Abstract
Non-ionizing radiation at 2.45 GHz may modify the morphology and expression of genes that codify heat shock proteins (HSP) in the thyroid gland. Diathermy is the therapeutic application of non-ionizing radiation to humans for its beneficial effects in rheumatological and musculo-skeletal pain processes. We used a diathermy model on laboratory rats subjected to maximum exposure in the left front leg, in order to study the effects of radiation on the nearby thyroid tissue. Fifty-six rats were individually exposed once or repeatedly (10 times in two weeks) for 30 min to 2.45 GHz radiation in a commercial chamber at different non-thermal specific absorption rates (SARs), which were calculated using the finite difference time domain technique. We used immunohistochemistry methods to study the expression of HSP-90 and morphological changes in thyroid gland tissues. Ninety minutes after radiation with the highest SAR, the central and peripheral follicles presented increased size and the thickness of the peripheral septa had decreased. Twenty-four hours after radiation, only peripheral follicles radiated at 12 W were found to be smaller. Peripheral follicles increased in size with repeated exposure at 3 W power. Morphological changes in the thyroid tissue may indicate a glandular response to acute or repeated stress from radiation in the hypothalamic–pituitary–thyroid axis. Further research is needed to determine if the effect of this physical agent over time may cause disease in the human thyroid gland.
Keywords: Thyroid, Gigahertz, specific absorption rate, heat shock protein, follicle, septa
Introduction
Diathermy, or heat therapy, is a medical application based on the use of microwaves at 2.45 GHz radiofrequency (RF) as a source of electromagnetic radiation for the treatment or rehabilitation of various chronic, musculo-skeletal, or rheumatological processes. Bio-stimulant heat from localized non-ionizing radiation up to 200 W is applied therapeutically in humans to reduce swelling and localized pain, relax muscles, and improve circulation and articular mobility. While the increased temperature generated by the interaction of microwave radiation with the body can prove beneficial, many of the effects of exposure to non-thermal radiation have yet to be determined. There are indications that non-ionizing radiation produces in biological systems effects that are not caused by temperature increase or non-thermal effects (the limits established by electromagnetic field [EMF] legislation refer only to thermal effects).1–3
Exposure of an area of the human body to microwaves also exposes the tissues nearest the area to non-ionizing radiation. Since shoulder pain is frequently treated with diathermy in rehabilitation, the nearby thyroid gland would be repeatedly exposed to microwaves during this type of treatment. Other sources of exposure to non-ionizing radiation in the 2.4–3 GHz bandwidth include a wide range of technological devices that are used every day, including wireless phones, Wi-Fi, Bluetooth,4,5 or medical applications such as magnetic resonance imaging.6
The thyroid gland is one of the most superficial vital organs and possibly more vulnerable to EMFs.7 Chronic exposure to microwaves at a RF of 2.45 GHz has been shown to significantly affect the hypothalamus–pituitary–thyroid (HPT) axis, provoking changes in body temperature, behavior, and thyroid hormone concentrations.8 Alterations in human and animal levels of thyroid stimulating hormone and other thyroid hormones have also been reported with chronic exposure to frequencies used in mobile telephones, such as 900 MHz.9,10
Heat shock protein (HSP) 90 is a chaperone protein regulating several client proteins involved in thyroid cancer development and the level of expression is higher than in normal tissues. This chaperone has emerged as an exciting target in the development of cancer chemotherapeutics.11,12 Recently, we discovered that repeated, acute subthermal radiation for 30 min at 2.45 GHz can alter cellular stress levels in rat hypothalamus13 and thyroid gland,14 without initially altering apoptotic capacity. Surprisingly, in spite of frequent direct and indirect exposure to non-ionizing radiation in human environments and indications that radiation provokes a degree of stress in thyroid cells, there is very little research describing morphological changes that point to precocious re-adjustments of the mammalian thyroid gland after close-range exposure to non-ionizing radiation at 2.45 GHz.
In this study, we used a diathermic model involving maximum direct radiation of the left shoulder of the animal in an experimental set-up connected to a Gigahertz transverse electromagnetic (GTEM) chamber. We hypothesized that localized exposure of rats to 2.45 GHz diathermic radiation would have detectable effects on thyroid gland morphology and provoke changes in thyroid cell expression of heat stress proteins. To test this hypothesis, we studied morphological changes in the female rat thyroid gland through immunohistochemical analysis of the stress protein known as HSP-90 in rat thyroid tissue after single or repeated (10 times in two weeks) exposure to 2.45 GHz RF. The radiation levels, time, and doses were similar to those applied to humans.
Materials and methods
Animals
All experiments were carried out according to European regulations on animal protection,15 the Declaration of Helsinki, and/or the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.16,17 All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Santiago de Compostela.
Adult female Sprague-Dawley rats were used in the study. The rats weighed 230–250 g, were housed in individual cages with free access to food and water, and were maintained at ±22℃ under a 12:12 h light/dark regimen. There is already evidence that estrogen may act directly to increase the proliferation and functioning of thyroid cells in female rats.18 We only used animals with a synchronized estrogen cycle (same age and weight, under the same light/dark conditions) in all experiments, in order to eliminate any confounding factor or endocrine interference.
Experimental design
A total of 56 female Sprague-Dawley rats were randomly placed in the following groups:
Group A: single exposure and studied after 90 min (n = 18): The rats were divided into three subgroups (n = 6); each rat was exposed to 30 mina of microwave radiation at three levels: 0 (control), 3, and 12 W.b The rats were kept alive for 90 minc and then euthanized and perfused with fixative.
Group B: single exposure and studied after 24 h (n = 18): The rats were divided into three subgroups (n = 6); each rat was exposed to 30 min of microwave radiation at three levels: 0 (control), 3, and 12 W.b The rats were kept alive for 24 hc and then euthanized and perfused with fixative.
