Abstract
The final product of adipogenesis is a functional adipocyte. This mature cell acquires the necessary machinery for lipid metabolism, loses its proliferation potential, increases its insulin sensitivity, and secretes adipokines. Multipotent mesechymal stromal cells have been recognized as a source of adipocytes both in vivo and in vitro. The in vitro adipogenic differentiation of human MSC (hMSC) has been induced up to now by using a complex stimulus which includes dexamethasone, 3-isobutyl-1-methylxanthine, indomethacin, and insulin (a classical cocktail) and evaluated according to morphological changes. The present work was aimed at demonstrating that the simultaneous activation of dexamethasone’s canonical signaling pathways, through the glucocorticoid receptor and CCAAT-enhancer-binding proteins (C/EBPs) and rosiglitazone through peroxisome proliferator-activated receptor gamma (PPAR-gamma) is sufficient yet necessary for inducing hMSC adipogenic differentiation. It was also ascertained that hMSC exposed just to dexamethasone and rosiglitazone (D&R) differentiated into cells which accumulated neutral lipid droplets, expressed C/EBP-alpha, PPAR-gamma, aP2, lipoprotein lipase, acyl-CoA synthetase, phosphoenolpyruvate carboxykinase, adiponectin, and leptin genes but did not proliferate. Glucose uptake was dose dependent on insulin stimulus and high levels of adipokines were secreted (i.e. displaying not only the morphology but also expressing mature adipocytes’ specific genes and functional characteristics). This work has demonstrated that (i) the activating C/EBPs and PPAR-gamma signaling pathways were sufficient to induce adipogenic differentiation from hMSC, (ii) D&R producing functional adipocytes from hMSC, (iii) D&R induce adipogenic differentiation from mammalian MSC (including those which are refractory to classical adipogenic differentiation stimuli). D&R would thus seem to be a useful tool for MSC characterization, studying adipogenesis pathways and producing functional adipocytes.
Keywords: Adipogenesis, adipogenic differentiation, mesechymal stem cell, multipotent stromal cell, functional adipocyte, adipokine
Introduction
The final product of adipogenesis is a functional adipocyte. This mature cell acquires the necessary machinery for lipid metabolism, loses its proliferation potential, increases its insulin sensitivity, and secretes adipokines.1–3 Molecular mechanisms behind adipogenic differentiation have been thoroughly studied in immortalized cells already committed to the adipogenic lineage (mouse 3T3-L1 and 3T3F442 cell lines).4 CCAAT/enhancer binding protein (C/EBPs) and peroxisome proliferator activated receptor isoform gamma (PPAR-gamma) activation in these preadipocytes triggers the expression of genes related to the mature phenotype5–8; exposure to molecules activating C/EBPs and PPAR-gamma thus results in preadipocyte maturation.3 To the best of our knowledge, no reports have shown that activating these transcription factors results in primary uncommitted cells’ adipogenic differentiation. Multipotent mesechymal stromal cells (also referred to as mesenchymal stem cells—MSC) have been recognized as undifferentiated adipocyte precursors.9–11 Cells’ adipogenic differentiation in vitro can be induced by culturing them at confluence and exposing them to a stimuli containing a glucocorticoid (dexamethasone), a phosphodiesterase inhibitor (3-isobutyl-1-methylxanthine—IBMX), a cyclooxygenase inhibitor (indomethacin) and insulin, hereafter referred to as the classic cocktail (CC).12 Each CC component activates a different signaling pathway which seems to be relevant for differentiation.2,13 Due to CC complexity, the molecular events associated with MSC differentiation into the adipogenic lineage have been difficult to elucidate.14–19 The adipocyte-like cells produced from CC-exposed MSC have been mainly characterized according to their morphology (the presence of oil droplets and fusiform transition to spherical form) and gene and protein expression of adipogenesis terminal phase markers.9,12,14,16,20,21 However, it is unknown whether adipocytes differentiate in vitro into functional cells.
Our work was aimed at developing a simple differentiation stimulus which would allow functional adipocytes to be produced from human MSC (hMSC). This involved evaluating the dependence of hMSC adipogenic differentiation on the activation of dexamethasone’s canonical signaling pathways through the glucocorticoid receptor (GR) and the rosiglitazone pathway through PPAR-gamma, assessing the presence and abundance of Oil Red O and Nile Red-stained cells, the subcellular localization of C/EBP-alpha and PPAR-gamma, and their mRNA levels in dexamethasone and rosiglitazone (D&R)-exposed cells in the absence or presence of inhibitors for both signaling pathways. The degree of functionality of adipocytes produced from D&R-exposed hMSC was evaluated by assessing their gene profile, proliferation potential, insulin sensitivity, and adipokine secretion. It was also ascertained whether mouse, rat, hamster, rabbit, dog, and/or human MSC exposed to D&R led to adipocyte-like cell production by assessing the presence and abundance of Oil Red O- and Nile Red-stained cells.
Materials and methods
MSC isolation, expansion, and characterization
hMSCs were isolated from remaining material after heparinized aspirates had been obtained from normal donors undergoing bone marrow harvest for allogeneic transplant. MSCs from mice (C57BL/6 strain), rats (Wistar), hamsters (Syrian Golden Hamster), and rabbits (New Zealand) were isolated from their femurs and tibias. MSCs from dogs (German shepherd breed) were isolated from heparinized aspirates taken from harvested bone marrow. The Clínica Alemana’s Ethics Committee (Santiago, Chile) approved using human leftover material for research. The Medicine Faculty’s Ethics Committee at the Clínica Alemana, Universidad del Desarrollo, approved the animal protocols. Bone marrow samples were diluted with phosphate-buffered saline (PBS, Gibco, Grand Island, NY) and spun at 400 × g for 7 min. Cells were seeded at 1 × 106 nucleated cells/cm2 density in alpha-minimal essential medium (alpha-MEM, Gibco, Grand Island, NY) supplemented with 10% (v/v) selected fetal bovine serum (HyClone, Logan, UT) and 80 mg/mL gentamicin (Laboratorio Biosano, Chile), hereafter referred to as alpha-10 medium. Non-adherent cells were removed one day later by replacing the culture medium. When fibroblastic-like cells foci were confluent, cells were detached with 0.25% (w/v) trypsin, 2.65 mM EDTA (Gibco, Grand Island, NY), and subcultured at 7 × 103 cells/cm2 density. Cultures were kept in a humidified atmosphere containing 5% (v/v) CO2 at 37℃. Adherent cells were immunophenotyped after two passages and characterized according to their adipogenic, chondrogenic, and osteogenic differentiation potential.22,23
MSC exposure to differentiation stimuli
MSCs were seeded at 2.5 × 104/cm2 density in alpha-10 medium. The medium was replaced one day later with alpha-10 supplemented with CC (1 µM dexamethasone [Sigma-Aldrich, Germany], 100 µg/mL IBMX (Calbiochem, La Jolla, CA), 100 µM indomethacin [Sigma-Aldrich, St. Louis, MO], and 0.2 U/mL insulin [Sanofi-Aventis, Frankfurt, Germany]), separate CC components, or 1 µM dexamethasone and 10 µM rosiglitazone (D&R, patent pending—PCT/CL2012/000076). Differentiation stimuli were replaced every two days. Cultures were maintained in a humidified atmosphere containing 5% (v/v) CO2 at 37℃.
