Abstract
Metallothionein (MT) gene therapy leads to resolution of liver fibrosis in mouse model, in which the activation of collagenases is involved in the regression of liver fibrosis. MT plays a critical role in zinc sequestration in the liver suggesting its therapeutic effect would be mediated by zinc. The present study was undertaken to test the hypothesis that zinc supplementation suppresses liver fibrosis. Male Kunming mice subjected to bile duct ligation (BDL) resulted in liver fibrosis as assessed by increased α-smooth muscle actin (α-SMA) and collagen I production/deposition in the liver. Zinc supplementation was introduced 4 weeks after BDL surgery via intragastric administration once daily for 2 weeks resulting in a significant reduction in the collagen deposition in the liver and an increase in the survival rate. Furthermore, zinc suppressed gene expression of α-SMA and collagen I and enhanced the capacity of collagen degradation, as determined by the increased activity of total collagenases and elevated mRNA and protein levels of MMP13. Therefore, the results demonstrate that zinc supplementation suppresses BDL-induced liver fibrosis through both inhibiting collagen production and enhancing collagen degradation.
Keywords: Zinc, bile duct ligation, liver fibrosis, Kunming mice, collagenases, metalloproteinases
Introduction
In an attempt to explore experimental therapy for liver fibrosis, we tested the efficacy of metallothionein (MT) gene therapy for carbon tetrachloride-induced liver fibrosis in mice.1 We used adenovirus-mediated human MT-IIA (hMT-IIA) gene transfection as our therapeutic approach. Mice treated with carbon tetrachloride for 4 weeks developed liver fibrosis that was reversible upon removal of the chemical stimulus, correlating with a high level of hepatic MT. In contrast, mice treated with carbon tetrachloride for 8 weeks developed irreversible liver fibrosis along with low levels of hepatic MT. Additionally, carbon tetrachloride treatment for 4 weeks resulted in irreversible liver fibrosis in MT knockout (MT-KO) mice. Adenoviral delivery of hMT-IIA gene reversed the fibrosis along with increased hepatocyte regeneration in both WT and MT-KO mice with irreversible fibrosis. In the MT treated liver, the activity of collagenases was significantly elevated.
MT is an important zinc-binding protein involved in zinc metabolism and homeostasis.2–5 The interaction of MT with a number of oxidants causes a release of zinc bound to the protein,3,6–8 and the released zinc would thus affect the activities of enzymes involved in fibrogenesis and fibrinolysis in the liver. Among the enzymes and proteins that require zinc for their activation are matrix metalloproteinases (MMPs), which have a zinc active center.9 Therefore, the activity of these zinc-dependent enzymes is highly dependent on the availability of zinc.9,10
Since MT gene therapy would increase the availability of zinc in the liver, it is reasonable to speculate that zinc supplementation would be also effective in the treatment of liver fibrosis in mice. Therefore, the present study was undertaken to test the hypothesis that zinc supplementation suppresses liver fibrosis. To accomplish this, we used a bile duct ligation (BDL)-induced irreversible liver fibrosis mouse model and initiated zinc supplementation after the development of liver fibrosis. We thus examined the therapeutic effect, as opposed to the preventive effect, of zinc on irreversible liver fibrosis.
Materials and methods
Animals and BDL
Male Kunming mice, 8 weeks old and weighing 30–35 g, were obtained from Chengdu Da-Shuo experimental animal breeding research center. This strain of mice was selected as they are less susceptible to the potentially lethal effects of BDL than other tested strains. Animals were allowed free access to standard AIN-76 rodent chow and mineral-free DD-water. All the mice were treated according to the experimental procedure approved by the Institutional Animal Care and Use Committee. BDL/sham operation was done as previously described.11 Briefly, mice were anesthetized by inhalation using 2–3% isofluorane mixed in oxygen. The abdomen was opened by a midline laparotomy and the bile duct was separated from portal vein and hepatic artery, then ligated by three pieces of 5.0 mersilk (Ethicon); two at the upstream of bile outflow and one at the downstream. The bile duct was cut above the last ligation site followed by abdomen closure using an inverted interrupted suture. In sham-operated controls, the bile duct was identified but not ligated or transected. To test the effect of zinc on BDL-induced liver fibrosis, mice were divided into four groups 4 weeks after the BDL surgery: (1) sham, (2) sham + zinc, (3) BDL, and (4) BDL + zinc. These mice received vehicle solution (group 1 and 3) or zinc sulfate solution (5 mg zinc/kg, group 2 and 4) by intragastric administration once a day for 2 weeks. At the end of the experiment, all mice were sacrificed, and liver and blood samples were collected for analyses.