Group C: repeated exposure and studied after 90 min (n = 20): Rats in this group were irradiated at 3 W for 30 min/day, for a total of 10 times in a two-week period. On the last day of exposure, the rats were irradiated and after 90 min were euthanized and perfused with fixative. They were then tested for HSP-90 expression. In the non-irradiated control group (n = 10), rats were immobilized for each of the 10 sessions and euthanized on the last day, following the same protocol as the irradiated animals.
Experimental radiation system protocol and description of the numerical simulations by finite difference time domain (FDTD) SAR estimation
Power was delivered by a controlled signal during the radiation procedure. The amplifier (AMP) output was connected to the directional coupler (DC) that fed the chamber (Schaffner GTEM 250, 1.25 m × 0.65 m × 0.45 m). The rat was positioned in the region of maximum field uniformity inside the chamber.
The DC made it possible to measure the input power (PIN) and reflected power (PREF) values. The PIN was read by a power meter that also monitored the purity of the input signal, while the PREF value was obtained directly by means of a spectrum analyzer. All the measuring instruments were certified and calibrated by Agilent Technologies. This exposure set-up provides good field uniformity in which the behavior of a traveler wave can be simulated. The system components and operation are described in detail in Jorge-Mora et al.19 The rat was immobilized in a methacrylate holder in such a way that maximum radiation could be applied to the left front leg (Figure 1).
Figure 1.
Representation of the position of the rat in the methacrylate holder. The left front leg is the point of maximum radiation. (A color version of this figure is available in the online journal.)
The specific absorption rate (SAR) values were estimated with the aid of SEM-CAD X20 and an FDTD-based software tool. A Sprague-Dawley model R8 numerical (voxel) phantom rat20 was used, weighing 198.3 g and composed of 60 different tissues assembled in 1.15 mm thick slices. Tissue morphologies were obtained by magnetic resonance imaging. The numerical phantom was radiated by a plane wave impinging its left side, with the magnetic field H parallel to its main axis.19 The electrical field value E was determined by equation (1)
| (1) |
where h is the septum height in the exposure zone (position of the MH), PTR ζ is the input power of the GTEM cell, PTR = PIN–PREF, Z0 = 50 Ώ is the input impedance of the cell, and ζ is the coefficient that depends on the ripple field in the position MH, which is considered to have a value of 2.21
The SARs were estimated by applying a correction factor to the values obtained from the numerical simulations, in proportion to the ratio between the weight of the model rat and the weights of the experimental rats, as specified by the following expression
| (2) |
where SARE is the estimated value of the experimental SAR, SARS is the SAR obtained during the simulation, WS = 198.3 (g) (the weight of the model rat), and WE (g) the weight of the experimental rat.
Tissue extraction and preparation
After radiation, the rats were left to rest for 90 min or 24 h and then euthanized for tissue extraction. The euthanasia procedure involved an intraperitoneal injection of sodium pentothal to diminish stress levels, followed by an overdose of ethyl ether just prior to euthanasia. Subsequently, the thyroid gland was extracted from each animal and submerged in a fixing solution using 10% formaldehyde in phosphate buffer.
Paraffin embedding
Four hours after fixation, the tissue was placed in the same solution using 10% formaldehyde in phosphate buffer to eliminate water, and all tissues were refrigerated at 4℃. After 24 h, the fixing agent was replaced with 70% ethanol (ethanol in water) twice at a 2 h interval and agitated for 12 h. This was then replaced with 96% ethanol twice at a 2 h interval and subsequently submerged in 100% ethanol twice at a 2 h interval. One hour later, the alcohol was replaced with 100% toluene and left for 12 h. For embedding, all tissues were dipped three times in liquid paraffin (paraplast plus, leica) that had been melted in an oven at 58℃. The pieces were submerged for 2 h each time, leaving the samples completely saturated by the paraffin. After the final immersion, the pieces were placed in a mold and allowed to cool and solidify to the appropriate consistency for cutting.
Tissue sectioning
Once all the tissues had been embedded in paraffin blocks, a microtome was used to make a series of 5 µm slices of the paraffin blocks; which were then attached to create a ribbon several millimeters long. Sections from each ribbon were selected at 120 µm intervals (every 24th section, 120/5) and placed on microscope slides for study. The slides were placed in an oven at 37℃ for at least 24 h; then the tissues were deparaffinated by incubating them in an oven at 60℃ for 30 min and subsequently submerging them in Xilol twice for 5 min each time.
Immunohistochemistry and counterstain for hematoxylin
The thyroid sections were pretreated for 1 h with 10% normal rabbit serum and 0.25% Triton X-100 in 0.02% potassium-phosphate-buffered saline (KPBS) and then exposed overnight at room temperature to polyclonal rabbit anti-HSP-90 antibody (1:1000 in KPBS, Santa Cruz Biotechnology). Sections were then rinsed with KPBS, incubated for 1 h with biotinylated goat anti-rabbit antibody (1:500 in KPBS, Vector, Burlingame, CA, USA), rinsed three times with KPBS, incubated for 30 min with an avidin–biotin–peroxidase complex prepared according to the manufacturer’s instructions (DAKO, Glostrup, Denmark), and labeled with 3,3’-diaminobenzidine in imidazole-HCl-buffered H2O2 solution (DAKO).
The sections were then hydrated in distilled water, and hematoxylin tincture was applied for 1–2 min, followed by a final 3 min rinse in water. This was followed by dehydration with 70, 96, and 100% alcohol, after which the samples were submerged in xylol and mounted.