Assessing adipogenic differentiation
Cells were stained with 60% (w/v) Oil Red O (Sigma-Aldrich, Germany) in isopropanol for 1 h at room temperature (RT). Once washed, cells were observed and photographed by light microscope (ECLIPSE TS100. Nikon, Japan); alternatively, cells were trypsinized, stained with 1 mg/mL Nile Red (Sigma-Aldrich, St. Louis, MO) for 30 min at RT and analyzed by flow cytometry (CYAN ADP, Dako Cytomation, Carpinteria, CA). Summit V 4.3 software was used for data acquisition and processing.
Cell viability
Cell viability was determined based on trypan blue dye exclusion criteria. Cells were trypsinized and a representative aliquot was diluted two times with 0.4% trypan blue (w/v). The number of total cells and dead cells was determined in a Neubauer chamber. CV (%) = (total cells–dead cell/total cells) × 100.
C/EBP-alpha and PPAR-gamma subcellular localization
Cells were fixed with 4% (v/v) paraformaldehyde (Merck, Germany) for 10 min at 4℃, washed, and permeabilized with 0.2% (v/v) Triton X-100 in PBS for 15 min at RT. Once blocked with PBS containing 5% (w/v) BSA and 0.1% (v/v) Triton X-100 for 1 h at RT, cells were washed and incubated with 1/50 rabbit polyclonal IgG anti-CEBP-alpha (Santa Cruz Biotechnology Inc, Santa Cruz, CA) or 1/50 chicken polyclonal IgY anti-PPAR-gamma (Gen Way Biotech Inc, San Diego, CA) overnight at 4℃. After three washes, cells were incubated with 1/100 goat anti-rabbit IgG serum conjugated with Alexa-fluor488 (Molecular Probes, Eugene, OR) or 1/100 donkey anti-chicken IgY serum conjugated with Cy2 (Jackson Immune Research, Baltimore, PA) for 1 h at RT. Cells were washed again, counterstained with Topro-3 (Invitrogen, Carlsbad, CA), mounted with mounting medium (DAKO, Carpinteria, CA), observed and photographed by LSM 510 confocal microscope (Zeiss, GmbH, Germany).
GR and PPAR-gamma inhibition
hMSCs were seeded at 2.5 × 104/cm2 density in the presence of D&R, with or without 10 µM RU486 (Sigma-Aldrich, St. Louis, MO), a GR antagonist,24 and/or 10 µM GW9662 (Sigma-Aldrich, St. Louis, MO), an inhibitor of PPAR-gamma25 D&R + inhibitors (D&R + I); the medium (D&R + I) was replaced every two days. RU486 and GW9662 were added separately or together at the indicated concentrations each 24 h. Cultures were maintained in a humidified atmosphere containing 5% (v/v) CO2 at 37℃.
Adipocyte-specific gene expression
Total RNA was isolated from cells using Trizol reagent (Invitrogen, Carlsbad, CA) and quantified by absorbance at 260 nm. One microgram total RNA was reverse transcribed with 200 U M-MLV reverse transcriptase (Promega, Madison, WI) and 300 pmol oligo-dT. Real-time PCR was performed in 20 µL final volume containing 50 ng cDNA, PCR LightCycler-DNA Master SYBRGreen reaction mix (Roche, Indianapolis, IN), 4 mM MgCl2, and 0.5 µM each specific primer (Supplementary Table S1) using a LightCycler thermocycler (Roche, Indianapolis, IN). Controls without reverse transcription were included to ensure that amplification was from mRNA and not from genomic DNA. Amplicons were characterized according to their melting temperature, determined by LightCycler thermocycler, and after their size had been evaluated after being electrophoresed on agarose gel (Supplementary Table S1). Each target gene’s mRNA level was standardized against the GAPDH mRNA level for each sample. The ΔCt method was used for mRNA quantification, expressed as arbitrary units (a.u.).26
Assessing proliferation potential
Cells were trypsinized and seeded at 1 × 104/cm2 density in alpha-10 medium. One day later the medium was supplemented with 25 µM BrdUrd (or not); two days later the cells were trypsinized, fixed, permeabilized, and stained with anti-BrdUrd-APC antibody, following the manufacturer’s instructions (BrdU Flow kit, BD Pharmingen, San Diego, CA). The cells were then stained with Nile Red and analyzed by flow cytometry (CYAN ADP, Dako Cytomation, Carpinteria, CA). Summit V 4.3 software was used for data acquisition and processing.
Determining insulin sensitivity
Culture media were replaced by alpha-MEM. The cells were washed with PBS 4 h later and incubated without or with 0.1, 1, or 10 nM insulin for 30 min at 37℃. Culture media were replaced by PBS containing 4 mM 2-deoxyglucose (2-DG) and 2 µCi/mL [H3]dT 2-DG and cells were incubated for 1 min at 37℃. Glucose transport was stopped by washing with cold PBS and freezing samples at −20℃. The cells were then lysed with formic acid (0.5 N, for 1 h at RT and for 30 min at RT). Lysates were diluted in 2 mL biodegradable scintillation solution. Radioactivity was measured on a LKB Rackbeta 1217 scintillation counter (LKB Instruments, INC, Rockville, MD) and standardized against protein concentration.27
Quantifying adipokine secretion
Culture media were replaced by alpha-MEM; the conditioned media were collected two days later. The level of adiponectin secreted by cells was determined by Quantikine human adiponectin/Acrp30 immunoassay and leptin level by Quantikine human leptin immunoassay (R&D System Inc, Minneapolis, MN).
Statistical analysis
The data have been presented as mean ± SEM. Analysis of variance (ANOVA) was used for multiple group comparisons, followed by Tukey’s post hoc test. p < 0.05 was considered to be statistically significant.
Results
D&R-induced adipogenic differentiation of hMSC
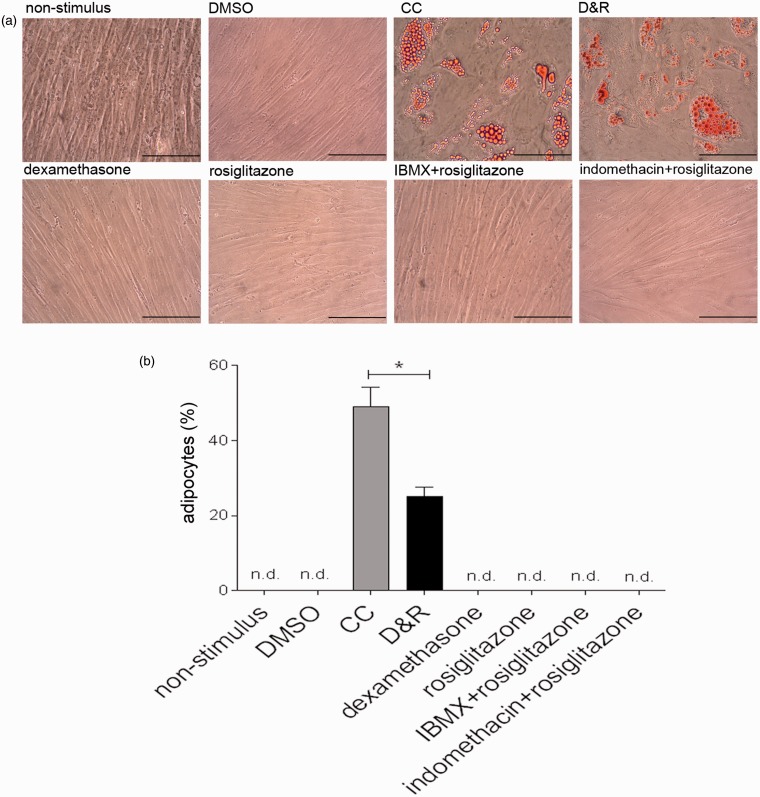
While D&R-exposed hMSC differentiated into adipocytes, cells exposed to alpha-10 medium supplemented with the vehicle (DMSO), dexamethasone, rosiglitazone, IBMX + rosiglitazone, or indomethacine + rosiglitazone remained undifferentiated (Figure 1(a)). Regarding adipogenic differentiation efficiency, a significant difference between CC and D&R (45 ± 5% cf 25 ± 3%, respectively) was observed regarding the production of cells accumulating neutral lipid droplets in their cytoplasm (Figure 1(b)). However, D&R triggered the adipogenesis of all hMSC samples tested (7/7).