Zinc concentrations in the blood and liver
Plasma and hepatic zinc concentrations were determined by atomic absorption spectrometry (Thermo Scientific iCE 3500) after lyophilization and digestion with nitric acid following the procedure described previously.12
Analysis of liver fibrosis
Collagen deposition was identified in formalin-fixed, paraffin-embedded liver tissue sections after Masson’s trichrome staining and Sirus red staining. Semi-quantification of collagen deposition was performed by randomly selecting five non-overlapping areas in each slide at x 100 magnification under Nikon Eclipse 80i microscope and the collected images were analyzed using Image-pro plus 6.0 software.
Measurement of hepatic hydroxyproline concentration
The hydroxyproline content in the liver tissue was measured by a hydroxyproline assay kit (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) according to the manufacture’s instruction. The absorbance of the purple complex formed was measured at 550 nm using a standard spectrophotometer. Hydroxyproline was used as the standard to calculate the sample content of free hydroxyproline. Values are expressed as hydroxyproline (µg)/liver tissue (mg).
Immunohistochemical analysis
Liver tissues were fixed with 4% paraformaldehyde and sectioned in 5 µm portions. The sections were deparaffinized in xylene and dehydrated in alcohol. After treatment with 3% (vol/vol) hydrogen peroxide in methanol to eliminate non-specific reactions, the samples were incubated with anti-α-SMA antibody (Abcam, USA) overnight at 4℃. Samples were then processed using a DAB kit (Zhongshan Golden Bridge Inc., Beijing, China) according to the manufacturer’s instruction. Phosphate-buffered saline was used as negative control. The cells with brown staining in the cytoplasm were considered positive.
Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from liver tissues with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. RNA concentration was quantified using a Gene Quant pro (GE Healthcare, Waukesha, WI). Complementary DNAs (cDNAs) were synthesized using an iScript cDNA Synthesis Kit (Bio-Rad, USA) in MJ mini personal thermal cycler (Bio-Rad, USA). The amount of cDNA corresponding to 50 ng of RNA was amplified using a SYBR green PCR kit (Bio-Rad, USA) with the primers for α-SMA, collagen type Iα1, collagen type IIIα1, MMP8, MMP13, transforming growth factor-β1, tissue inhibitors of metalloproteinase 1, tissue inhibitors of metalloproteinase 2, and β-actin. The primer sequences (Table 1) were designed and synthesized by Invitrogen (Invitrogen Trading Co., Ltd, Shanghai, China).
Table 1.
Primer sequences used for real-time RT-PCR
| β-actin | 5’-AGATTACTGCTCTGGCTCCTAGC-3’ | (Forward primer) |
| 5’-ACTCATCGTACTCCTGCTTGCT-3’ | (Reverse primer) | |
| α-SMA | 5’-TCTGCCTCTAGCACACAACTG-3’ | (Forward primer) |
| 5’-CTAGGCCAGGGCTACAAGTT-3’ | (Reverse primer) | |
| COL1A1 | 5’-AGGCCACGCATGAGCCGAAG-3’ | (Forward primer) |
| 5’-GCCATGCGTCAGGAGGGCAG-3’ | (Reverse primer) | |
| COL3A1 | 5’-ACCTGCAGGACCCACTGGCA-3’ | (Forward primer) |
| 5’-GACCACGCCCACCGGGAAAG-3’ | (Reverse primer) | |
| MMP8 | 5’-TGCCTCGATGTGGAGTGCCTGA-3’ | (Forward primer) |
| 5’-GCCCTTGACAGCTGTGGCGT-3’ | (Reverse primer) | |
| MMP13 | 5’-ACAGGCTCCGAGAAATGCAA-3’ | (Forward primer) |
| 5’-CCACATCAGGCACTCCACAT-3’ | (Reverse primer) | |
| TGF-β1 | 5’-TTGCCCTCTACAACCAACACAA-3’ | (Forward primer) |
| 5’-GGCTTGCGACCCACGTAGTA-3’ | (Reverse primer) | |
| TIMP1 | 5’-TGATTTCCCCGCCAACTCCGC-3’ | (Forward primer) |
| 5’-TGCCAGAGATGCAAAGGGGGC-3’ | (Reverse primer) | |
| TIMP2 | 5’-AGCCCTCCCTGAGCCGTGTT-3’ | (Forward primer) |
| 5’-AGAGCCAAACCGAGCCGTGC-3’ | (Reverse primer) |
Western blot analysis of proteins
Livers were removed and immediately stored in liquid nitrogen. Frozen livers were homogenized in lysis buffer (50 mM Tris–HCl, pH 8, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, and 0.1% SDS) supplemented with 1% complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and 1 mM phenylmethanesulfonyl fluoride. The homogenates were centrifuged at 12,000 g/min for 20 min at 4℃. The supernatants were carefully collected and protein content was determined using Pierce® BCA Protein Assay Kit (Pierce Biotechnology, Rockford, USA). A 50 µg protein sample was resolved in 10% SDS-PAGE and transferred to polyvinylidene fluoride membrane (Bio-Rad, USA). Transferred proteins were blocked with 5% non-fat dry milk in Tris–HCl buffer solution containing Tris–HCl (50 mM), NaCl (150 mM), and Tween-20 (0.1%) for 1 h at room temperature. The blots were then incubated with respective primary antibodies (anti-α-SMA, anticollagen I, Abcam, USA) in blocking solution, according to the vender’s recommendations. After incubation, the blots were washed with TBS-T six times for 5 min each followed by incubation with appropriate secondary antibody for 1 h. Target proteins were visualized using a chemiluminescent horseradish peroxidase substrate (Millipore, USA) and analyzed by densitometry using Quantity One Software (Bio-Rad, USA).