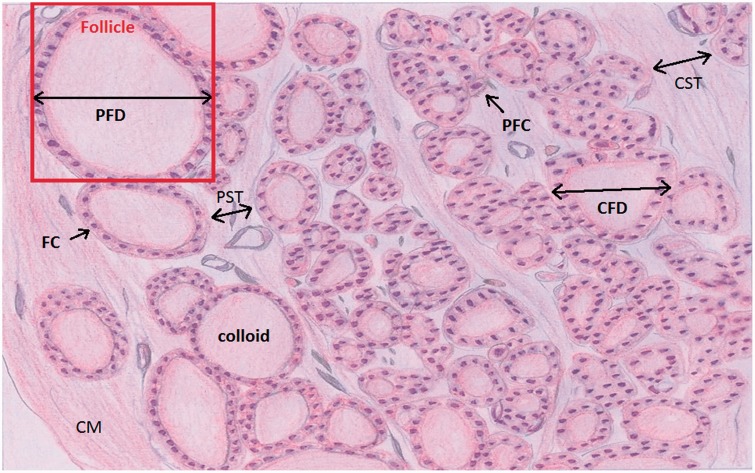
The samples were examined under a conventional brightfield microscope to observe the follicular cells, connective tissue, and colloid of the thyroid gland (see Figure 2).
Figure 2.
Diagram of rat thyroid tissue stained with Hematoxylin-Eosin (H&E). Anatomical areas are identified in all groups studied: peripheral follicle diameter (PFD), central follicle diameter (CFD), peripheral septa thickness (PST), and central septa thickness (CST). Also identified are the capsular membrane (CM); follicle, colloid, follicle cell (FC); and parafollicle cell (PFC). All morphological parameters were studied and measured in this experiment. (A color version of this figure is available in the online journal.)
Histological parameters, quantification, and statistical analysis
The results in the text and figures are expressed as averages and standard error of the mean (SEM) (p < 0.05). The following statistical tests were applied:
- One-way ANOVA was used to study the SAR values (Table 1), followed by a Holm–Sidak test.
- • Morphological measurements included the largest diameters of the central (surrounding the center or intersection of the X and Y axes of the preparation) and peripheral follicle (next to the capsular membrane) (Figure 2), and the thickness of the of central and peripheral connective tissue septa in the HSP-90 sections, at 40× magnification. Four sections (one section every 120 µm) of the tissue mounted in paraffin blocks were measured per animal and group, and the following statistical tests were done:
The statistical significance of differences in histological parameters between acute exposure groups A and B was assessed using three-way analysis of variance (ANOVA) based on: (a) radiation (no radiation, radiation), (b) power (3 or 12 W), and (c) time postradiation (90 min or 24 h). Two-way ANOVA was applied for radiation × time after exposure, along with a one-way ANOVA for radiation only.
-
Two-way ANOVA was used to evaluate significant differences in parameters (diameter or partition size) between repeated exposure in group C and single exposure in group A, followed by a Holm–Sidak multiple comparison test. The factors taken into consideration were power used during exposure to radiation (non-radiated, 0 W or radiated, 3 W) and number of exposures (repeated or single exposure).
The total quantitative differences in color intensity between the HSP-90 immunohistochemical preparations were measured by software in the optical microscope and the following statistical tests were used:
One-way or two-way ANOVA was used to evaluate significant differences in HSP-90 immunohistochemical sample color intensity. One-way ANOVA was conducted for power between acute exposure groups (A and B), followed by a Holm–Sidak multiple comparison test. Two-way ANOVA between single exposure group A (3 W) and repeated exposure group C was conducted for power (non-radiated, 0 W; radiated, 3 W) and exposure (single or repeated).
Table 1.
Thyroid and body SAR values for the experimental rats, calculated from the power (P) and electrical field (E)
| Experimental measurement of specific absorption rate by FDTD |
||||
|---|---|---|---|---|
| Mean SAR in thyroid (W/kg) | Peak SAR in 1 g thyroid | Mean SAR in body (W/kg) | Peak SAR in 1 g body | |
| Acute single exposure | ||||
| P = 3 W E = 40.28 V/m | 0.102 ± 12.10–3 | 0.076 ± 4.10–3(2*) | 0.0364 ± 19.10–3(2*) | 0.180 ± 9.10–3(2*) |
| P = 12 W E = 80.56 V/m | 0.429 ± 12.10–3(1,3*) | 0.340 ± 10.10–3(1,3*) | 0.161 ± 4.10–3(1,3*) | 0.795 ± 2.10–3(1,3*) |
| Repeated exposure | ||||
| P = Repeated 3 W E = 40.28 V/m | 0.107 ± 6.10–3(2*) | 0.0809 ± 14.10–4(2*) | 0.0382 ± 7.10–3(2*) | 0.189 ± 3.10–3(2*) |
The SAR values were compared by one-way ANOVA differences between power (P) and electric field (E), and by an a posteriori Dunn’s test. For multiple comparisons, differences were considered significant at p < 0.05. *Indicates significant differences between (P = 3)1, (P = 12)2, and repeated (P = 3)3.
Transformations of natural logarithms were applied as needed to obtain normality and homoscedasticity. Tukey, Bonferroni HSD, or Holm–Sidak tests were used in a posteriori comparisons.
Results
SAR
The mean power absorbed by the rats in the three groups was estimated by equation (2), along with the average weight, mean SAR ± SEM in midbrain and body, and averaged peak SAR ± SEM for 1 g of brain or body at 2.45 GHz frequency in a Shaffner GTEM cell. One-way ANOVA (for different levels of radiation and mean SAR in thyroid or body and peak SAR averaged for 1 g of thyroid or body tissue) revealed significant differences in all SAR values (p ≤ 0.001). Table 1 shows the mean values of the average SAR in rat thyroid and body, as well as the mean values of the peak SAR per 1 g of thyroid and body, obtained from individual measurements of rats exposed to different powers (P) and electric fields (E). The increase in average and maximum SAR values is directly proportional to the initial power (P) and the electric field value (E) for each subgroup.
Morphological analysis of the thyroid
Results of morphological measurements in the thyroid gland
A and B groups
In animals exposed to an acute, single dose of radiation (groups A and B), morphometric parameters in the thyroid tissue showed increased size in the central and peripheral follicle diameters of rats irradiated at 3 and 12 W, with respect to non-irradiated animals. Thickness of the peripheral wall or septa showed a temporary decrease in size (90 min after exposure, but not after 24 h), but no changes in size were observed in the central septa of irradiated animals.
