Abstract
The pathophysiology of ischemic myocardial injury involves cellular events, reactive oxygen species, and an inflammatory reaction cascade. The zinc complex of acetylsalicylic acid (Zn(ASA)2) has been found to possess higher anti-inflammatory and lower ulcerogenic activities than acetylsalicylic acid (ASA). Herein, we studied the effects of both ASA and Zn(ASA)2 against acute myocardial ischemia. Rats were pretreated with ASA (75 mg/kg) or Zn(ASA)2 (100 mg/kg) orally for five consecutive days. Isoproterenol (85 mg/kg, subcutaneously [s.c.]) was applied to produce myocardial infarction. After 17–22 h, animals were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally [i.p.]) and both electrical and mechanical parameters of cardiac function were evaluated in vivo. Myocardial histological and gene expression analyses were performed. In isoproterenol-treated rats, Zn(ASA)2 treatment normalized significantly impaired left-ventricular contractility index (Emax 2.6 ± 0.7 mmHg/µL vs. 4.6 ± 0.5 mmHg/µL, P < 0.05), increased stroke volume (30 ± 3 µL vs. 50 ± 6 µL, P < 0.05), decreased systemic vascular resistance (7.2 ± 0.7 mmHg/min/mL vs. 4.2 ± 0.5 mmHg/min/mL, P < 0.05) and reduced inflammatory infiltrate into the myocardial tissues. ECG revealed a restoration of elevated ST-segment (0.21 ± 0.03 mV vs. 0.09 ± 0.02 mV, P < 0.05) and prolonged QT-interval (79.2 ± 3.2 ms vs. 69.5 ± 2.5 ms, P < 0.05) by Zn(ASA)2. ASA treatment did not result in an improvement of these parameters. Additionally, Zn(ASA)2 significantly increased the mRNA-expression of superoxide dismutase 1 (+73 ± 15%), glutathione peroxidase 4 (+44 ± 12%), and transforming growth factor (TGF)-β1 (+102 ± 22%). In conclusion, our data demonstrate that oral administration of zinc and ASA in the form of bis(aspirinato)zinc(II) complex is superior to ASA in preventing electrical, mechanical, and histological changes after acute myocardial ischemia. The induction of antioxidant enzymes and the anti-inflammatory cytokine TGF-β1 may play a pivotal role in the mechanism of action of Zn(ASA)2.
Keywords: Myocardial ischemia, acetylsalicylic acid, zinc complex of acetylsalicylic acid, cardiac function, isoproterenol, antioxidant
Introduction
Myocardial ischemia is caused by a critical imbalance between the coronary oxygen supply and the metabolic demand of the myocardium.1 Among the various proposed mechanisms, increased migration of neutrophils to the myocardium and oxidative stress resulting from increased production of free radicals associated with decreased levels of antioxidants have been implicated in the pathophysiology of ischemic myocardial injury.2 An important consequence of myocardial ischemia is the occurrence of potentially lethal heart function impairment. Therapeutic intervention targeting free radical generation, inflammation and/or enhancement of endogenous antioxidant enzymes may reduce ischemic injury.
Acetylsalicylic acid (ASA; trade name aspirin), a traditional non-steroidal anti-inflammatory drug, has been used for many years as an antipyretic, analgesic, antithrombotic, and anti-inflammatory agent. Its prophylactic administration substantially attenuates the development of myocardial necrosis in animals induced by catecholamines.3 Additionally, ASA has been shown to exert a protective effect on isoproterenol-induced cardiac injury4 and epinephrine-induced myocardial necrosis in dogs.5 However, its principal ulcerogenicity has limited its widespread application. Zinc ions, on the other hand, are known to possess anti-ulcerogenic6 as well as anti-inflammatory effects.7 It has been demonstrated that administration of zinc partially protects the heart against isoproterenol-induced heart injury8 and Powell et al. demonstrated that zinc improves post-ischemic recovery of isolated rat hearts.9,10 Zn(ASA)2, a 2:1 complex of ASA to zinc, was therefore synthesized and characterized.11 It possesses several advantages over uncomplexed ASA: (a) the ulcerogenicity of ASA may be further reduced by direct gastroprotective action of zinc, (b) zinc may increase the anti-inflammatory effects of ASA, and (c) zinc will be present in a better tolerated form.
Isoproterenol hydrochloride, a synthetic catecholamine and non-specific β-adrenergic agonist, has been found to cause myocardial infarction in mammalians when administered in supramaximal doses.12 The acute phases of myocardial infarction and repair are comparable with those occurring in patients, e.g. changes in serum enzymes,13 electrocardiographic alterations14 as well as histological changes.15 Generation of highly cytotoxic free radicals through auto-oxidation of catecholamines has been implicated as one of the important causative factors of myocardial necrosis.
One of the long-term treatments of patients with heart failure due to ischemic cardiomyopathy includes ASA medication. We hypothesized that the use of zinc and ASA in the form of bis(aspirinato)zinc(II) complex will improve the effects of ASA and may be a new treatment concept for ischemic heart disease. In the present study, we studied the effects of ASA and Zn(ASA)2 administration on different aspects of cardiac damage caused by isoproterenol in an in vivo rat model of myocardial ischemia. Despite the fact that Zn(ASA)2 possesses anti-inflammatory activity, to our knowledge, its protective effects on acute myocardial ischemia have not been studied.