Determination of collagenase activity
The total enzyme activity of collagenases MMP1, 8, and 13 was determined in liver homogenates using an EnzCheck Gelatinase/Collagenase Assay kit (Molecular Probes, USA) according to the manufacturer’s instruction. Fluorescently labeled collagen type I was used as substrate with purified collagenase from clostridium histolyticum as the control. Fluorescence (excitation 495 nm, emission 515 nm) was measured using a microplate reader (BioTek Synergy2).
Statistics
The data were analyzed using SPSS/19.0 software and comparisons between groups were made using one way analysis of variance followed by Dunnett’s T3 test. The value P < 0.05 was considered as statistically significant.
Results
Effect of zinc supplementation on zinc concentrations in the blood and liver
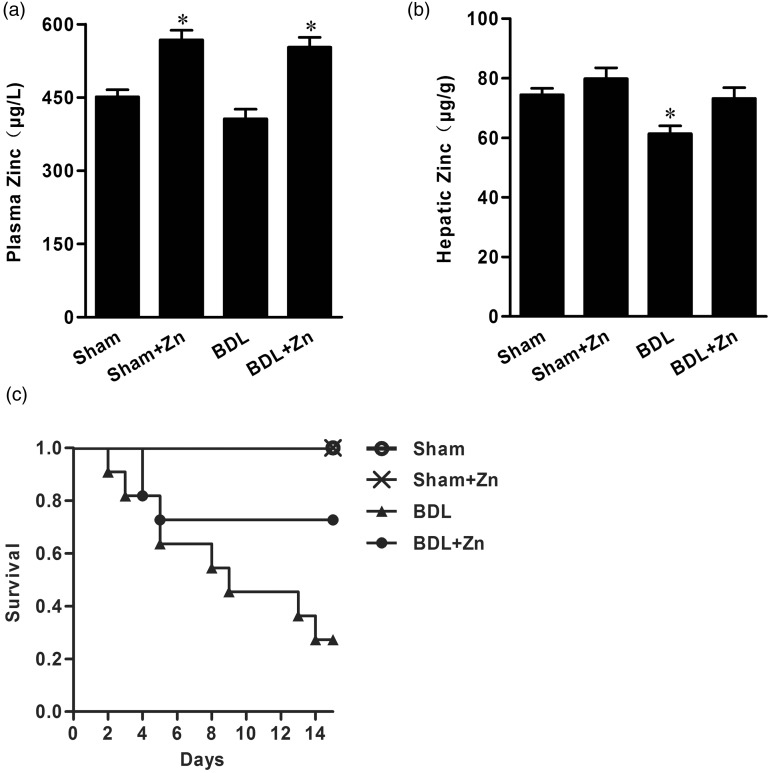
BDL significantly depressed zinc concentrations in the liver, but only slightly decreased zinc concentrations in the blood. Zinc supplementation not only replenished zinc concentrations in the liver, but also increased zinc concentrations in the blood in BDL mice (Figure 1). In contrast, zinc supplementation significantly increased zinc concentrations in the blood, but did not affect liver concentrations in the sham-operated control mice (Figure 1).
Figure 1.
Zinc concentrations in blood (a) and liver tissue (b) in the sham-operated and BDL groups with or without zinc supplementation and changes in mortality rates of mice within these groups (c). Mice were subjected to BDL for 4 weeks and then divided into four groups as shown. Zinc supplementation started at the end of 4 weeks after BDL and lasted for 2 weeks. Animals were harvested at the end of 2 weeks of zinc supplementation. Data were obtained from six mice in each treatment group (A and B) and expressed as mean ± SD; *significantly different from sham-operated controls (P < 0.05). The mortality in the BDL only group at the end of the experiment (n = 30 each group) was significantly different from other groups (P < 0.05)
Effect of zinc supplementation on survival and liver fibrosis in BDL mice
A gradual increase in animal death was observed in the BDL mice, and zinc supplementation markedly suppressed the mortality (Figure 1). The survival rate was 27% in the mice subjected to BDL without zinc supplementation. After zinc supplementation for 2 weeks the survival rate increased to 72%.