Central follicle diameter values indicated time after exposure (p < 0.001) to be the only significant factor in and of itself. Radiation/non-radiation or power levels (3 or 12 W) were not significant when analyzed by three-way ANOVA. The effect of different levels of radiation was dependent on time after exposure (p < 0.001). The effect of radiation was not dependent on the power level (p = 0.896) nor was the effect of power dependent on time after exposure (p = 0.392) (Table 2).
Table 2.
Results of three-way ANOVA (radiation × power × time after irradiation) for central follicle diameter in thyroid gland
| Effects | F | DF | P-value |
|---|---|---|---|
| Rad | 49.04 | 1 | <0.001 |
| P | 0.0004 | 1 | 0.982 |
| T | 0.149 | 1 | 0.700 |
| Rad × P | 0.0004 | 1 | 0.982 |
| Rad × T | 11.92 | 1 | <0.001 |
| P × T | 0.861 | 1 | 0.355 |
| Rad × P × T | 0.861 | 1 | 0.355 |
DF: degrees of freedom; F: Fisher–Snedecor F statistic; p-value: level of significance.
Ninety minutes after radiation at 0.102 ± 12.10–3 or 0.429 ± 12.10–3 SAR, central follicle diameters in the thyroid gland were significantly larger than in non-radiated animals (p < 0.001 in both cases). Central follicle diameters decreased significantly 24 h after radiation at 3 W power, compared to animals studied 90 min after exposure (p < 0.05). There was also a significant decrease in the central follicle diameters 24 h after radiation at 0.102 ± 12.10–3 or 0.429 ± 12.10–3 SAR compared to control (non-radiated) animals (p = 0.007, p = 0.045, respectively). See Tables 6 and 7.
Table 6.
Thyroid gland average values ± SEM in µm for central follicle diameter (CFD), peripheral follicle diameter (PFD), central septa thickness (CST) and peripheral septa thickness (PST), ninety minutes (90 min) and twenty-four hours (24 h) postexposure in non-radiated (NO RAD) and radiated (RAD) animals at 2.45 GHz radiofrequency, 3 W power (p < 0.05, two-way ANOVA followed by Tukey’s or Bonferroni t-test)
| 90 min postexposure |
24 h postexposure |
||||||
|---|---|---|---|---|---|---|---|
| NO RAD | RAD | NO RAD | RAD | ||||
| Measures (µm) | N | P value | P value | ||||
| CFD | 28 | 53 ± 4 | 83 ± 3 | <0.001 | 63 ± 4 | 70 ± 3 | 0.181 |
| PFD | 28 | 105 ± 6 | 130 ± 5 | 0.003 | 104 ± 6 | 110 ± 6 | 0.462 |
| CST | 28 | 22 ± 2 | 23 ± 2 | 0.765 | 21 ± 2 | 22 ± 2 | 0.542 |
| PST | 28 | 39 ± 5 | 18 ± 4 | 0.004 | 32 ± 5 | 23 ± 5 | 0.219 |
Table 7.
Thyroid gland of the average values ± SEM in µm for central follicle diameter (CFD), peripheral follicle diameter (PFD), central septa thickness (CST), and peripheral septa thickness (PST), ninety minutes (90 min) and twenty-four hours (24 h) postexposure in non-radiated (NO RAD) and radiated (RAD) animals at 2.45 GHz radiofrequency, 12 W power (p < 0.05, two-way ANOVA followed by Tukey’s or Bonferroni t-test)
| 90 min postexposure |
24 h postexposure |
||||||
|---|---|---|---|---|---|---|---|
| NO RAD | RAD | NO RAD | RAD | ||||
| Measures (µm) | N | P value | P value | ||||
| CFD | 30 | 53 ± 4 | 78 ± 4 | <0.001 | 63 ± 4 | 75 ± 4 | 0.375 |
| PFD | 30 | 105 ± 7 | 130 ± 7 | 0.024 | 104 ± 7 | 159 ± 7 | <0.001 |
| CST | 30 | 22 ± 2 | 22 ± 2 | 0.93 | 21 ± 2 | 28 ± 2 | 0.010 |
| PST | 30 | 39 ± 6 | 13 ± 6 | 0.003 | 32 ± 5 | 22 ± 5 | 0.291 |
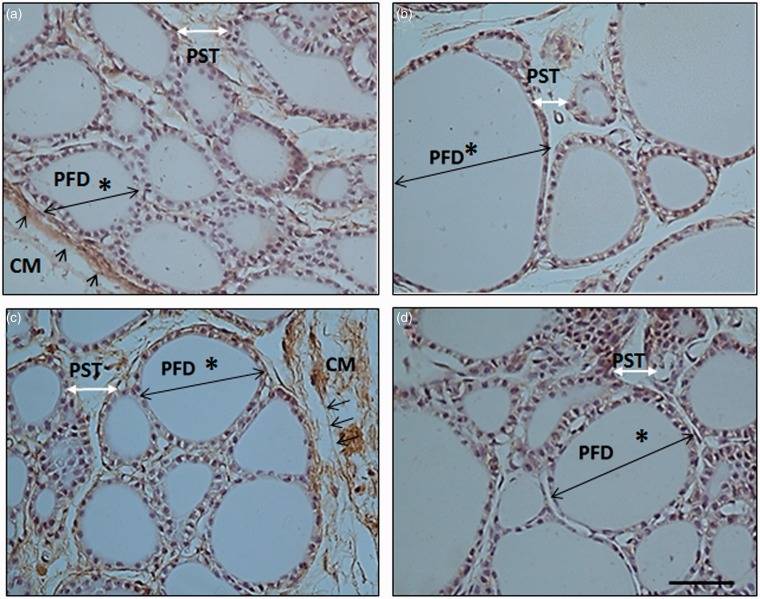
Results for measurements of peripheral follicle diameters indicated that more direct morphological effects can be observed in the most superficial part of the gland. Both radiation and power after exposure were statistically significant factors (p < 0.001 and p = 0.014, respectively).The effect of radiation was dependent on the power level (p = 0.014) but not on time after exposure (p = 0.887) (see Table 3). Thus, the size increase in the peripheral follicles was larger in radiated animals with respect to non-radiated animals 90 min after exposure, at both power levels (p = 0.003 at 3 W and p = 0.024 at 12 W) (See Figure 5(a),(b)). However, a positive influence of time, with observable increments in the size of the peripheral follicle only occurred when the power was increased. At 12 W power significant differences were observed between radiated and non-radiated animals (p < 0.001) (See Figure 5(c),(d)) (Tables 6 and 7).