Materials and methods
Animals
Male Sprague-Dawley rats (250–350 g; Charles River, Sulzfeld, Germany) were housed in a room at 22 ± 2℃ under 12-h light/dark cycles and were fed a standard laboratory rat diet and water ad libitum. The rats were acclimatized for at least one week before experiments. All animals received humane care in compliance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86–23, revised 1996). This investigation was reviewed and approved by the ethics committee of the Land Baden-Württemberg for Animal Experimentation.
Experimental myocardial infarction
Isoproterenol was injected subcutaneously into rats (85 mg/kg, at a volume of 0.1 mL/100 g body weight) at an interval of 24 h for two consecutive days to induce myocardial injury.16
Experimental groups
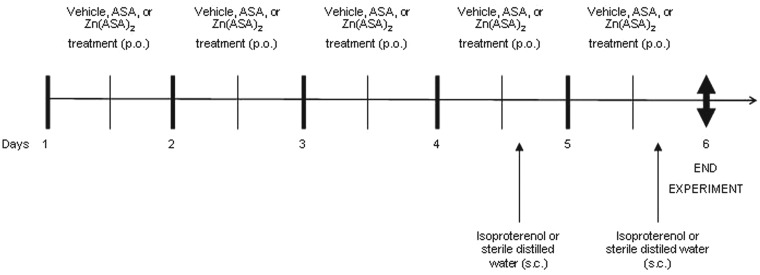
Rats were randomly divided into five groups: (a) the control group received methylcellulose vehicle for five days and sterile distilled water on days four and five (n = 7); (b) the isoproterenol group received methylcellulose vehicle for five days and isoproterenol on days four and five (n = 12); (c) the ASA + isoproterenol group received ASA for five days and isoproterenol on days four and five (n = 8); (d) the Zn(ASA)2 + isoproterenol group received Zn(ASA)2 for five days and isoproterenol on days four and five (n = 13); and (e) the Zn(ASA)2 group received Zn(ASA)2 for five days and sterile distilled water on days four and five (n = 8). The experiment was stopped 17–22 h after the last administration of drugs. The experimental protocol is shown in Figure 1.
Figure 1.
Experimental protocol. Rats were pretreated with ASA (75 mg/kg) or Zn(ASA)2 (100 mg/kg) orally for five consecutive days. Isoproterenol was injected subcutaneously into rats (85 mg/kg) at an interval of 24 h on days 4 and 5 to induce myocardial injury
Electrocardiography
Rats were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally [i.p.]) at 17–22 h after the last administration of drugs and kept in a supine position on heating pads maintaining their core temperature (measured via a rectal probe) at 37℃. Standard 12-lead electrocardiograms were monitored for 10 min and recorded using needle electrodes placed subcutaneously. All leads were connected to a standard direct-writing recorder (Mortara Instrument, WI). The ECG analysis was evaluated in lead II including the following measurements: ST-segment elevation, PQ- and QT-intervals. QT-interval, measured from the onset of the QRS complex to the end of the T wave, was corrected using normalized Bazett’s formula adjusted for rats (nQTc = QT/(RR/f)1/2).17 Electrocardiography was analyzed in a blinded fashion.
In vivo hemodynamic measurements
After ECG recording, the animals were tracheotomized and intubated and artificially ventilated. A polyethylene catheter was inserted into the left external jugular vein for fluid administration. A 2F microtip pressure–volume catheter was inserted into the right carotid artery and advanced into the ascending aorta. After stabilization for 5 min, arterial blood pressure was recorded and the catheter was advanced into the left-ventricle under pressure control. With the use of a special pressure–volume analysis program (PVAN, Millar Instruments, Houston, TX), heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, stroke volume, and systemic vascular resistance were calculated. Left-ventricular (LV) pressure–volume relations were assessed by transiently compressing the inferior vena cava. The slope Emax of the LV end-systolic pressure–volume relationship, a load-independent index of LV contractility, was calculated.
At the end of each experiment, 0.1 mL hypertonic saline (5%) was injected using the central venous line, and from the shift of pressure–volume relations parallel conductance volume was calculated by PVAN pressure–volume analysis software and used for correction of absolute LV volume.18
Histopathology
Hearts were fixed in buffered paraformaldehyde solution (4%) and embedded in paraffin. Five-micrometer thick sections were stained with hematoxylin and eosin to determine the histopathological changes in the degree of inflammation and edema.
Biochemical estimation
Blood collected from the rats into lithium heparin tubes was immediately centrifuged, and the plasma separated. Cardiac troponin-T concentrations were determined by automatic biochemistry analyzer.
Quantitative real-time polymerase chain reaction (PCR)
The apex of explanted hearts was further used for gene expression analyses. Total RNA was isolated with the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany). RNA concentration and purity were determined photometrically (230 nm, 260 nm and 280 nm). Reverse transcription was performed with the QuantiTect Reverse Transcription Kit (Qiagen) using 800 ng RNA in a total volume of 20 µL. Quantitative real-time PCR was performed with the Light-Cycler480 system using the LightCycler480 Probes Master and Universal ProbeLibrary probes (Roche, Mannheim, Germany) (Table 1). The conditions for PCR were as follows: 95℃ for 10 min (1 cycle), 95℃ for 10 s, 60℃ for 30 s (single; 45-cycle quantification), 40℃ for 10 s (1 cycle). The efficiency of the PCR reaction was confirmed by standard curve analysis. Sample quantifications were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression by using a pool of all cDNA’s (positive calibrator). Evaluation was performed with the Light Cycler 480 SW 1.5 software (Roche).