Figure 1.
D&R-induced hMSC adipogenic differentiation. hMSCs were exposed to non-stimulus, DMSO, CC, D&R, dexamethasone, rosiglitazone, IBMX + rosiglitazone, or indomethacin + rosiglitazone. Adipocyte presence and abundance were assessed on day 14 by Oil Red O (a) and Nile Red (b) staining, respectively. Representative data regarding seven different hMSC donors. Cell viability >95%. Scale bar: 100 µm. n.d. below the assay detection limit.*: p < 0.05 (ANOVA, Tukey). (A color version of this figure is available in the online journal.)
D&R-induced adipogenic differentiation of other mammalian MSC
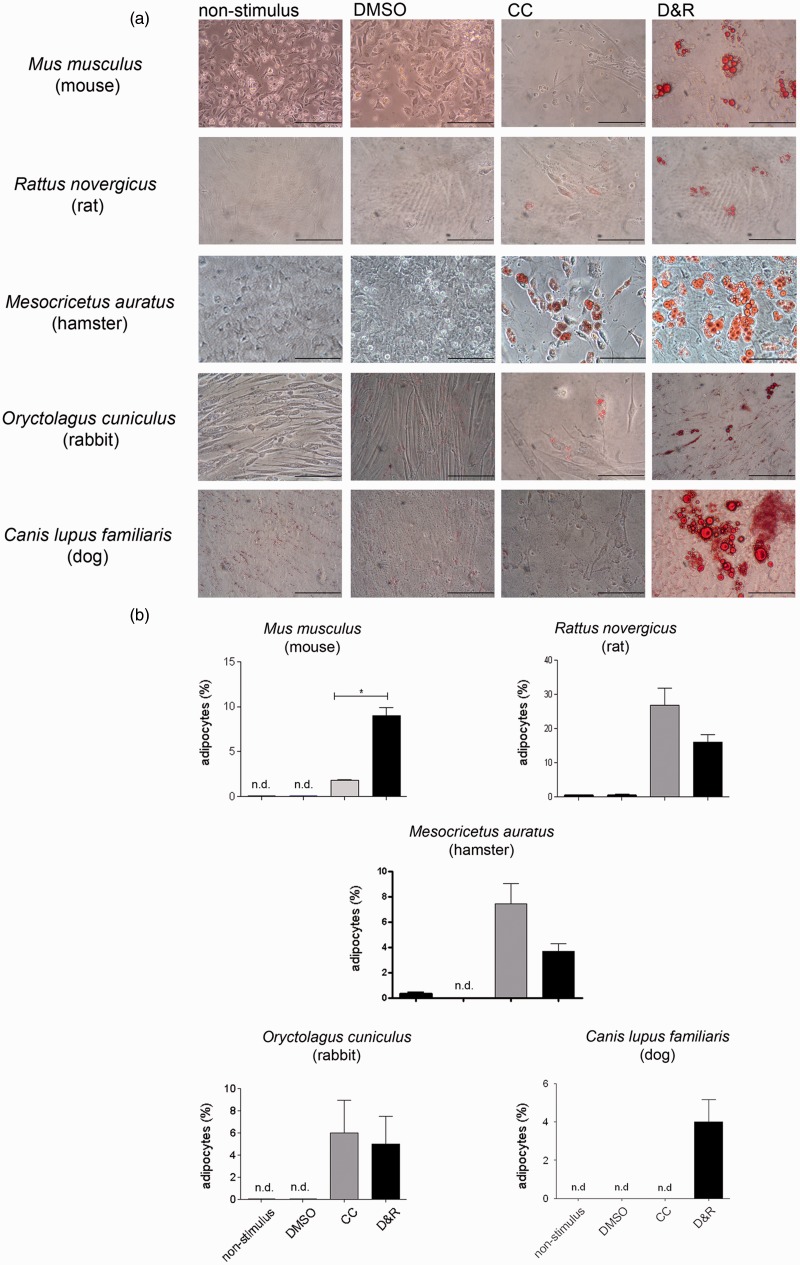
MSC from mice, rats, hamsters, rabbits, and dogs D&R exposed produced adipocytes whereas CC scarcely promoted mouse and dog MSC differentiation in the same conditions (Figure 2(a) and (b)).
Figure 2.
D&R induced the adipogenic differentiation of MSC from different mammalian species. MSCs isolated from mice, rats, hamsters, rabbits, and dogs were exposed to non-stimulus, DMSO, CC, or D&R. Adipocyte presence and abundance were assessed on day 14 by Oil Red O (a) and Nile Red (b) staining, respectively. Representative data regarding seven different hMSC donors. Cell viability >95%. Scale bar: 100 µm. n.d. below the assay detection limit.*: p < 0.05 (ANOVA, Tukey). (A color version of this figure is available in the online journal.)