Table 3.
Results of three-way ANOVA (radiation × power × time after irradiation) for peripheral follicle diameter in thyroid gland
| Effects | F | DF | P-value |
|---|---|---|---|
| Rad | 30.383 | 1 | <0.001 |
| P | 6.219 | 1 | 0.014 |
| T | 0.00290 | 1 | 0.957 |
| Rad × P | 6.219 | 1 | 0.014 |
| Rad × T | 0.0204 | 1 | 0.887 |
| P × T | 6.022 | 1 | 0.015 |
| Rad × P × T | 6.022 | 1 | 0.015 |
DF: degrees of freedom; F: Fisher–Snedecor F statistic; p-value: level of significance.
Figure 5.
Photographs of rat thyroid tissue with HSP-90 inmunohistochemistry show how peripheral follicles increased in size after acute exposure. Photos a and b: animals euthanized 90 min after exposure to 0 W (control) and 3 W, respectively (group A); c and d: animals euthanized 24 h after exposure to 0 W (control) and 12 W, respectively (group B). Asterisk * indicates peripheral follicle diameter (PFD), single arrows indicate capsular membrane (CM), double white arrows indicate peripheral septa thickness (PST); magnification 40×, calibration bar = 50 µm. (A color version of this figure is available in the online journal.)
Analysis of the septa thickness of the connective tissue next to the central follicles showed time after radiation to be the only potentially influencing factor (p < 0.003). There were no significant interactions between any two or all three factors of radiation, power level, and time after exposure (Table 4). Similarly, there were no statistically significant differences between radiated and non-radiated animals exposed to different SAR levels or in septa thickness 90 min versus 24 h after radiation (Tables 6 and 7).
Table 4.
Results of three-way ANOVA (radiation × power × time after irradiation) for septa wall thickness in thyroid gland
| Effects | F | DF | P-value |
|---|---|---|---|
| Rad | 2.926 | 1 | 0.089 |
| P | 0.737 | 1 | 0.392 |
| T | 0.496 | 1 | 0.482 |
| Rad × P | 0.718 | 1 | 0.398 |
| Rad × T | 2.306 | 1 | 0.131 |
| P × T | 1.455 | 1 | 0.230 |
| Rad × P × T | 1.482 | 1 | 0.225 |
DF: degrees of freedom; F: Fisher–Snedecor F statistic; p-value: level of significance.
Comparison of mean values for peripheral septa thickness revealed significant differences at different levels of radiation (p < 0.001), with a statistically significant interaction of radiation × time after exposure (p < 0.001). However, neither power level nor time after exposure showed significant effects on the thickness of the peripheral septa nor were there significant interactions between any two or all three factors of radiation, power level, and time after exposure (Table 5). Ninety minutes after exposure to both SAR levels (0.102 ± 12.10–3 or 0.429 ± 12.10–3), a statistically significant decrease in the thickness of the peripheral septa was observed with respect to non-radiated animals (p = 0.010 and p = 0.024, respectively). Radiated animals recovered septa thickness 24 h after exposure to 12 W (p < 0.05) and the difference between radiated and non-radiated animals disappeared, regardless of the SAR level applied. See Tables 6 and 7.
Table 5.
Results of three-way ANOVA (including radiation × power × time postirradiated) for peripheral septa thickness in thyroid gland
| Effects | F | DF | P-Value |
|---|---|---|---|
| Rad | 24.262 | 1 | <0.001 |
| P | 1.398 | 1 | 0.239 |
| T | 2.431 | 1 | 0.121 |
| Rad × P | 1.009 | 1 | 0.317 |
| Rad × T | 5.394 | 1 | 0.022 |
| P × T | 0.569 | 1 | 0.452 |
| Rad × P × T | 0.869 | 1 | 0.353 |
DF: degrees of freedom; F: Fisher–Snedecor F statistic; p-value: level of significance.