Table 1.
The sequence for the forward (F) and reverse (R) primers (from 5’ to 3’) and Universal Probe Library (UPL) probes
| Assay | Sequence | UPL probes |
|---|---|---|
| Cyclooxygenase-2 | F: 5’-GACGAGCGACTGTTCCAAAC-3’ R: 5’-GGAACTGCTGGTTGAAAAGC-3’ | 115 |
| GAPDH | F: 5’-CTACCCACGGCAAGTTCAAT-3’ R: 5’-ATTTGATGTTAGCGGGATCG-3’ | 111 |
| GPx-4 | F: 5’-TGGGAAATGCCATCAAATG-3’ R: 5’-CGGCAGGTCCTTCTCTATCA-3’ | 25 |
| SOD-1 | F: 5’-GGTCCAGCGGATGAAGAG-3’ R: 5’-GGACACATTGGCCACACC-3’ | 5 |
| TGF-β1 | F: 5’-TCAGACATTCGGGAAGCAGT-3’ R: 5’-ACGCCAGGAATTGTTGCTAT-3’ | 56 |
GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GPx-4: glutathione peroxidase 4; SOD-1: superoxide dismutase 1; TGF-β1: transforming growth factor β1.
Chemical reagents
ASA was purchased from Sigma Aldrich Chemie GmbH (Steinheim, Germany) and Zn(ASA)2 was synthesized and kindly provided by Prof. Hiroyuki Yasui, Kyoto Pharmaceutical University, Japan. ASA and Zn(ASA)2 were suspended in 0.5% methylcellulose (Caesar & Loretz GmbH, Hilden, Germany) and administrated orally at the dose of 75 mg/kg (the same amount of ASA as in the Zn(ASA)2 complex) and 100 mg/kg (1 mL/100 g of body weight), respectively. Isoproterenol hydrochloride (Sigma Aldrich Chemie GmbH, Steinheim, Germany) was dissolved in sterile distilled water.
Statistical analysis
Statistical analysis was performed by using the Origin 7.0 (Microcal Software, Northampton). All data are expressed as means ± standard error of the mean (SEM). Means between groups were compared by one-way ANOVA followed by an unpaired t-test with Bonferroni correction for multiple comparisons. A value of P < 0.05 was considered statistically significant.
Results
Heart rate and arterial blood pressures
Isoproterenol administration resulted in increased heart rates when compared to control animals. Pre-treatment with either ASA or Zn(ASA)2 did not have any effect on this parameter. Values of systolic blood pressure, diastolic blood pressure, and mean arterial pressure remained unchanged in all experimental groups (Table 2).
Table 2.
Heart rate and arterial blood pressures.a
| Control | Iso | Iso + ASA | Iso + Zn(ASA)2 | Zn(ASA)2 | |
|---|---|---|---|---|---|
| Heart rate (beats/min) | 412 ± 14 | 464 ± 13b | 518 ± 28b | 481 ± 17b | 414 ± 8c |
| Systolic blood pressure (mmHg) | 123 ± 10 | 108 ± 3 | 97 ± 5 | 101 ± 5 | 124 ± 6 |
| Diastolic blood pressure (mmHg) | 96 ± 9 | 85 ± 3 | 77 ± 5 | 82 ± 4 | 96 ± 7 |
| Mean arterial pressure (mmHg) | 105 ± 9 | 92 ± 3 | 83 ± 5 | 88 ± 4 | 105 ± 7 |
Iso: isoproterenol; ASA: acetylsalicylic acid; Zn(ASA)2: zinc complex of acetylsalicylic acid.
Values are expressed means ± SEM. n = 7 in the control, ASA + Iso and Zn(ASA)2 groups, n = 11 in the Iso group, n = 12 in the Zn(ASA)2 + Iso group.
P < 0.05 vs. control.
P < 0.05 vs. Iso.
Zn(ASA)2 improves histopathological lesions of cardiac tissues
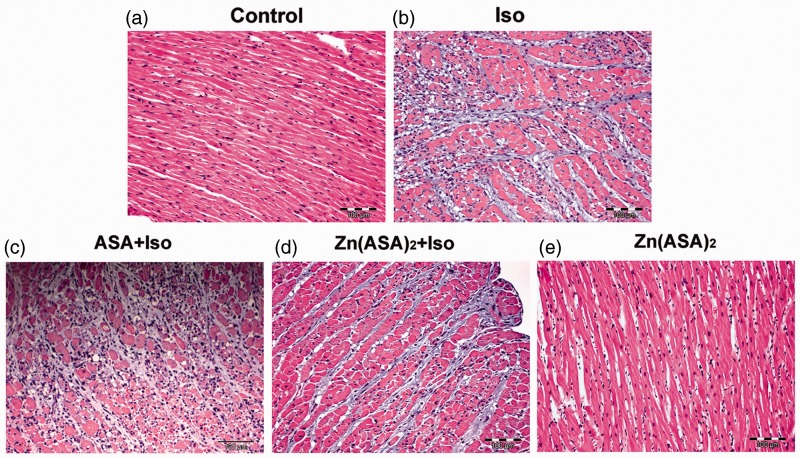
Compared to the control group (Figure 2a), cardiac tissues from isoproterenol-treated rats revealed myofibrillar degeneration, the most distinct at lumen-near areas of the left ventricle, continuously diminishing towards the epicardium and almost completely absent in the septal area and right ventricle (data not shown). This impairment is related to a dense inflammatory infiltrate and interstitial edema (Figure 2b). Pre-treatment with ASA did not reduce tissue edema and leukocytes infiltration (Figure 2c). However, treatment with Zn(ASA)2 led to a decreased amount of inflammatory cells in the tissues with less impairment of cardiomyocytes. Zn(ASA)2 failed to decrease myocardial edema (Figure 2d). Drug-treated animals showed normal appearance of cardiac muscle fibers (Figure 2e). Table 3 shows the effects of Zn(ASA)2 and ASA on the degree of histological changes in myocardial tissues.