The simultaneous activation of dexamethasone canonical signaling pathways through GR and C/EBPs and rosiglitazone through PPAR-gamma was necessary for inducing hMSC adipogenic differentiation
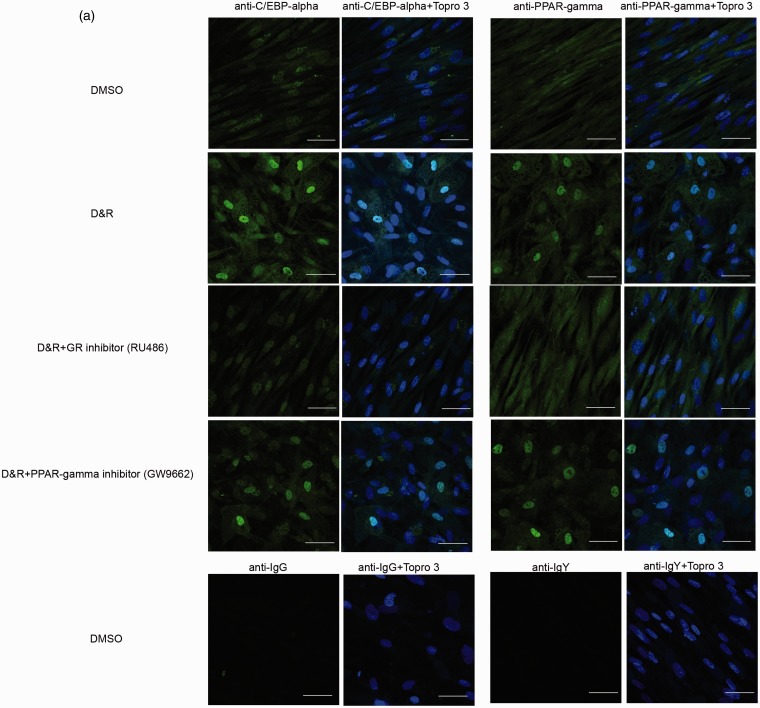
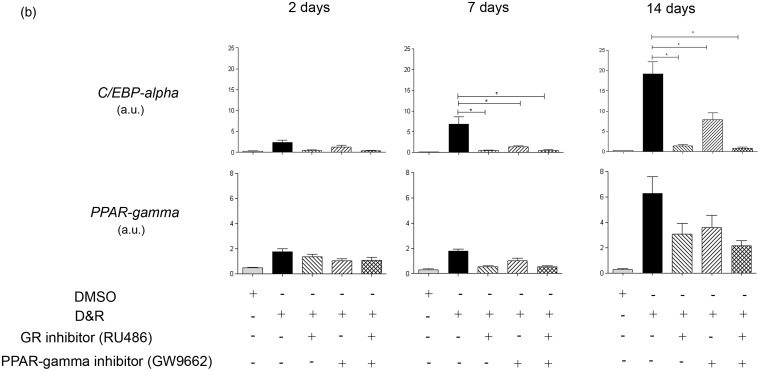
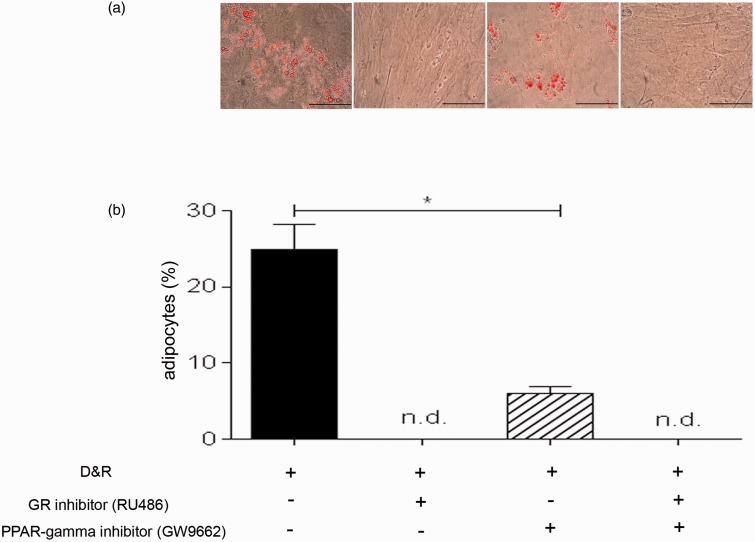
C/EBP-alpha and PPAR-gamma were found in the nucleus of differentiated cells when D&R-exposed (Figure 3(a)). Both transcription factors remained in hMSC cytoplasm in the presence of the GR inhibitor, regardless of whether they were D&R exposed. GW9662 (used at concentrations selectively inhibiting PPAR-gamma) reduced the number of cells in which C/EBP-alpha and PPAR-gamma became located in the nucleus (Figure 3(a)). Regarding C/EBP-alpha and PPAR-gamma gene expression, it was observed that exposure to RU486 and/or GW9662 resulted in significant inhibition of C/EBP-alpha by day 7 following treatment, while no significant changes were observed regarding PPAR-gamma expression levels at any of the times studied here (Figure 3(b)). GR inhibition resulted in the complete abolishment of hMSC adipogenic differentiation (Figure 4(a) and (b)). Partial inhibition caused by D&R-triggered differentiation was observed when PPAR-gamma was blocked by GW9662 (Figure 4(a) and (b)); similarly, the genes associated with the adipocyte mature phenotype were not expressed or were so at very low levels when GR or PPAR-gamma was inhibited, respectively (Supplementary Figures 1 and 2). The simultaneous activation of GR and C/EBPs and PPAR-gamma was thus necessary for inducing D&R-triggered hMSC adipogenic differentiation.
Figure 3.
Dexamethasone canonical signaling pathway through GR and rosiglitazone signaling pathway through PPAR-gamma became activated when hMSCs were D&R exposed. hMSCs were D&R exposed in the absence or presence of a GR inhibitor (RU486) and/or a PPAR-gamma inhibitor (GW9662). C/EBP-alpha and PPAR-gamma subcellular localization was assessed by immunocytofluorescence on day 14 (a). Representative data regarding seven different hMSC donors. C/EBP-alpha and PPAR-gamma mRNA levels were assessed on days 2, 7, and 14 by RT-PCR. (b) Scale bar: 50 µm.*: p < 0.05 (ANOVA, Tukey). (A color version of this figure is available in the online journal.)
Figure 4.
Activating dexamethasone canonical signaling pathways through GR and rosiglitazone through PPAR-gamma was necessary to induce hMSC adipogenic differentiation. hMSCs were D&R exposed in the absence or presence of a GR inhibitor (RU486) and/or a PPAR-gamma inhibitor (GW9662). The presence and abundance of adipocytes were assessed on day 14 by Oil Red O (a) and Nile Red (b) staining, respectively. Representative data regarding seven different hMSC donors. Cell viability >95%. Scale bar: 100 µm. n.d. below the assay detection limit.*: p < 0.05 (ANOVA, Tukey). (A color version of this figure is available in the online journal.)