Group C animals compared to group A animals exposed to 3 W power
Results of analysis of thyroid tissues showed decreased size in the central and peripheral follicles of animals subjected to repeated exposure with respect to animals subjected to single, acute exposure at a similar SAR level. However, the septa thickness did not appear to be altered in animals that underwent repeated exposure. The results of two-way ANOVA for radiation × number of exposures are as follows:
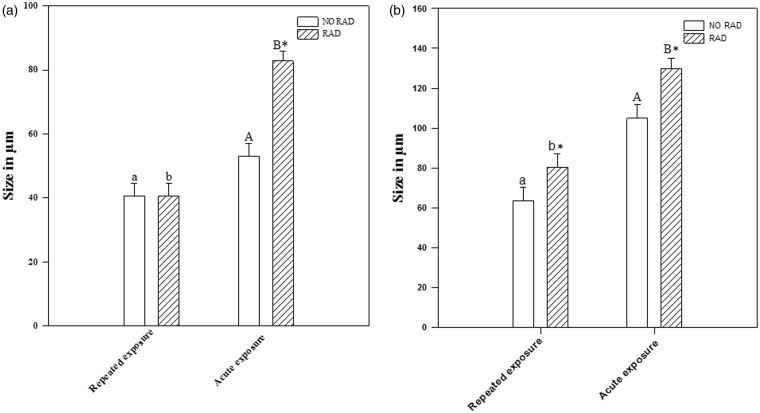
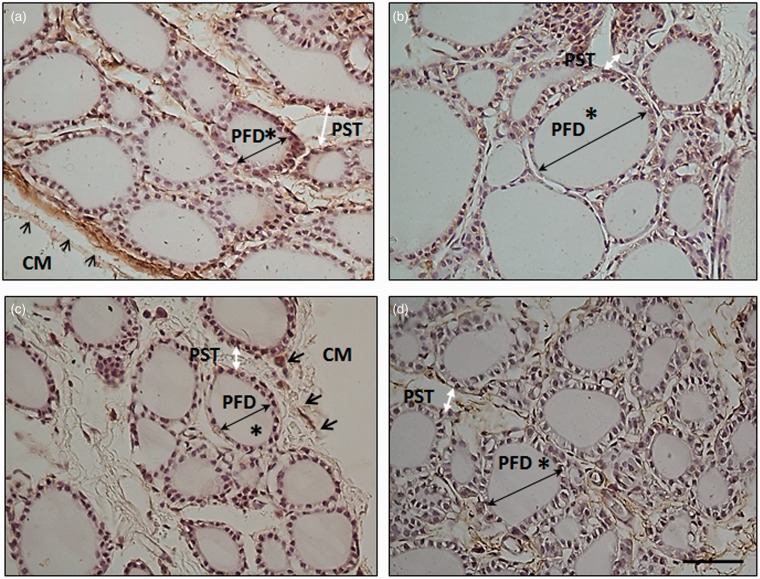
Radiation increased the diameter of the central follicles after single, acute exposure (p < 0.001), but not after repeated exposure. There were no significant differences between animals exposed repeatedly to radiation and non-radiated animals (p = 0.984), but a significant increase was observed in the central follicle diameters of animals exposed to a single, acute dose of radiation, compared to non-radiated animals (p < 0.001). See Figures 3(a) and 6.
Figure 3.
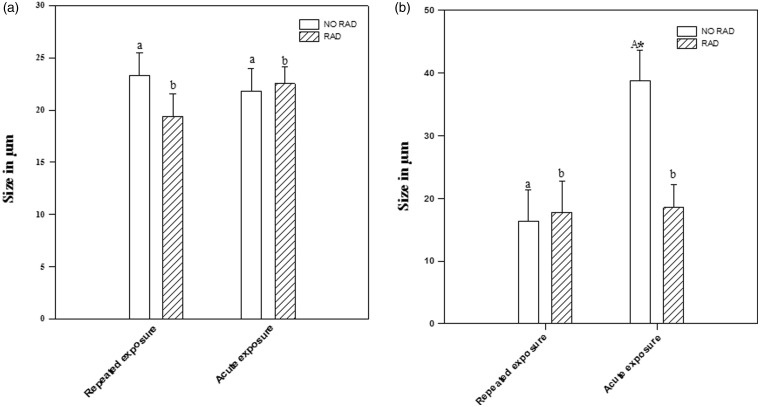
No significant differences were detected in the size of the central follicle diameter, but there was a significant size increase in the diameter of the peripheral follicle between non-radiated animals and those radiated with repeated exposure. In both cases, central and peripheral follicle diameters are greater with acute than with repeated exposure. Values of (a) central follicle diameter (CFD) and (b) peripheral follicle diameter (PFD) in non-radiated (NO RAD) and radiated (RAD) rats in group C (repeated exposure) and group A (acute single exposure). Asterisk * indicates statistically significant differences between radiated/non-radiated; a, b indicate statistically significant differences between repeated exposure and single exposure rats (p < 0.05, two-way ANOVA, followed by Tukey’s test)
Figure 6.
Photographs of rat thyroid tissue with HSP-90 inmunohistochemistry show increased size of peripheral follicles after single and repeated exposure. Photos a and b: animals euthanized 90 min after exposure to 0 W (control) and 3 W, respectively. c and d: animals two weeks after repeated exposure to 0 W (control) and 3 W. Asterisk * indicates peripheral follicle diameter (PFD), single arrows indicate capsular membrane (CM), double white arrows indicate peripheral septa thickness (PST); magnification 40×, calibration bar = 50 µm. (A color version of this figure is available in the online journal.)
The effect of radiation was clearly evident in the increased size of the peripheral follicles in animals subjected to single and repeated exposure, when compared to non-radiated animals (p = 0.008 and p = 0.043, respectively). The effect of radiation on peripheral follicles was more intense in single than in repeated exposure (p < 0.001). See Figures 3(b) and 6.
Radiation had no appreciable effect on the septa thickness in the central connective tissue in single or repeated exposure. No significant differences were observed between radiated and non-radiated animals (p = 0.151). See Figures 4(a) and 6.
Figure 4.
No significant differences were observed in central or peripheral septa thickness between non-radiated animals and those radiated with repeated exposure. Reduced size was only observed in the peripheral septa of animals subjected to acute exposure. The histograms represent the values of (a) central septa thickness (CST), and (b) peripheral septa thickness (PST) in no radiated rats (NO RAD) and radiated rats (RAD) of group C, with repeated exposure and group A with acute exposure. *Means statistically significant enter radiated/no radiated and a, b means statistically significant enter repeated exposure and acute exposure rats (p < 0.05, two-way ANOVA followed by Tukey’s test
In the peripheral septa of the animal thyroid glands, there were no significant effects from repeated exposure to radiation (p = 0.854). However, peripheral septa thickness in animals exposed to a single, acute dose was inferior to that of non-radiated animals (p = 0.005). See Figures 4(b) and 6.
Distribution of HSP-90 immunohistochemical markers in the thyroid gland
Distribution of HSP-90 expression was studied in the connective tissue of the capsular and interlobular membranes and in the follicular and parafollicular cells of the thyroid. In thyroid gland exposed to acute EMF radiation at 2.45 GHz, the distribution of HSP-90 expression varied as a function of the power applied. The color intensity was quantitatively determined for each of the HSP-90 immunohistochemistry preparations for thyroid between groups A and B and groups A and C, to establish the statistically significant differences by one-way or two-way ANOVA. Immunomarking indicated two levels of distribution of HSP-90 expression in the thyroid gland: (a) in the septa of the supportive structure of the gland, forming part of the connective tissue fibers and in the follicular and capsular membranes; (b) in the cytoplasm of the follicular and parafollicular cells. Color intensity decreased with SAR level or repeated exposure.