Figure 2.
Zinc complex of acetylsalicylic acid (Zn(ASA)2) improves histopathological lesions of cardiac tissues in isoproterenol (Iso)-induced myocardial ischemia in rats. Histopathological examinations of myocardium (hematoxylin and eosin staining) in each group. Magnification x100; scale bar = 100 µm. ASA indicates acetylsalicylic acid. n = 7 in the control group, n = 12 in the Iso group, n = 9 in the ASA + Iso group, n = 13 in the Zn(ASA)2 + Iso group, and n = 8 in the Zn(ASA)2 group. (A color version of this figure is available in the online journal.)
Table 3.
Effects of zinc complex of acetylsalicylic acid (Zn(ASA)2) and acetylsalicylic acid (ASA) on the degree of histopathological changes in cardiac tissues in isoproterenol-induced myocardial ischemia in rats
| Edema | Inflammation | |
|---|---|---|
| Control | A | A |
| Isoproterenol | ++ | +++ |
| ASA + isoproterenol | ++ | +++ |
| Zn(ASA)2 + isoproterenol | ++ | ++ |
| Zn(ASA)2 | A | A |
A: no changes; ++: moderate changes; +++: marked changes.
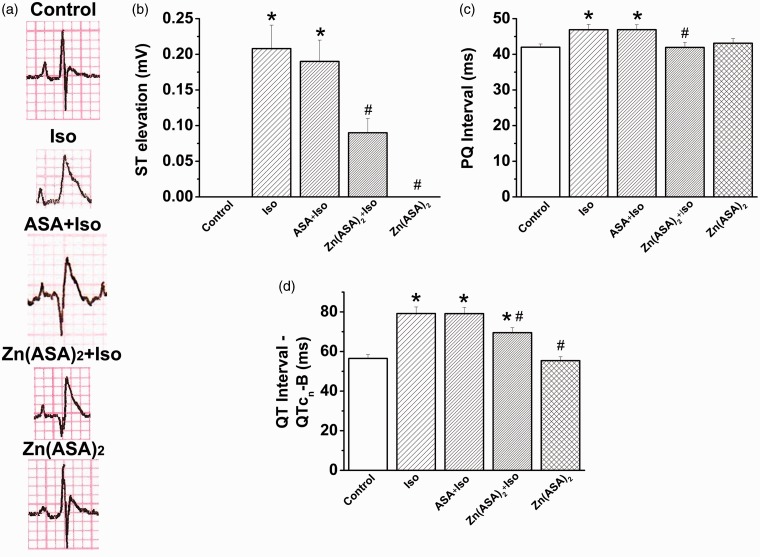
Zn(ASA)2 improves electrocardiogram patterns
On ECG recordings, isoproterenol-treated animals showed a significant elevation of ST-segments, elongated PQ- and corrected QT-intervals in comparison with control animals (Figure 3a–d). The altered ECG of isoproterenol-treated animals was not improved by ASA. However, pre-treatment with Zn(ASA)2 significantly restored the changes in ECG-parameters induced by isoproterenol (Figure 3).
Figure 3.
Zinc complex of acetylsalicylic acid (Zn(ASA)2) improves electrocardiogram patterns in isoproterenol (Iso)-induced myocardial ischemia in rats. (a) Representative surface 12-lead ECG tracings, (b) ST-segment elevation, (c) PQ-interval, and (d) corrected QT-interval. *P < 0.05 vs. control, #P < 0.05 vs. Iso. ASA indicates acetylsalicylic acid. n = 6 in the control and Zn(ASA)2 groups, n = 7 in the Iso group, n = 8 in the ASA + Iso group, n = 10 in the Zn(ASA)2 + Iso group. (A color version of this figure is available in the online journal.)