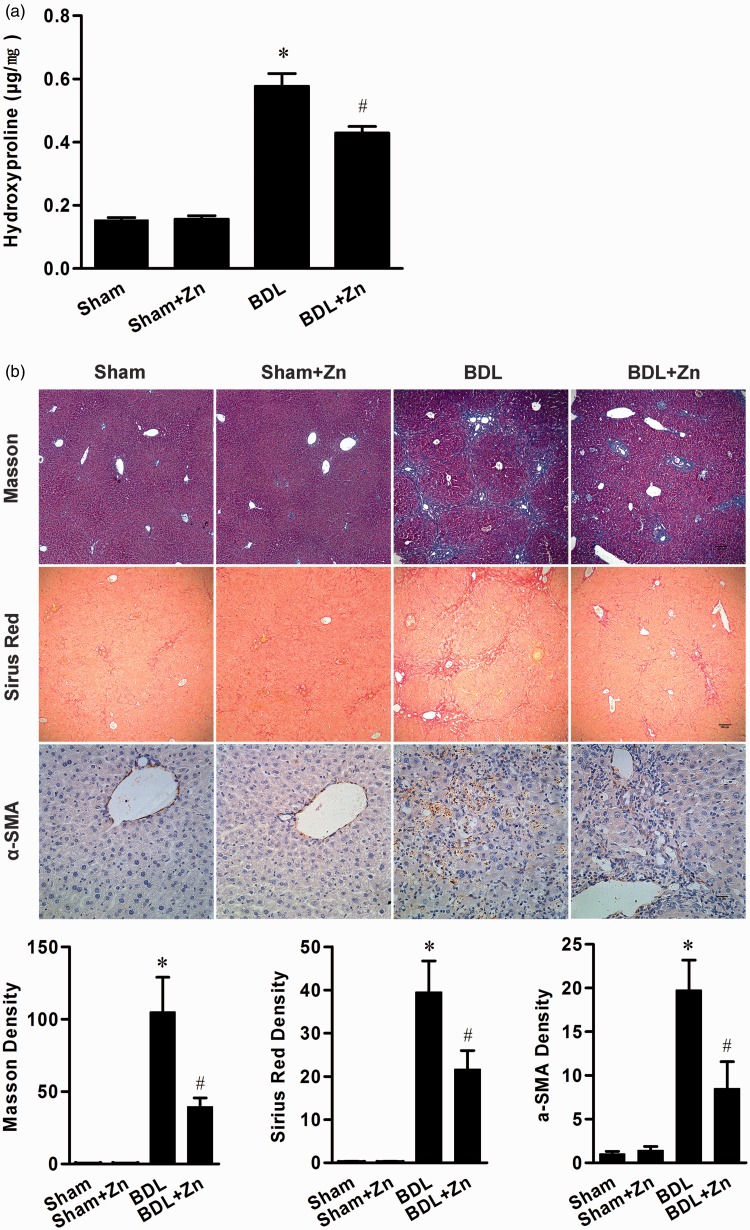
Zinc supplementation significantly reduced collagen deposition in the liver as revealed by Masson and Sirus red staining and semi-quantitative analysis (Figure 2). In addition, immunohistochemical analysis showed a remarkable α-SMA-positive staining in the liver of the BDL mice, which was significantly reduced after zinc supplementation (Figure 2). Furthermore, zinc supplementation significantly reduced hydroxyproline concentrations in the livers of BDL mice (Figure 2) confirming the suppression effect of zinc on BDL-induced liver fibrosis.
Figure 2.
Effect of zinc supplementation in the sham-operated and BDL groups on liver hydroxyproline content (a), and collagen and α-SMA deposition (b). Immunostaining of collagen and α-SMA (b) appeared positive in BDL mice and semi-quantitative analysis revealed a significant increase in collagen content and α-SMA concentrations, which were significantly reduced by zinc supplementation. Data were obtained from nine mice in each treatment group (a) and expressed as mean ± SD. The mean density of Masson’s trichrome staining, Sirus red staining, and α-SMA staining was obtained from six animals; three different section slides from each mouse and the images of five randomized and non-overlapping areas at x100 (Masson’s trichrome staining, Sirus red staining) or x400 (α-SMA staining) magnification in each slide were captured and calculated. Data are shown as the mean ± SEM; *significantly different from sham-operated controls (P < 0.05), #significantly different from both sham-operated controls and BDL treated only (P < 0.05). (A color version of this figure is available in the online journal.)