Adipocytes produced from D&R-exposed hMSC were functional
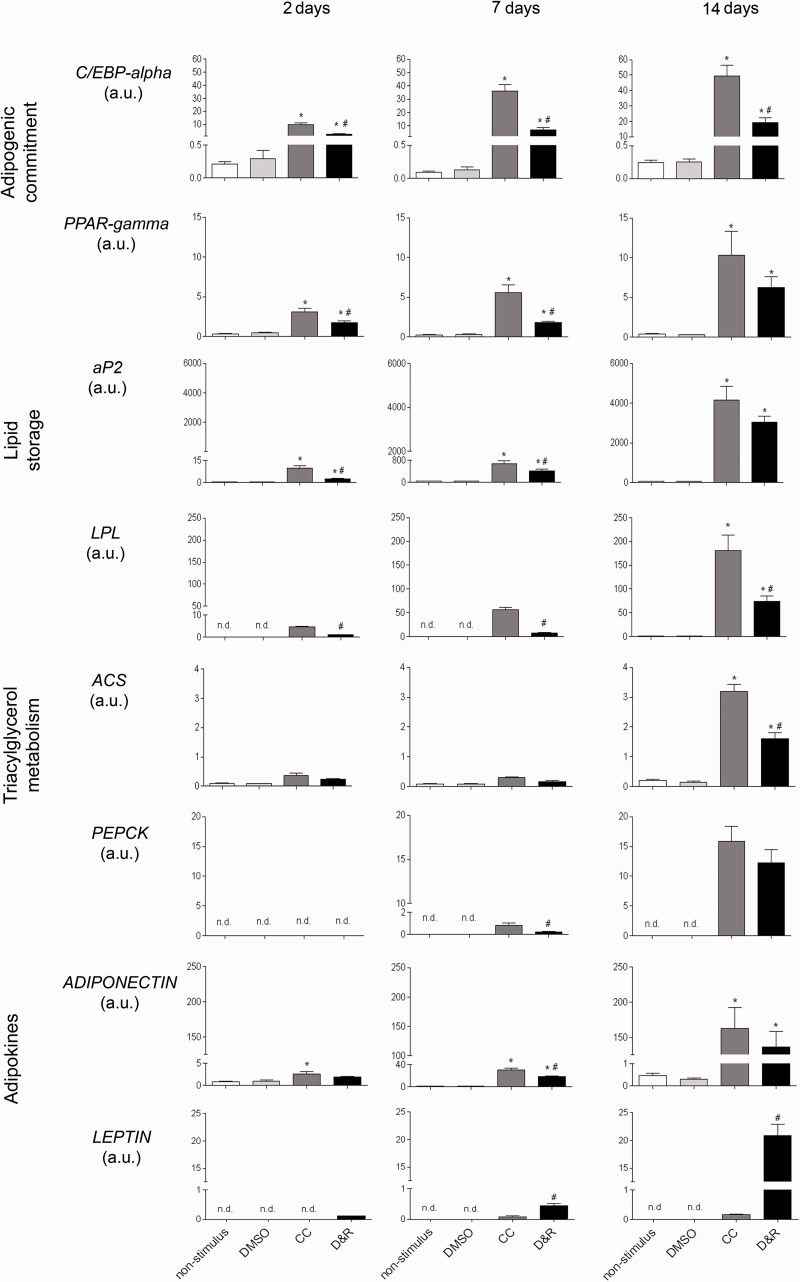
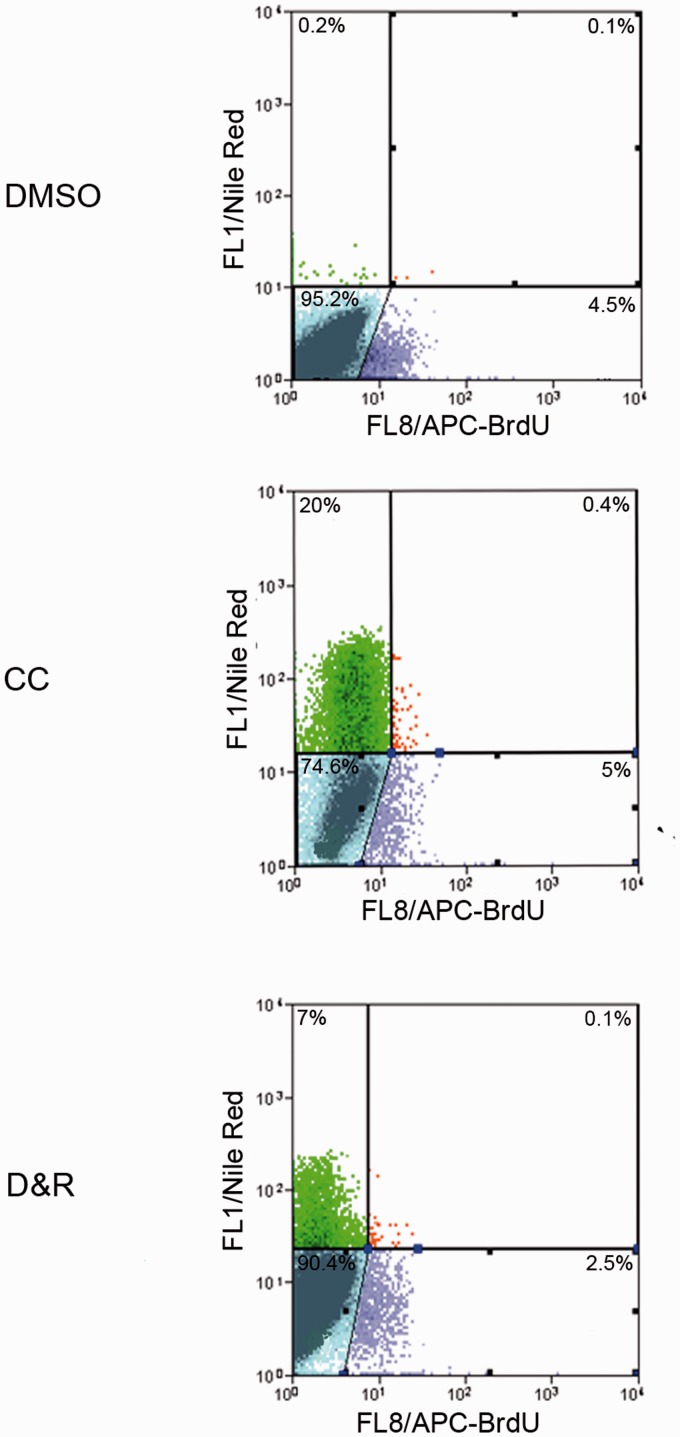
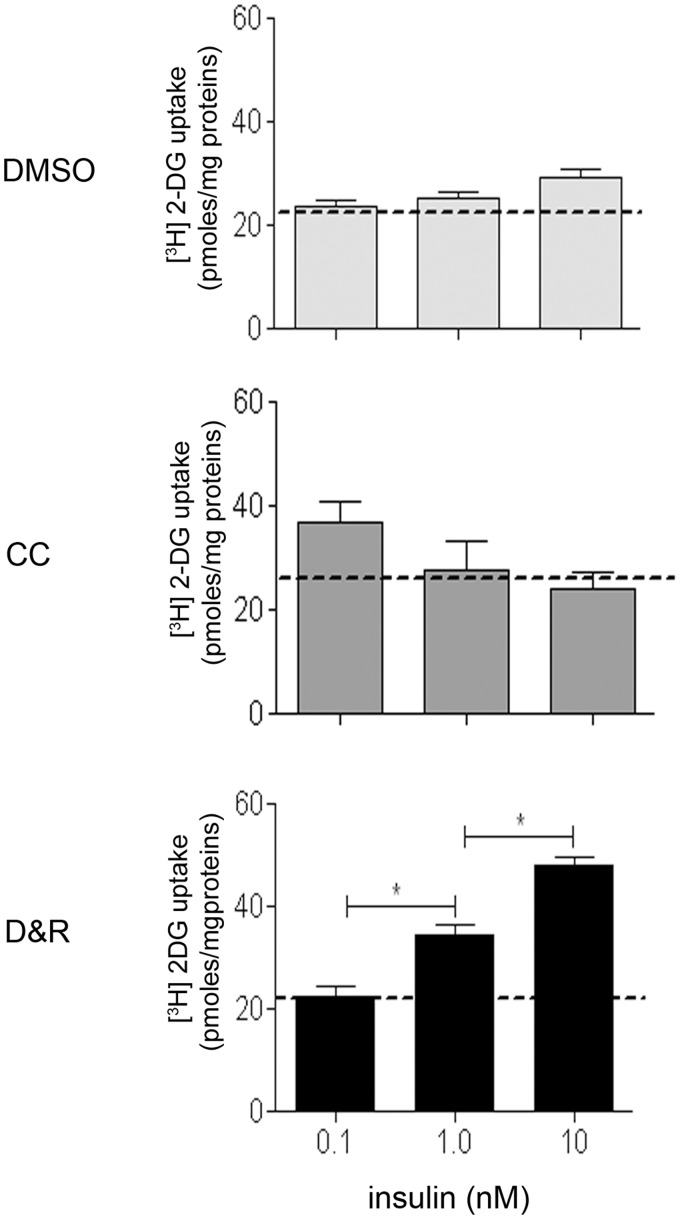
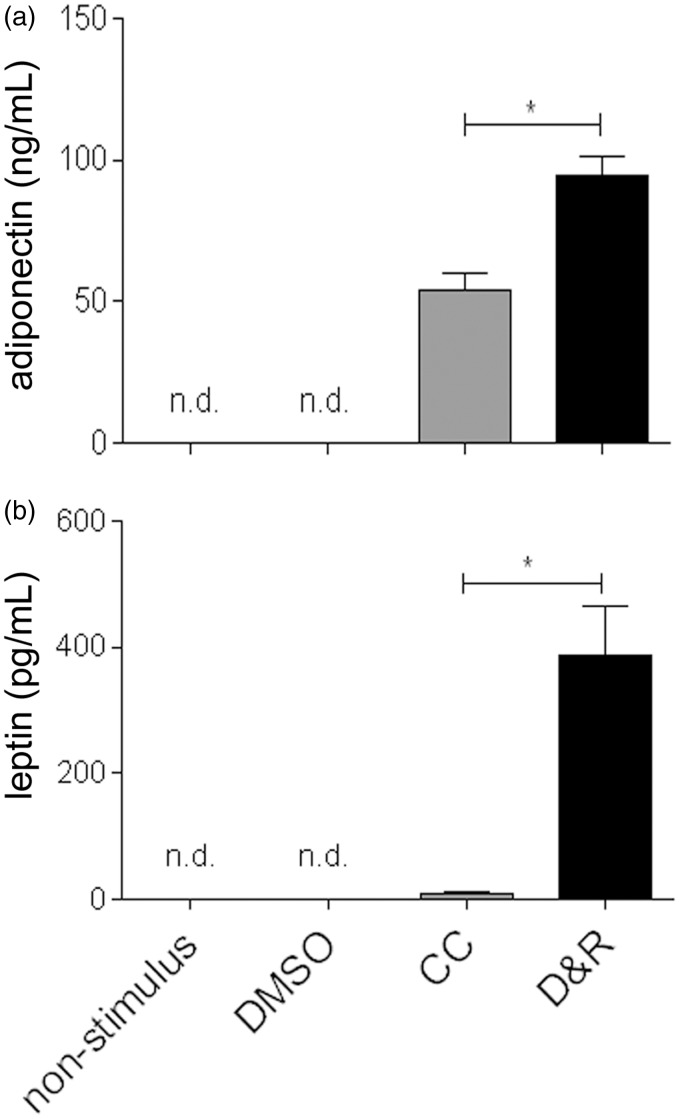
As seen in Figure 5, hMSC exposed to CC or D&R gradually expressed genes related to adipogenic commitment (C/EBP-alpha and PPAR-gamma), lipid storage (adipocyte protein2 [aP2] and lipoprotein lipase [LPL]), triacylglycerol metabolism (acyl-CoA synthase and phosphoenolpyruvate carboxykinase (PEPKC)), and adipokines (adiponectin and leptin), reaching maximum expression on day 14. Most mRNAs assessed were more abundant in hMSC exposed to CC than to D&R; such difference correlated with the higher percentage of adipocytes found in the former (Figure 1). Nonetheless, adiponectin levels were similar, and those of leptin were significantly higher in D&R-exposed hMSC compared to cells exposed to CC. When the data were standardized against adipocyte percentage, the difference was two and 250 times higher for adiponectin and leptin, respectively. When proliferation potential was assessed, it was observed that regardless of the stimulus used, adipocytes produced from hMSC (Nile Red positive cells) were unable to proliferate (BrdUrd negative) (Figure 6); however, undifferentiated hMSC proliferated in the same culture conditions (Nile Red negative, BrdUrd positive). Regarding insulin sensitivity, our results showed that undifferentiated hMSC did not incorporate a glucose response to insulin (Figure 7). The same was observed for adipocytes produced with CC. Furthermore, glucose uptake did not significantly vary at different insulin concentrations in these cells. Interestingly, D&R-exposed hMSC incorporated glucose in response to insulin in a dose-dependent way. It was also observed that undifferentiated cells secreted neither adiponectin nor leptin and hMSC exposed to CC secreted adiponectin and marginally, leptin (Figure 8). Adipocytes produced from D&R-exposed hMSC secreted both adipokines. Data standardized against adipocyte percentage revealed that D&R resulted in adiponectin- and leptin-secretion levels four and 800 times higher than CC, respectively.
Figure 5.
Adipocytes produced from D&R-exposed hMSC expressed functional adipocyte-specific genes. hMSCs were exposed to non-stimulus, DMSO, CC, or D&R. Adipocyte marker gene mRNA levels were assessed by RT-PCR on days 2, 7, and 14. Representative data regarding seven different hMSC donors. n.d. below the assay detection limit.*: p < 0.05, versus DMSO. #: p < 0.05, versus CC (ANOVA, Tukey)