The statistically significant differences were as follows:
HSP-90 expression in animals subjected to single exposure appeared to be more intense in the capsular and interlobular connective tissue 90 min after radiation (group A) at 0.102 ± 12.10–3 SAR (p = 0.025), but evidence of this diminished when 0.429 ± 12.10–3 SAR was applied (p ≤ 0.05) (Table 8, Figure 5). Twenty-four hours after radiation, group B presented very low expression at 12 W (Table 8). Group C animals exposed repeatedly to microwave radiation presented decreased HSP-90 expression in both capsular and interlobular connective tissue with respect to animals exposed once (p ≤ 0.001) (Table 9, Figure 6).
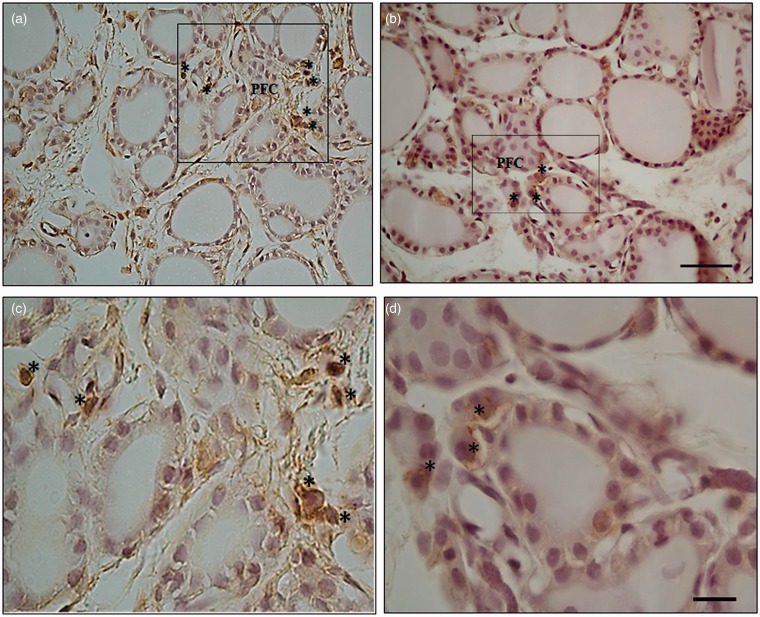
In group A, measurements taken 90 min after radiation showed expression of an immunopositive marker for HSP-90 in the follicular and parafollicular cells after exposure to 0.102 ± 12.10–3 SAR. However, expression of this protein was weak after exposure to 0.429 ± 12.10–3 SAR. Expression of HSP-90 in follicular and parafollicular cells was very low 24 h after single-dose radiation in group B at both SAR levels. Expression was virtually null in animals subjected to repeated exposure (group C). See Figure 7.
Table 8.
Measure of color intensity in samples taken 90 min or 24 h after acute exposure
| Time postexposure | Power (P) |
||
|---|---|---|---|
| 0 W | 3 W | 12 W | |
| 90 min | 148 ± 2 | 152 ± 1* | 144 ± 2* |
| 24 h | 145 ± 2 | 151 ± 1* | 139 ± 1* |
The optical density values were compared by one-way ANOVA and differences between power levels with an a posteriori Holm–Sidak test. For multiple comparisons, differences were considered significant at p ≤ 0.05. * Indicates significant differences between power P = 0 and P = 3 or 12 W.
Table 9.
Measure of color intensity on samples after single/repeated exposure
| Non-irradiated/irradiated | Number of exposure |
|
|---|---|---|
| Single | Repeated exposure | |
| Control | 148 ± 2 | 141 ± 2 |
| Irradiated | 152 ± 1*,$ | 141 ± 1 |
The optical density values were compared two-way ANOVA differences between number of exposure × non-irradiated/irradiated rats and a posteriori Bonferroni test. For multiple comparisons, differences considered significant at p ≤ 0.05.
Indicates significant differences between single control and irradiated animals.
Indicates significant differences between animals subjected to single and repeated exposure.
Figure 7.
Photographs of rat thyroid tissue with HSP-90 inmunohistochemistry show differences in expression in parafollicular cells in animals euthanized 90 min after exposure to 3 and 12 W at 40× magnification (photos a and c) and at 100× magnification (photos b and d). Asterisks * indicate parafollicular cells (PFC). Calibration bars (a, c) = 40 µm; (b, d) = 20 µm. (A color version of this figure is available in the online journal.)
Discussion
In this study using an experimental diathermy model on Sprague-Dawley rats, we found that the interaction of non-ionizing radiation at a frequency of 2.45 GHz caused modifications in the morphology of the thyroid gland tissue and in the distribution of the constituent cellular stress protein known as HSP-90. The morphology of the thyroid gland underwent the following changes due to radiation:
The size of central and peripheral follicles increased and the thickness of the peripheral septa decreased 90 min after single exposure. After 24 h, central follicles had decreased in size, but hypertrophy was still present in the peripheral follicles of thyroid gland exposed to the higher SAR level.
Repeated stimulus of the thyroid gland at the lower SAR level triggered adaptation and an increase in the size of peripheral follicles.
The observed localization of the expression of this protein in the supportive tissue of the septa, specifically in the fibers and in the capsular and lobular membranes suggests that this stress protein constitutes an important component of glandular architecture and is probably dedicated to maintaining glandular structure and morphology. The distribution of HSP-90 in thyroid membranes and cells was diminished after single (if the SAR and time after radiation increased) and repeated exposure to radiation.
Our work describes for the first time the effects of single and repeated exposure to 2.45 GHz RF on the morphology of Sprague-Dawley rat thyroid gland. Published studies to date have described histopathological alterations in thyroid tissue of experimental animals exposed to extremely low frequency (ELF) (50 Hz)22,23 or in thyroid hormone levels7,9,10 in humans or animals exposed at ELF or RF.