Zinc supplementation on α-SMA and collagen I expression in the liver
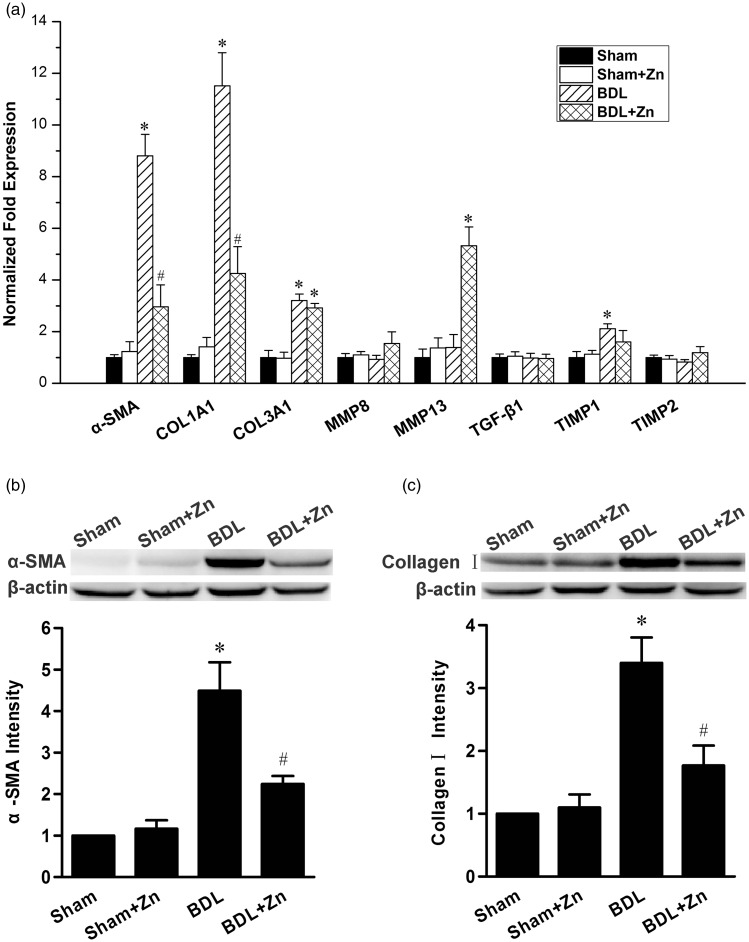
To further understand the effect of zinc supplementation on BDL-induced liver fibrosis, the expressions of α-SMA and collagen I in the livers of mice subjected to BDL with or without zinc supplementation were assessed by real-time RT-PCR and Western blot analysis. The mRNA levels of α-SMA and collagen Iα1 were significantly increased in the BDL mice, which were significantly suppressed by zinc (Figure 3(a)). Western blot analysis showed the same effect, i.e. zinc supplementation significantly reduced the protein levels of α-SMA (Figure 3(b)) and total collagen I (Figure 3(c)).
Figure 3.
Gene expression profile of the livers of mice in the sham-operated and BDL groups with or without zinc supplementation (a) and the protein content of α-SMA (b) and collagen I (c) in the livers. The mRNA levels for different proteins as labeled were determined by real-time PCR (a). Western blot analyses for α-SMA (b) and collagen I (c) were also subjected to semi-quantitative analysis as shown. The data were obtained from seven mice in each group (a) and four mice in each treatment (b and c) and expressed as mean ± SD; *significantly different from sham-operated controls (P < 0.05), #significantly different from sham controls and BDL only (P < 0.05)
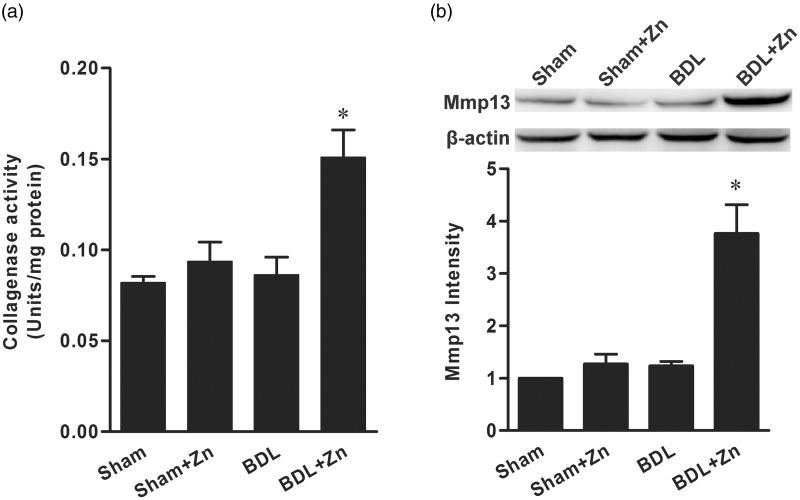
Zinc supplementation on the activity of collagenases and the expression of MMP13
To probe possible mechanisms by which zinc supplementation suppressed the progression of liver fibrosis induced by BDL, we measured the effect of zinc supplementation on the activity of collagenases (MMP1, 8, and 13) in the liver. The total activity of collagenases in the BDL mice supplemented with zinc was significantly elevated in comparison with that of the BDL mice or sham-operated controls (Figure 4). Zinc supplementation did not affect the activity of collagenases in the livers of sham-operated controls. Furthermore, the mRNA and protein levels of MMP13 were significantly increased in response to zinc supplementation in the BDL mice, but not in the sham-operated controls (Figure 4).