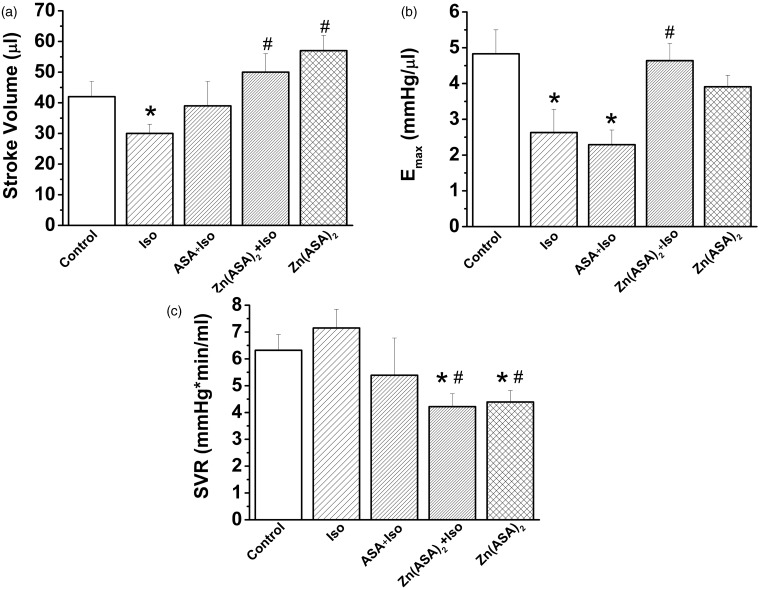
Zn(ASA)2 improves cardiac pump function
Isoproterenol treatment was associated with a significant decrease in systolic performance when compared to control animals, as reflected by decreased stroke volume and the slope Emax of the end-systolic pressure–volume relationship (Figure 4a,b). Pre-treatment with Zn(ASA)2 resulted in a significant increase in these parameters indicating improved LV contractility. Moreover, compared to the isoproterenol group, Zn(ASA)2 treatment induced a significant decrease in systemic vascular resistance (Figure 4c). ASA treatment did not result in an improvement of stroke volume and the slope Emax of the end-systolic pressure–volume relationship (Figure 4a, b).
Figure 4.
Zinc complex of acetylsalicylic acid (Zn(ASA)2) improves cardiac pump function in isoproterenol (Iso)-induced myocardial ischemia in rats. (a) Stroke volume, (b) the slope Emax of the left-ventricular end-systolic pressure–volume relationship, and (c) systemic vascular resistance. *P < 0.05 vs. control, #P < 0.05 vs. Iso. ASA indicates acetylsalicylic acid. n = 7 in the control, ASA + Iso and Zn(ASA)2 groups, n = 11 in the Iso group, n = 12 in the Zn(ASA)2 + Iso group
Zn(ASA)2 did not decrease elevated plasma cardiac troponin-T levels
Plasma levels of cardiac troponin-T in the isoproterenol group were significantly increased compared to the control and Zn(ASA)2 groups (isoproterenol: 4138 ± 1125 pg/mL vs. control: 257 ± 49 pg/mL vs. Zn(ASA)2: 164 ± 32 pg/mL, P < 0.05). Treatment with either Zn(ASA)2 or ASA did not decrease the plasma levels of this enzyme (Zn(ASA)2 + isoproterenol: 5603 ± 833 pg/mL; ASA + isoproterenol: 7338 ± 742 pg/mL).
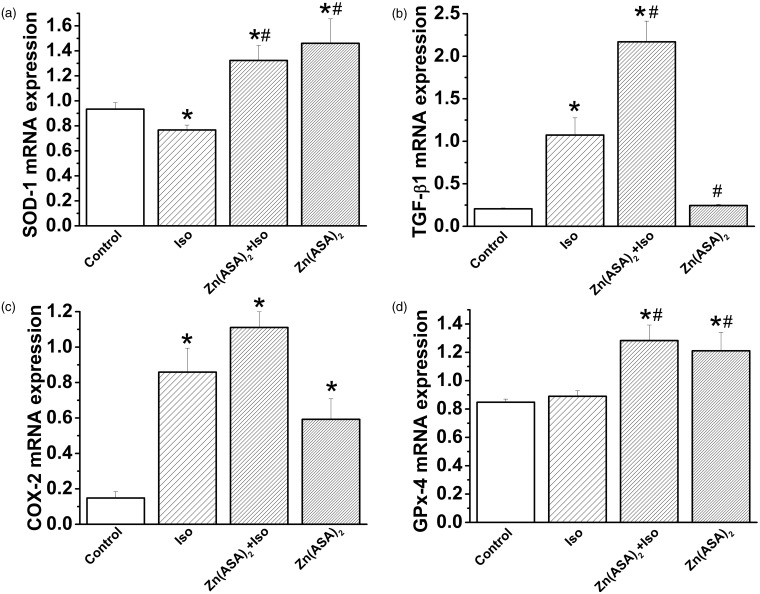
Zn(ASA)2 regulates myocardial gene expression
Administration of isoproterenol significantly decreased superoxide dismutase (SOD)-1 and increased transforming growth factor (TGF)-β1 and cyclooxygenase (COX)-2 gene expression when compared to the control group (Figure 5a–c). Pre-treatment with Zn(ASA)2 significantly increased isoproterenol-induced changes of SOD-1, TGF-β1, and glutathione peroxidase-4 mRNA-levels (Figure 5a, b, and d). Treatment of non-infarcted rats with Zn(ASA)2 significantly upregulated SOD-1, COX-2, and glutathione peroxidase-4 mRNA-expression (Figure 5a, c, and d).
Figure 5.