We chose to experimentally examine small animals at 2.45 GHz RF because of the wide range of potential applications, from therapeutics to tissue diathermy (this frequency resonates with H2O, facilitating greater penetration) to telecommunications involving WIFI, UMTS, or Bluetooth. We used subthermal SAR levels of 0.102 ± 12.10–3 and 0.429 ± 12.10–3 W/kg at 2.45 GHz in the right front leg, near the thyroid gland, to ensure that the non-ionizing radiation would not cause direct thermal effects to the gland.24 Research of this type requires immobilization of the animal, which itself has been found to generate a certain amount of stress.14,25 It must also be noted that radiation can catalyze single or repetitive activation of different neuron populations in rat hypothalamus,13,26 which intervene in the HPT axis. We cannot therefore assume that the effects of non-ionizing radiation to the thyroid are limited to its tissues; it must be treated as part of a system with multiple, interacting entry points. Other studies have described how microwave radiation at 2.45 GHz affects brain physiopathology and provokes changes in cerebral functioning and behavior. In the present study, the thyroid system is directly or indirectly affected by alterations in the HPT axis as well as by biochemical changes in the thyroid itself due to exposure to microwaves.7
The follicles are a functional unit of the thyroid and their size varies according to biological activity. However, follicle size is not uniform: peripheral follicles are larger than central follicles due to different amounts of colloid.27 The increase in the size of peripheral and/or central follicles after single or repeated exposure to radiation is a significant finding that is corroborated by other authors. Rajkovic et al.23 and Eşmekaya et al.28 described hypothyroidism or follicular increase at the expense of colloid in animals exposed to RF or ELF for 2–3 months. This might be explained by a transitory state of inactivity in the rat thyroid due to the radiation, which sets off a chain reaction to suppress the elimination or replacement of thyroglobulin in colloid with a compensatory increase in the follicular diameter and/or hypothyroidism.29 The increase in peripheral follicle size after single or repeated exposure to 2.45 GHz indicates that radiation can penetrate and stimulate the thyroid, which could be harmful. If it were to persist, it could alter functioning, which has recently been described by other authors.23,28
Experimental morphological studies of the thyroid gland in the aging process have reported an association between increased follicle size and decreased thyroid function in physiological conditions.30 However, exogenous participation was found to be involved in the transitory imbalance that appears in the autocrine regulation of the thyroid follicles in some physiological states and thyroid pathologies.31 This suggests that non-ionizing radiation could constitute another environmental toxin that affects the morphological functioning of the thyroid gland.32 Deficient thyroid functioning has potentially serious repercussions: it can provoke cardiovascular alterations in adults,33 affect fetal–neonatal development in humans34 and rodent models,35 or give rise to anomalous development in the brain.36
Recent research has described how EMFs can constitute external sources for the formation of free radicals in blood cells,37 the brain,38 spermatozoids,39 and myocardial tissue.40 The thyroid gland is by nature an oxidative organ, and when additional oxidative abuse is caused by exogenous pro-oxidants (ionizing radiation would be the most significant), damage to the macromolecules in the gland increases, possibly leading to thyroid pathology or cancer.41 In spite of this, a direct relation between thyroid cancer and exposure to EMFs has not yet been established.42 However, the search is ongoing for biomarkers in thyroid diseases that would make early detection, diagnosis, and intervention possible. HSP-90 is physiologically essential in cellular processes such as hormone signaling and control, proliferation, and differentiation of the cellular cycle.43 In prior studies, we described a decrease in HSP-90 and 70 due to acute radiation at 2.45 GHz in the thyroid gland, with no apparent effect in the apoptotic activity of thyroid cells.14 HSP-90 is known to play a modulatory role against thyroid cancer11 due to its primarily antiapoptotic function.12 In the present work, we have observed how, after 30 min exposure, the immunoreactivity of HSP-90 is histologically distributed throughout the thyroid gland in places where kinase proteins had previously been activated,44 between the capsular and lobular membranes and in the follicular and parafollicular cells. Other experiments have described how exposure to EMFs causes morphological alterations in the thyroid gland, affecting the epithelium, connective tissue, follicular and interfollicular cells, and mastocytes.22,23,45 The qualitative distribution of HSP-90 markers (in membranes and cytoplasm of follicular and parafollicular cells) in key functional and histological areas of the thyroid gland indicates that it is not randomly situated but rather it is distributed in such a way as to preserve the integrity of the colloid and membranes in conditions of cellular stress. Immunohistochemical expression of this protein in cells and membranes diminishes as power increases or with repeated exposure. This leads us to think that the cellular stress response is probably related to the combined activity of other systems that interact alternatively to produce a synergistic effect in some cases and an antagonistic effect in others.46 Misa et al.14 used rectal temperature to quantify stress in animals before and after radiation, finding stress to be evident only at the highest SAR level. Here we should keep in mind the balance between thyroid hormone stimulation caused by microwaves and that due to the negative impact of stress caused by immobilization.25 Both are provoked by cortisol and possibly by estrogens,18 which are related to HSPs and can influence cellular stress balance in the thyroid.
Repeated exposure to radiation intensifies stress in the immobilized animals, and signs of facilitation in the HPT axis have been observed in response to inhibitory feedback due to elevated corticoid levels.13 After repeated exposure to radiation, the thyroid gland exhibited significant hypertrophy of peripheral follicles when compared to control animals, and peripheral follicles were significantly smaller in size than in animals exposed to a single dose. The effect of radiation seemed to be less manifest in gland morphology as the animal became accustomed to repeated exposure. Contrary to this, cellular damage in the thyroid gland was directly related to the SAR level and/or number of exposures applied to the tissue. The parafollicular or C cells are responsible for regulation of the follicular cells, facilitating the interrelation of the two endocrine populations.47 The number and distribution of the parafollicular or C cells in the thyroid gland can be modified and is occasionally contradictory. