, Srivastava AK, Bhui K, Roy P, Chaturvedi PK, Shukla Y. Resveratrol and black tea polyphenol combination synergistically suppress mouse skin tumors growth by inhibition of activated MAPKs and p53. PLoS One 2011; 6: e23395–e23395. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Meth 1986; 89: 271–7. [DOI] [PubMed] [Google Scholar]
- 19.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth 1991; 139: 271–9. [DOI] [PubMed] [Google Scholar]
- 20.Morrone S. ANNEXIN V [CD-ROM], Rome: Purdue Cytometry, 1997. [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55. [DOI] [PubMed] [Google Scholar]
- 22.In K, Park J, Park H. Resveratrol at high doses acts as an apoptotic inducer in endothelial cells. Cancer Res Treat 2006; 38: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou HC, Chou FP, Sheen HM, Lin TM, Yang CH, Huey-Herng Sheu W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin Chim Acta 2006; 364: 196–204. [DOI] [PubMed] [Google Scholar]
- 24.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58: 621–81. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N, Adhami VM. Resveratrol causes WAF-1/p21-mediated G (1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 2001; 7: 1466–73. [PubMed] [Google Scholar]
- 26.Adhami VM, Afaq F, Ahmad N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun 2001; 288: 579–85. [DOI] [PubMed] [Google Scholar]
- 27.Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun 2005; 32: 433–40. [DOI] [PubMed] [Google Scholar]
- 28.Ming ZJ, Hu Y, Qiu YH, Cao L, Zhang XG. Synergistic effects of beta-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC-7721 cells. Phytomedicine 2010; 17: 575–80. [DOI] [PubMed] [Google Scholar]
- 29.Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol 2002; 8: 841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrieres J. The French Paradox; Lessons for other countries. Heart 2004; 90: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms. Int J Oncol 2003; 23: 17–28. [PubMed] [Google Scholar]
- 32.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006; 1: 2315–9. [DOI] [PubMed] [Google Scholar]
- 33.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–8. [DOI] [PubMed] [Google Scholar]
- 34.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci 2008; 82: 348–58. [DOI] [PubMed] [Google Scholar]
- 35.Celio LA, DiGregorio GJ, Ruch E, Pace JN, Piraino AJ. 5-Fluorouracil concentrations in rat plasma, parotid saliva, and bile and protein binding in rat plasma. J Pharm Sci 1983; 72: 597–9. [DOI] [PubMed] [Google Scholar]
- 36.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther 2002; 302: 369–73. [DOI] [PubMed] [Google Scholar]
- 37.Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 2002; 33: 387–98. [DOI] [PubMed] [Google Scholar]