Zinc complex of acetylsalicylic acid (Zn(ASA)2) regulates myocardial gene expression in isoproterenol (Iso)-induced myocardial ischemia in rats. Quantitative PCR analysis of myocardial mRNA levels of (a) superoxide dismutase (SOD)-1, (b) transforming growth factor (TGF)-β1, (c) cyclooxygenase (COX)-2, and (d) glutathione peroxidase (GPx)-4. *P < 0.05 vs. control, #P < 0.05 vs. Iso. n = 6 in the control group, n = 10 in the Iso group, n = 13 in the Zn(ASA)2 + Iso group, and n = 8 in the Zn(ASA)2 group
Discussion
In the present study, we investigated the effects of both ASA and Zn(ASA)2 on isoproterenol-induced myocardial injury in rats. We and other groups have previously reported that the administration of isoproterenol at suprapharmacological dosages results in impaired cardiac function.16,19,20 We demonstrated that oral administration of zinc and ASA in the form of bis(aspirinato)zinc(II) complex for five consecutive days is superior to ASA in preventing acute myocardial ischemia based on electrical and mechanical changes and histological evaluation.
After isoproterenol treatment, we found a significant decrease in the stroke volume that can be attributed to depressed LV contractility. Further, we found a decreased Emax, a well-established load-independent contractility index,21 suggesting decreased systolic performance. Increased heart rate observed in isoproterenol-treated rats can reduce the myocardial blood supply and cause ischemia. Even though ASA treatment did not result in an improvement of these parameters, pre-treatment of rats with Zn(ASA)2 restored altered cardiac hemodynamics. Additionally, pre-treatment of isoproterenol-administrated rats with Zn(ASA)2 significantly decreased systemic vascular resistance.
The ECG is an important tool for the diagnosis of myocardial ischemia and infarction. In the present study, we assessed the ischemic damage after isoproterenol administration by ischemic repolarization changes as shown by a significant elevation of the ST-segment. Moreover, we observed an increased corrected QT-interval following isoproterenol injection, a predictor for ventricular tachyarrhythmia,22 that occurred while the heart rate was normal, suggesting that the ventricular repolarization time had lengthened. These data showed that isoproterenol has the potential to induced cardiac arrhythmias. In this study, Zn(ASA)2 treatment in isoproterenol-injected rats prevented the pathological alterations in the ECG, suggestive of its cell membrane protecting effects. ASA alone failed to protect the heart against myocardial ischemia. The release of intracellular cardiac enzymes or markers such as troponin-T, lactate dehydrogenase, creatine kinase into the circulation reflects major cellular membrane damage and/or death of cardiomyocytes.23,24 Even though our study demonstrated improved cardiac function and ECG patterns after myocardial ischemia, the increased plasma levels of cardiac troponin-T were not reduced by administration of Zn(ASA)2 or ASA, indicating no improvement in the degree of myocardial necrosis. Since myocardial necrosis occurs diffusely, assessment of infarct size with classical methods (such as TTC staining) is not reliable in this model. However, our observations support the view that improved cardiac performance by Zn(ASA)2 may be partially due to the rescue of cardiomyocytes from border zones and remote regions of infarcted hearts. Zn(ASA)2 treatment therefore may improve the function of cardiac cells and lead to an improved global cardiac performance. In this experimental setting, the dose of 75 mg/kg corresponding to the same amount of ASA as in the Zn(ASA)2 complex, did not provide the same cardioprotective effect as observed by Zn(ASA)2 treatment. It has been shown that oral treatment with ASA for four days in a dose of 200 mg/kg body weight effectively prevents the formation of confluent foci of injury to the myocardium induced by isoproterenol.25 Additionally, another study suggested that per os pre-treatment with 300 mg/kg of aspirin for one week can effectively prevent ventricular tachyarrhythmias and ventricular fibrillation during acute myocardial ischemia in rats.26 Taken together, our data suggest that a lower dose of ASA is required when ASA is complexed with zinc(II) to protect myocardial injury induced by isoproterenol speculating that gastrointestinal side effects may be reduced. The superior protective ability of Zn(ASA)2 treatment perhaps is not surprising. Anti-inflammatory studies (using carrageenan-induced hind paw edema method) showed that the zinc complex is 2.64 times more potent and lower ulcerogenicity as compared to aspirin itself.11
TGF-β, pleiotropic cytokine with multifunctional actions, is involved in cardiac injury, repair and remodeling and its activation may be important in suppressing expression of pro-inflammatory cytokines and chemokines in the infarcted myocardium resulting in resolution of the inflammatory infiltrate.27,28 In the present study, treatment of infarcted rats with Zn(ASA)2 upregulated TGF-β1 mRNA-expression, suggesting that Zn(ASA)2 also possesses TGF-β mediated effects that may be important in the regulation of post-infarction inflammatory response. In line with these observations, our histopathological examination of cardiac tissues of infarcted rats treated with Zn(ASA)2 revealed a reduced inflammatory cellular infiltration and further confirms the cardioprotective action of its oral administration in rats with myocardial ischemia. It is thus tempting to speculate that compared to ASA, the zinc complex of ASA might increase its potency as anti-inflammatory agent in acute myocardial ischemia. It has also been shown that the copper complex of ASA possesses a similar anti-inflammatory spectrum, but a more potent anti-inflammatory activity than ASA.29 However, in our hands, Zn(ASA)2 administration did not influence the upregulation of COX-2 mRNA expression induced by isoproterenol treatment, indicating that its effects do not implicate COX-2 regulation.