Figure 6.
Adipocytes produced from D&R-exposed hMSC had no proliferation potential. hMSCs were exposed to DMSO, CC, or D&R. Cells were subcultured on day 14 in conditions which allowed proliferation; cells were incubated with BrdUrd one day later, and immunocytofluorescence was performed two days later. Representative data regarding seven different hMSC donors.*: p < 0.05 (ANOVA, Tukey). (A color version of this figure is available in the online journal.)
Figure 7.
Adipocytes produced from D&R-exposed hMSC were sensitive to insulin. hMSCs were exposed to DMSO, CC, or D&R. Cells were treated with increasing concentrations of insulin on day 14 and [H3]dT 2-DG uptake was assessed. Representative data regarding three different hMSC donors. Dotted lines indicate base uptake for each condition.*: p < 0.05 (ANOVA, Tukey)
Figure 8.
Adipocytes produced from D&R-exposed hMSC secreted adiponectin and leptin. hMSCs were exposed to non-stimulus, DMSO, CC, or D&R. Adiponectin (a) and leptin (b) levels in conditioned media were assessed by ELISA on day 14. Representative data regarding seven different hMSC donors.*: p < 0.05 (ANOVA, Tukey)
Discussion
Adipogenesis involves two steps: the commitment of an undifferentiated stem cell to the adipogenic lineage followed by the maturation of a preadipocyte into a functional adipocyte.1,3,28 While MSCs are recognized as a source of adipocytes in vivo and in vitro, the characterization of the cellular and molecular events leading to their adipogenic differentiation has been poorly studied as have the functional stages (gene expression profile, proliferation potential, insulin sensitivity, and adipokine secretion) of the cells so produced. This paper has shown that exposure to D&R, but not to each of its components separately, induced hMSC adipogenic differentiation (Figure 1(a) and (b)). These results were consistent with Ninomiya’s work which showed that rosiglitazone treatment alone was not sufficient for inducing functional adipocytes from hMSC.20 Furthermore, an attempt was made to ascertain whether D&R was sufficient to induce adipogenic differentiation in MSC obtained from other mammals commonly used as research models. The results showed that D&R induced adipogenic differentiation in all the species tested here, even in those where CC appeared to be refractory (Figure 2(a) and (b)).
D&R-induced adipogenesis in hMSC depended on C/EBP-alpha and PPAR-gamma activation (Figures 3 and 4). This was consistent with previous report which have shown in vivo and in vitro that C/EBPs and PPAR-gamma are crucial in inducing and maintaining the adipose phenotype and also with the fact that these transcription factors are activated in MSC induced to the adipogenic lineage in the presence of CC.9,13,18,19,29 Since hMSC exposed to dexamethasone did not differentiate into adipocytes (Figure 1(a) and (b)), C/EBP’s activation was not sufficient to trigger adipogenesis in uncommitted cells and required the simultaneous activation of PPAR-gamma. Further support for the fact that D&R activates dexamethasone and rosiglitazone’s canonical signal pathways was provided by the increased expression of both transcription factors in the nucleus and hMSC became differentiated into adipogenic lineage (Figure 3(a)) accompanied by a significant increase in their gene expression (Figure 3(b)), whereas C/EBP-alpha and PPAR-gamma expression decreased in the presence of the GR inhibitor (RU486) and was preferably located in the cytoplasm of undifferentiated hMSC. This led to hypothesizing that C/EBP-alpha and PPAR-gamma quantification in the nuclear fraction of D&R-exposed hMSC could complement the results observed in immunocytofluorescence experiments.
Furthermore, interdependency between these two signal pathways was revealed since PPAR-gamma mRNA levels did not increase in the presence of the GR inhibitor (Figure 3(b)). Interestingly, while GR inhibition led to a complete blockade of adipogenic differentiation from hMSC (Figure 4(a) and (b)), PPAR-gamma inhibition led to a partial reduction of such differentiation (Figure 4(a) and (b)). It has been reported that dexamethasone promotes dissociation and degradation of the mSin3A/HDAC1 complex (repressing C/EBP-beta) in 3T3-L1 cells.30,31 Activated C/EBP-beta triggered C/EBP-alpha and PPAR-gamma expression, inducing and maintaining the adipose phenotype.32,33 The above and the results obtained here led to suggesting that hMSC adipogenic differentiation could involve a mechanism similar to that described in 3T3-L1 cells. Consequently, C/EBP-alpha inhibition triggered by RU486 led to an early blocking of the C/EBP-beta and C/EBP-delta-mediated signaling pathway cascade, C/EBP-delta having been described as a complementary transcription factor regarding C/EBP-beta function, thereby preventing C/EBP-alpha and PPAR-gamma activation and blocking hMSC adipogenesis (Figure 4).34,35
Furthermore, GW9662 inhibition of PPAR-gamma led to a significant reduction of hMSC differentiation (even though this was partial) (Figure 4(a) and (b)). Similar results were observed when assessing PPAR-gamma gene expression in the presence of GW9662 (Figure 3(b)). These results could have been due to several factors. The literature has reported that regulation of transcription factors, PPAR-gamma type (nuclear hormone receptor) occurs mainly through ligand binding, and these induce or inhibit these transcription factors’ transcriptional activity.36 Although there is also evidence that other mechanisms may modulate PPAR-gamma activation, repression, and intracellular distribution, such as the action of co-activators and/or phosphorylation, which are independent of ligand binding.37–39 Some of these mechanisms may thus account for the partial inhibition of the PPAR-gamma pathway by GW9662. Furthermore, it has been described that PPAR-gamma has elements of response to other transcription factors, including C/EBP-alpha, which would become active at the moment of inhibiting the PPAR-gamma pathway by exerting positive control over this transcription factor,40 resulting in partial inhibition of PPAR-gamma expression and adipogenic differentiation.
The GW9662 inhibitor concentration used was insufficient to completely block the PPAR-gamma pathway. Considering that GW9662 acts as a competitive inhibitor and its half-life is about 2 h and rosiglitazone is about 20 h.41 Potential inhibition at different GW9662 concentrations increased by 5–10 times above the rosiglitazone concentration was evaluated; the results showed that at 10 times higher inhibitor concentration, D&R-induced adipogenic differentiation became completely suppressed, while inhibition was 99% at a fivefold higher concentration (Supplementary Figure S3A and B). Regarding PPAR-gamma gene expression, it was observed that both concentrations significantly inhibited this transcription factor’s expression levels (Supplementary Figure S4); concentrations greater than 50 µM GW9662 were harmful for cells. These results demonstrated that the rosiglitazone and GW9662 concentration ratio was decisive in terms of obtaining efficient inhibition of the PPAR-gamma pathway. A 10 µM rosiglitazone concentration was used here, given that this concentration led to obtaining the highest percentage of hMSC adipogenic differentiation (Supplementary Figure S5A and B). These results were consistent with Wang’s work which showed that the adipogenic effect of rosiglitazone was maximized at doses of 10 and 30 µM, in D1 cells a multipotential cell line derived from mouse bone marrow.21 Whereas 10 µM GW9662 was determined according to inhibitor specificity since it has been reported that higher than 10 µM concentrations of this inhibitor may act as agonists for other signaling pathways.25
As no changes were observed in PPAR-gamma gene expression levels at 10 µM GW9662, we asked ourselves whether GW9662 (at this concentration) would regulate this gene’s transcriptional activity. This led to evaluating the expression of target genes (i.e. aP2, LPL, PEPKC, and adiponectin) forming part of a mature adipocyte’s gene profile.1,3,42,43 The results showed a significant inhibition of the expression of all target genes tested 14 days after beginning adipogenic activation with D&R (Supplementary Figure 2A). Although GW9662 was insufficient to completely inhibit the PPAR-gamma pathway, the results demonstrated a direct interaction between the C/EBP-alpha and PPAR-gamma pathways and that both pathways were crucial for inducing hMSC adipogenesis.
Regarding the maturation stage, it was found that adipocytes produced from D&R-exposed hMSC had the gene profile characteristic of functional adipocytes (Figure 5); mRNA abundance was lower in D&R-exposed hMSC than in those exposed to CC at all the times studied here. This was an unexpected result because the percentage of Nile Red-stained cells was lower when hMSCs were triggered by D&R rather than CC (Figure 1(b)); nonetheless, there was no difference for adiponectin mRNA and the opposite was observed for leptin mRNA (Figure 5). As adipokine expression is a marker of final maturation, these mRNA level differences might be attributed to adipocyte functionality. Accordingly, adipocytes produced from D&R-exposed hMSC had no proliferation potential (Figure 6) and glucose uptake was dose dependent on insulin stimulus (Figure 7). These results agreed with properties in terms of function and maturation described for adipocytes in vivo in normal conditions, while undifferentiated cells and adipocytes produced from CC-exposed hMSC did not share these characteristics.
The pertinent literature has reported that an ability to incorporate glucose regarding insulin is an exclusive property of adipocytes which have completed their maturation and which would be in the final stages of adipogenesis.44–46 The foregoing and our results thus suggested that adipocytes produced by CC exposure would be immature adipocytes which had not acquired glucose transport mechanisms and dysfunctional adipocytes given an alteration in the same mechanism. The ultimate test of mature adipocyte functionality would be its ability to secrete adipokines. While adipocytes produced from CC-exposed hMSC marginally secreted adiponectin and did not secrete leptin, those produced with D&R abundantly secreted both adipokines (Figure 8). Such differences could be attributed to the different degrees of cell adipogenic differentiation; adipocytes produced with CC were partially differentiated (immature), therefore lacking the machinery to induce leptin expression and secretion (Figures 5 and 8). Regarding adipocyte functionality, the literature has reported that beta adrenergic agonists, lipolytic hormones (ACTH and thyrotropin-stimulating hormone), catecholamines, and analogs of camp (caffeine, theophyl-line, IBMX) interfere with both adipokines’ synthesis and secretion.47,48 The low level of leptin secreted by adipocytes differentiated with CC may thus have been due to the presence of IBMX producing dysfunctional adipocytes.