Figure 4.
The effect of zinc supplementation on the activity of collagenases (a) and the protein content of MMP13 (b) in mice subjected to BDL versus the sham-operated control. The data were obtained from six mice in each treatment group (a) and three mice in each group (b) and expressed as mean ± SD; *significantly different from sham-operated controls (P < 0.05)
Discussion
We previously demonstrated that MT gene therapy reversed liver fibrosis induced by carbon tetrachloride in a mouse model.1 It is important to understand the mechanism by which MT gene therapy imparts reversibility on irreversible liver fibrosis. Since interstitial collagenases including MMP1, 8, and 13 are important metalloproteases involved in the liver fibrinolysis, several studies have evaluated the role of collagenases in the recovery of established liver fibrosis. For instance, human MMP8 delivered by an adenovirus vector (AdMMP8) was shown to effectively reverse liver fibrosis.13 The same AdMMP8 system was also shown to be effective in reducing liver fibrosis in bile duct-ligated cirrhosis rats.14 A similar approach using MMP1 delivered by an AdMMP1 construct was also effective in reversing liver fibrosis in rats.15
Treatment with carbon tetrachloride for 4 weeks causes liver fibrosis, which is reversible upon secession of the treatment. Therefore, to define the effect of intervention on the progression and regression of liver fibrosis requires the concomitant presence of carbon tetrachloride. However, continuous treatment with carbon tetrachloride during the intervention confounds the interpretation of the result; the direct and indirect effects are indistinguishable. In contrast, BDL for 4 weeks causes irreversible liver fibrosis. Therefore, the improvement after intervention in BDL mice is not confounded by the self-recovery process or through interaction with the cause. A negative feature of this approach is severe mortality in several strains of mice (including parent strains of 129, C57BL/6, and FVB/N), which impedes observation of the progression and intervention following BDL-induced liver fibrosis. The strain of Kunming mice is much more tolerant of the lethality induced by BDL allowing a long-term observation after BDL treatment. This model provides a convenient experimental tool for more comprehensive understanding of BDL-induced liver fibrosis and potential intervention. We took advantage of the hardiness of these mice with respect to BDL treatment to observe the intervention effect of zinc supplementation.
Zinc supplementation increased its blood levels in both sham-operated and BDL mice suggesting that zinc absorption is not affected by BDL and that increasing zinc availability through oral supplementation in secondary biliary cirrhosis is possible. Further, zinc levels were replenished in the BDL mice, but zinc supplementation did not increase hepatic zinc concentrations in the sham-operated mice. This suggests the regulation of hepatic zinc homeostasis in BDL mice remains functional in secondary biliary cirrhosis and that increasing the availability of zinc in the blood replenishes the depleted zinc in the liver.
It is important to note that zinc supplementation remarkably increased the survival of BDL mice; especially when supplementation was introduced after BDL-induced liver fibrosis (4 weeks after BDL) rather than prior to BDL treatment. This simple intervention is fairly achievable in clinical setting. Provided that the regulation of hepatic homeostasis remains functional, oral zinc supplementation would improve the condition of secondary biliary cirrhosis. The suppressed progression of liver fibrosis through zinc supplementation was observed by reduced collagen deposition, decreased hydroxyproline accumulation, and diminished α-SMA staining in the liver. These factors indicate that intervention is effective in suppressing the progression of secondary biliary cirrhosis, although it is also possible that this treatment can reverse BDL-induced liver fibrosis.
Several prior studies examined the effects of multiple intervention procedures on BDL- or carbon tetrachloride-induced liver fibrosis in mice or rats.16–21 In addition to the gene therapies for liver fibrosis described earlier,13–16,18 approaches include blocking the fibrogenesis signaling pathways with treatments such as rapamycin in rat model of BDL-induced biliary cirrhosis.21 However, these intervention procedures were all applied immediately following BDL and demonstrated preventive effect on liver fibrosis.