In the ischemic myocardium, the generation and accumulation of reactive oxygen species is increased and the anti-oxidant defense mechanisms against oxygen free radicals (such as superoxide anion, H2O2, hydroxyl radicals) of cardiac myocytes are altered. In the present study, we demonstrated that Zn(ASA)2 resulted in a significant increase in SOD-1 mRNA-expression, one of first line defense anti-oxidant enzymes and glutathione peroxidase-4 mRNA-level, indicating that the expressional induction of anti-oxidant enzymes might be an important component of its cardioprotective effect. In a previous work, the copper complex of ASA has been ascribed an anti-oxidative effect in a rat model of aging-associated cardiovascular dysfunction.30 However, Ott et al. showed that cobalt–aspirin complex leaves the serine residue untouched and acetylates other sites instead that may result in a different activity spectrum for the drug.31
In summary, our data demonstrate the superiority of zinc complex of ASA to ASA in preventing postischemic myocardial dysfunction. Induction of anti-oxidant enzymes and the anti-inflammatory cytokine TGF-β1 may play a pivotal role in the mechanism of action of Zn(ASA)2. Other mechanisms involved in the protection of Zn(ASA)2 in acute ischemic injury need to be explored.
Study limitations
Isoproterenol, a well-known inducer of myocardial ischemia,32 is widely used as a model to evaluate cardioprotective drugs.33 Although this rat model does not exactly reflect the clinical situation in terms of coronary occlusion and regional myocardial infarction, administration of isoproterenol has been reported to produce “infarct-like” damage in experimental animals. Another limitation of this study was that we did not assess the long term effects of Zn(ASA)2 treatment on the LV remodeling but confined the investigation solely to the acute effects of Zn(ASA)2 administration after acute myocardial ischemia. Additionally, quantitative measurements of inflammatory mediators and oxidative stress need to be elucidated in future work.
Acknowledgments
This study was supported by the Land Baden-Württemberg, Germany, by the Medical Faculty of the University of Heidelberg, Germany (to Dr S Korkmaz and Dr K Hirschberg), and by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to Dr T Radovits). The expert technical assistance of Karin Sonnenberg, Patricia Kraft, Lutz Hoffmann and Nadine Weiberg is gratefully acknowledged. We thank Alice Lehner for her help in histological studies.
The authors declare that there is no conflict of interest.
Author contributions
SK participated in research design, in the performance of the research, in data analysis, and in the writing of the manuscript. AA participated in research design, in the performance of the research, in data analysis, and in the writing of the manuscript. SL participated in the performance of the research and in data analysis. TR participated in research design, in data analysis, in the writing of the manuscript. PH participated in the performance of the research and in data analysis. EB participated in data analysis and in the writing of the manuscript. KH participated in data analysis and in the writing of the manuscript. SL participated in data analysis and in the writing of the manuscript. YY participated in data analysis and in the writing of the manuscript. HY participated in data analysis and in the writing of the manuscript. MK participated in research design and in the writing of the manuscript. GS participated in research design, in the performance of the research, in data analysis, and in the writing of the manuscript.
References
- 1.Whellan DJ. Heart failure disease management: implementation and outcomes. Cardiol Rev 2005; 13: 231–9. [DOI] [PubMed] [Google Scholar]
- 2.Sahna E, Deniz E, Bay-Karabulut A, Burma O. Melatonin protects myocardium from ischemia-reperfusion injury in hypertensive rats: role of myeloperoxidase activity. Clin Exp Hypertens 2008; 30: 673–81. [DOI] [PubMed] [Google Scholar]
- 3.Haft JI, Kranz PD, Albert FJ, Fani K. Intravascular platelet aggregation in the heart induced by norepinephrine. Microscopic studies. Circulation 1972; 46: 698–708. [DOI] [PubMed] [Google Scholar]
- 4.Karmazyn M, Moffat MP, Beamish RE, Dhalla NS. Comparative effects of acetylsalicylic acid (ASA) and sulfinpyrazone on isoproterenol-induced heart damage. J Pharmacol Exp Ther 1981; 218: 764–70. [PubMed] [Google Scholar]
- 5.Kraikitpanitch S, Haygood CC, Baxter DJ, Yunice AA, Lindeman RD. Effects of acetylsalicylic acid, dipyridamole, and hydrocortisone on epinephrine-induced myocardial injury in dogs. Am Heart J 1976; 92: 615–22. [DOI] [PubMed] [Google Scholar]
- 6.Troskot B, Simicevic VN, Dodig M, Rotkvic I, Ivankovic D, Duvnjak M. The protective effect of zinc sulphate pretreatment against duodenal ulcers in the rat. Biometals 1997; 10: 325–9. [DOI] [PubMed] [Google Scholar]
- 7.Shih CJ, Chiou YL. Zinc sulfate inhibited inflammation of Der p2-induced airway smooth muscle cells by suppressing ERK1/2 and NF-kappaB phosphorylation. Inflammation 2013; 36: 616–24. [DOI] [PubMed] [Google Scholar]