It has usually been considered that neutral fat droplet accumulation in a cell’s cytoplasm is indicative of terminal adipogenic differentiation. However, it has been shown that cells having such morphology fail to express mature adipocyte genes, proliferate, and can become dedifferentiated.49 A combined analysis was thus used which included morphological, genetic, and functional parameters to assess the degree of maturation. A minimum stimulus was defined which would induce adipogenesis from undifferentiated MSC. Compared to CC, D&R has several advantages; it is simpler, induces the differentiation of MSC from several mammalian species including those refractory to CC, and allows functional adipocytes to be produced from hMSC. This new adipogenic stimulus should be a useful tool for MSC characterization, studying adipogenesis pathways and producing functional adipocytes which might be used in cell therapy, for example in plastic and reconstructive surgery.
Acknowledgements
We would like to thank Mrs Valeska Simon for technical assistance with flow cytometry and Mr Jason Garry for editing the English version of the manuscript.
This work was supported by Direccion de Investigacion, Universidad del Desarrollo grant number 80.11.013 to D.C.
Authors’ contributions
DC conceived and carried out the experiments and analyzed the data, FE, ME, MA-R, and CP carried out the experiments; LS analyzed the data; and PC conceived the study, analyzed, and interpreted data. All the authors were involved in writing the paper and gave their final approval to the first and revised versions.
Conflict of interest
DC and PC are the inventors concerned in the aforementioned patent application filed by the Universidad del Desarrollo.
References
- 1.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol 2013; 92: 229–36. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–96. [DOI] [PubMed] [Google Scholar]
- 3.Moreno-Navarrete JM and Fernández-Real JM. Adipocyte differentiation chapter 2, In springer science + business media LLC. In: Symonds ME (ed.) Adipose Tissue Biology 2012, pp.17–23.
- 4.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010; 235: 1185–93. [DOI] [PubMed] [Google Scholar]
- 5.Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landshulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev 1989; 3: 1323–35. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 1991; 5: 1538–52. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor (published erratum appears in. Cell 1995; 80: 1147–56). [DOI] [PubMed] [Google Scholar]
- 8.Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 1995; 9: 168–81. [DOI] [PubMed] [Google Scholar]
- 9.Janderova L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. ObesRes 2003; 11: 65–74. [DOI] [PubMed] [Google Scholar]
- 10.Won Park K, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab 2008; 8: 454–7. [DOI] [PubMed] [Google Scholar]
- 11.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135: 240–9. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7. [DOI] [PubMed] [Google Scholar]
- 13.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung SC, Chang CF, Ma HL, Chen TH, Low-Tone Ho L. Gene expression profiles of early adipogenesis in human mesenchymal stem cells. Gene 2004; 340: 141–50. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Lee BH, Park SA, Kim CW. The proteomic analysis of an adipocyte differentiated from human mesenchymal stem cells using two-dimensional gel electrophoresis. Proteomics 2006; 6: 1223–29. [DOI] [PubMed] [Google Scholar]
- 16.Ylostalo J, Smith JR, Pochampally RR, Matz R, Sekiya I, Larson BL, Vuoristo JT, Prockop DJ. Use of differentiating adult stem cells (marrow stromal cells) to identify new downstream target genes for transcription factors. Stem Cells 2006; 24: 642–52. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JA, Ko KM, Park HS, Lee J, Jang C, Jeon CJ, Koh GY, Kim H. Membrane proteomic analysis of human mesenchymal stromal cells during adipogenesis. Proteomics 2007; 7: 4181–91. [DOI] [PubMed] [Google Scholar]
- 18.Qian SW, Li X, Zhang YY, Huang HY, Liu Y, Sun X, Tang QQ. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Dev Biol 2010; 10: 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Invest 2011; 91: 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ninomiya Y, Sugahara-Yamashita Y, Nakachi Y, Tokuzawa Y, Okazaki Y, Nishiyama M. Development of a rapid culture method to induce adipocyte differentiation of human bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2010; 394: 303–8. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Haile A, Jones LC. Rosiglitazone-induced adipogenesis in a bone marrow mesenchymal stem cell line—biomed 2011. Biomed Sci Instrum 2011; 47: 213–21. [PubMed] [Google Scholar]
- 22.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 1999; 181: 67–73. [DOI] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–17. [DOI] [PubMed] [Google Scholar]
- 24.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol 2007; 27: 2266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002; 41: 6640–50. [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–8. [DOI] [PubMed] [Google Scholar]
- 27.Rojas S, Rojas R, Lamperti L, Casanello P, Sobrevia L. Hyperglycaemia inhibits thymidine incorporation and cell growth via protein kinase C, mitogen-activated protein kinases and nitric oxide in human umbilical vein endothelium. Exp Physiol 2003; 88: 209–19. [DOI] [PubMed] [Google Scholar]
- 28.Prokesch A, Hackl H, Hakim-Weber R, Bornstein SR, Trajanoski Z. Novel insights into adipogenesis from omics data. Curr Med Chem 2009; 16: 2952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab 2009; 20: 107–14. [DOI] [PubMed] [Google Scholar]
- 30.Wiper-Bergeron N, Salem HA, Tomlinson JJ, Wu D, Haché RJG. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5. Proc Natl Acad Sci USA 2007; 104: 2703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Haché RJG. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J 2003; 22: 2135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 2002; 16: 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W, Chambon P, Metzger D. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA 2004; 101: 4543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 1996; 16: 4128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J 1997; 16: 7432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol 1998; 10: 384–91. [DOI] [PubMed] [Google Scholar]
- 37.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996; 274: 2100–3. [DOI] [PubMed] [Google Scholar]
- 38.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 1997; 272: 5128–32. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama TE, Baumann CT, Sakai S, Hager GL, Gonzalez FJ. Selective intranuclear redistribution of PPAR isoforms by RXR alpha. Mol Endocrinol 2002; 16: 707–21. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt SF, Jørgensen M, Chen Y, Nielsen R, Sandelin A, Mandrup S. Cross species comparison of C/EBPα and PPARγ profiles in mouse and human adipocytes reveals interdependent retention of binding sites. BMC Genomics 2011; 12: 152–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol 2011; 127: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipLabel storage in vivo. Diabetes Care 2004; 27: 1660–7. [DOI] [PubMed] [Google Scholar]
- 43.Haakonsson AK, Stahl Madsen M, Nielsen R, Sandelin A, Mandrup S. Acute genome-wide effects of rosiglitazone on PPARγ transcriptional networks in adipocytes. Mol Endocrinol 2013; 27: 1536–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia de Herreros A, Birnbaum MJ. The regulation by insulin of glucose transporter gene expression in 3T3 adipocytes. J Biol Chem 1989; 264: 9885–90. [PubMed] [Google Scholar]
- 45.Mastick CC, Aebersold R, Lienhard GE. Characterization of a major protein in GLUT4 vesicles. Concentration in the vesicles and insulin-stimulated translocation to the plasma membrane. J Biol Chem 1994; 269: 6089–92. [PubMed] [Google Scholar]
- 46.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 2010; 298: E141–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol 2002; 283: C244–50. [DOI] [PubMed] [Google Scholar]
- 48.Delporte M-L, Funahashi T, Takahashi M, Matsuzawa Y, Brichard SM. Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J 2002; 367: 677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shigematsu M, Watanabe H, Sugihara H. Proliferation and differentiation of unilocular fat cells in the bone marrow. Cell Struct Funct 1999; 24: 89–100. [DOI] [PubMed] [Google Scholar]