In an attempt to examine the effect of zinc treatment on ongoing liver fibrosis, Kono et al.22 used polaprezinc to treat thioacetamide (TAA)-induced liver fibrosis in rats. The rats were treated with TAA for 20 weeks and polaprezinc was administrated daily during the last 10 weeks. It was found that polaprezinc treatment reduced liver fibrosis. Interestingly, zinc sulfate administrated in the equal quantities of zinc found in polaprezinc failed to show any protective effect on TAA-induced liver fibrosis. This may be due to direct or indirect interaction between TAA and zinc sulfate that limits zinc availability, which interferes with the effect of zinc on liver fibrosis similar to the effect of zinc on carbon tetrachloride-induced liver fibrosis.
The present study reveals that zinc suppresses the progression of liver fibrosis in BDL mice through inhibition of collagen production and deposition, as well as through enhanced collagen degradation. First, we observed that zinc supplementation suppressed the expression of collagen I, as determined by reduced levels of mRNA and protein. Second, the levels of mRNA and protein of α-SMA were also significantly reduced. Since up-regulation of both collagen I and α-SMA is involved in the fibrogenesis in the liver, zinc suppression on their expression suggests a common inhibitory mechanism in the BDL mice. The understanding of this inhibitory mechanism will be included in our future studies.
The increase in the activity of collagenases by zinc supplementation plays a critical role in the regression of liver fibrosis. Inhibition of collagen production leads to depressed collagen deposition. However, the preexisting collagen deposition requires a degradation process for its removal. It was known that zinc is a critical co-factor for the activity of collagenases.9,23 Therefore, it is not unexpected that replenishment of zinc pool in the BDL liver increased activity of collagenases. However, this is not the only mechanism for the enhanced activity. Zinc supplementation also increased the expression of MMP13, a major collagenase, as demonstrated by the increase in both mRNA and protein levels. It should be noted that zinc alone does not trigger up-regulation of collagenases, as demonstrated by that unaltered expression of MMP13 and the consistent activity of collagenases following zinc supplementation in the sham-operated controls.
The present study demonstrates the role of zinc in blocking the progression of BDL-induced liver fibrosis. However, a comprehensive understanding of the mechanism by which zinc suppresses liver fibrosis remains limited. While this study clearly demonstrated zinc suppression of the progression of BDL-induced liver fibrosis, it cannot exclude the possibility that zinc reverses liver fibrosis in the presence of BDL. Differentiating these modes of zinc action requires further studies. Additionally, it is important to understand the mechanism by which zinc suppresses the expression of pro-fibrotic genes in BDL mice. Furthermore, the up-regulation of MMP13 and perhaps other MMPs is another important role of zinc that requires further mechanistic understanding. Lastly, zinc supplementation may affect copper transportation in the liver. In particular, copper accumulation in the liver is a factor for liver fibrosis. Zinc replenishment in the liver may help copper excretion. These unsolved and emerging issues will be the foci of our future studies.
In summary, the present study exploits the high tolerance of Kunming mice to the lethal action of BDL to understand zinc suppression of BDL-induced liver fibrosis and the subsequent lethality. Zinc supplementation not only suppressed liver fibrosis, but it also remarkably improved the survival of mice subjected to BDL. The suppression of liver fibrosis was well correlated with the suppressed collagen production and deposition and enhanced activity of collagenases. However, it remains to be determined whether zinc supplementation reverses liver fibrosis and how zinc down-regulates the expression of pro-fibrotic genes and up-regulates the collagenolytic genes.
Acknowledgements
The authors thank Dr Kefei Chen for advising the establishment of BDL-induced liver fibrosis in Kunming Mice. This study was supported by Sichuan University West China Hospital.