- 8.Chvapil M, Owen JA. Effect of zinc on acute and chronic isoproterenol induced heart injury. J Mol Cell Cardiol 1977; 9: 151–9. [DOI] [PubMed] [Google Scholar]
- 9.Powell SR. The antioxidant properties of zinc. J Nutr 2000; 130: 1447S–54S. [DOI] [PubMed] [Google Scholar]
- 10.Powell SR, Hall D, Aiuto L, Wapnir RA, Teichberg S, Tortolani AJ. Zinc improves postischemic recovery of isolated rat hearts through inhibition of oxidative stress. Am J Physiol 1994; 266: H2497–507. [DOI] [PubMed] [Google Scholar]
- 11.Singla AK, Wadhwa H. Zinc-aspirin complex - synthesis, physicochemical and biological evaluation. Int J Pharm 1994; 108: 173–85. [DOI] [PubMed] [Google Scholar]
- 12.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol 1985; 17: 291–306. [DOI] [PubMed] [Google Scholar]
- 13.Wexler BC. Myocardial infarction in young vs old male rats: pathophysiologic changes. Am Heart J 1978; 96: 70–80. [DOI] [PubMed] [Google Scholar]
- 14.Wexler BC, Greenberg BP. Effect of exercise on myocardial infarction in young vs. old male rats: electrocardiograph changes. Am Heart J 1974; 88: 343–50. [DOI] [PubMed] [Google Scholar]
- 15.Chagoya de Sanchez V, Hernandez-Munoz R, Lopez-Barrera F, Yanez L, Vidrio S, Suarez J, Cota-Garza MD, Aranda-Fraustro A, Cruz D. Sequential changes of energy metabolism and mitochondrial function in myocardial infarction induced by isoproterenol in rats: a long-term and integrative study. Can J Physiol Pharmacol 1997; 75: 1300–11. [PubMed] [Google Scholar]
- 16.Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Pali S, Seidel B, Zollner S, Karck M, Szabo G. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation 2009; 120: 677–86. [DOI] [PubMed] [Google Scholar]
- 17.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol 2010; 641: 187–92. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 2008; 3: 1422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Yu X, Qu S, Xu H, Han B, Sui D. Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. Eur J Pharmacol 2010; 636: 121–5. [DOI] [PubMed] [Google Scholar]
- 20.Zhou R, Xu Q, Zheng P, Yan L, Zheng J, Dai G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol 2008; 586: 244–50. [DOI] [PubMed] [Google Scholar]
- 21.Suga H. Cardiac energetics: from E(max) to pressure-volume area. Clin Exp Pharmacol Physiol 2003; 30: 580–5. [DOI] [PubMed] [Google Scholar]
- 22.Sides GD. QT interval prolongation as a biomarker for torsades de pointes and sudden death in drug development. Dis Markers 2002; 18: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luscher MS, Thygesen K, Ravkilde J, Heickendorff L. Applicability of cardiac troponin T and I for early risk stratification in unstable coronary artery disease. TRIM Study Group. Thrombin inhibition in myocardial ischemia. Circulation 1997; 96: 2578–85. [DOI] [PubMed] [Google Scholar]
- 24.Ravkilde J, Nissen H, Horder M, Thygesen K. Independent prognostic value of serum creatine kinase isoenzyme MB mass, cardiac troponin T and myosin light chain levels in suspected acute myocardial infarction. Analysis of 28 months of follow-up in 196 patients. J Am Coll Cardiol 1995; 25: 574–81. [DOI] [PubMed] [Google Scholar]
- 25.Martynyuk RA, Semenova LA, Tsellarius YG. Effect of acetylsalicylic acid on development of infarct-like myocardial necroses induced in rats by a single injection of isoproterenol. Bull Exp Bio Med 1975; 79: 31–3. [PubMed] [Google Scholar]
- 26.Ahn YK, Cho JG, Park WS, Kim NH, Kim JW, Kim SH, Cho JH, Park JH, Jeong MH, Park JC, Kang JC. The effects of antiplatelet agents in the prevention of ventricular tachyarrhythmias during acute myocardial ischemia in rats. Jpn Heart J 1999; 40: 79–86. [DOI] [PubMed] [Google Scholar]
- 27.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2007; 74: 184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 2008; 103: 485–92. [DOI] [PubMed] [Google Scholar]
- 29.Chohan ZH, Iqbal MS, Iqbal HS, Scozzafava A, Supuran CT. Transition metal acetylsalicylates and their anti-inflammatory activity. J Enzyme Inhib Med Chem 2002; 17: 87–91. [DOI] [PubMed] [Google Scholar]
- 30.Radovits T, Gero D, Lin LN, Loganathan S, Hoppe-Tichy T, Szabo C, Karck M, Sakurai H, Szabo G. Improvement of aging-associated cardiovascular dysfunction by the orally administered copper(II)-aspirinate complex. Rejuvenation Res 2008; 11: 945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott I, Kircher B, Bagowski CP, Vlecken DH, Ott EB, Will J, Bensdorf K, Sheldrick WS, Gust R. Modulation of the biological properties of aspirin by formation of a bioorganometallic derivative. Angew Chem Int Ed Engl 2009; 48: 1160–3. [DOI] [PubMed] [Google Scholar]
- 32.Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 2007; 230: 178–88. [DOI] [PubMed] [Google Scholar]
- 33.Karthick M, Stanely Mainzen Prince P. Preventive effect of rutin, a bioflavonoid, on lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction in rats. J Pharm Pharmacol 2006; 58: 701–7. [DOI] [PubMed] [Google Scholar]