Authors’ contributions
Participated in research design: FS, YJK
Conducted experiments: FS, QS, XHX, WLH
Performed data analysis: FS, YJK
Contributed to the writing of the manuscript: YJK, FS
References
- 1.Jiang Y, Kang YJ. Metallothionein gene therapy for chemical-induced liver fibrosis in mice. Mol Ther 2004; 10: 1130–9. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL. The function of metallothionein. Neurochem Int 1995; 27: 23–33. [DOI] [PubMed] [Google Scholar]
- 3.Spahl DU, Berendji-Grun D, Suschek CV, Kolb-Bachofen V, Kroncke KD. Regulation of zinc homeostasis by inducible NO synthase-derived NO: nuclear metallothionein translocation and intranuclear Zn2+ release. Proc Natl Acad Sci USA 2003; 100: 13952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez MM, Hill GM, Link JE, Raney NE, Tempelman RJ, Ernst CW. Pharmacological zinc and phytase supplementation enhance metallothionein mRNA abundance and protein concentration in newly weaned pigs. J Nutr 2004; 134: 538–44. [DOI] [PubMed] [Google Scholar]
- 5.Cherian MG, Kang YJ. Metallothionein and liver cell regeneration. Exp Biol Med (Maywood) 2006; 231: 138–44. [DOI] [PubMed] [Google Scholar]
- 6.Feng W, Benz FW, Cai J, Pierce WM, Kang YJ. Metallothionein disulfides are present in metallothionein-overexpressing transgenic mouse heart and increase under conditions of oxidative stress. J Biol Chem 2006; 281: 681–7. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol 2003; 184: 337–47. [DOI] [PubMed] [Google Scholar]
- 8.Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr 2003; 133: 1460S–2S. [DOI] [PubMed] [Google Scholar]
- 9.Seltzer JL, Jeffrey JJ, Eisen AZ. Evidence for mammalian collagenases as zinc ion metalloenzymes. Biochim Biophys Acta 1977; 485: 179–87. [DOI] [PubMed] [Google Scholar]
- 10.Anttinen H, Puistola U, Pihlajaniemi T, Kivirikko KI. Differences between proline and lysine hydroxylations in their inhibition by zinc or by ascorbate deficiency during collagen synthesis in various cell types. Biochim Biophys Acta 1981; 674: 336–44. [DOI] [PubMed] [Google Scholar]
- 11.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, Burt AD, Wilson CL, Anstee QM, Barter MJ, Masson S, Elsharkawy AM, Mann DA, Mann J. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med 2012; 18: 1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo X, Dong D, Sun M, Xie H, Kang YJ. Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS One 2013; 8: e67549–e67549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Banuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther 2002; 9: 127–34. [DOI] [PubMed] [Google Scholar]
- 14.Siller-Lopez F, Sandoval A, Salgado S, Salazar A, Bueno M, Garcia J, Vera J, Galvez J, Hernandez I, Ramos M, Aguilar-Cordova E, Armendariz-Borunda J. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 2004; 126: 1122–33;. discussion 949. [DOI] [PubMed] [Google Scholar]
- 15.Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 2003; 124: 445–58. [DOI] [PubMed] [Google Scholar]
- 16.Cong M, Liu T, Wang P, Fan X, Yang A, Bai Y, Peng Z, Wu P, Tong X, Chen J, Li H, Cong R, Tang S, Wang B, Jia J, You H. Antifibrotic effects of a recombinant adeno-associated virus carrying small interfering RNA targeting TIMP-1 in rat liver fibrosis. Am J Pathol 2013; 182: 1607–16. [DOI] [PubMed] [Google Scholar]
- 17.Sant’Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant 2011; 20: 441–53. [DOI] [PubMed] [Google Scholar]
- 18.Hu PF, Chen H, Zhong W, Lin Y, Zhang X, Chen YX, Xie WF. Adenovirus-mediated transfer of siRNA against PAI-1 mRNA ameliorates hepatic fibrosis in rats. J Hepatol 2009; 51: 102–13. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Ochi T, Shimada H, Inagaki K, Fujita I, Nii A, Moffat MA, Katragadda M, Violand BN, Arch RH, Masferrer JL. Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol Res 2010; 40: 1128–41. [DOI] [PubMed] [Google Scholar]
- 20.Ping J, Gao AM, Qin HQ, Wei XN, Bai J, Liu L, Li XH, Li RW, Ao Y, Wang H. Indole-3-carbinol enhances the resolution of rat liver fibrosis and stimulates hepatic stellate cell apoptosis by blocking the inhibitor of kappaB kinase alpha/inhibitor of kappaB-alpha/nuclear factor-kappaB pathway. J Pharmacol Exp Ther 2011; 339: 694–703. [DOI] [PubMed] [Google Scholar]
- 21.Biecker E, De Gottardi A, Neef M, Unternahrer M, Schneider V, Ledermann M, Sagesser H, Shaw S, Reichen J. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther 2005; 313: 952–61. [DOI] [PubMed] [Google Scholar]
- 22.Kono T, Asama T, Chisato N, Ebisawa Y, Okayama T, Imai K, Karasaki H, Furukawa H, Yoneda M. Polaprezinc prevents ongoing thioacetamide-induced liver fibrosis in rats. Life Sci 2012; 90: 122–30. [DOI] [PubMed] [Google Scholar]
- 23.Williams DH, Murray EJ. Specific amino acid substitutions in human collagenase cause decreased autoproteolysis and reveal a requirement for a second zinc atom for catalytic activity. FEBS Lett 1994; 354: 267–70. [DOI] [PubMed] [Google Scholar]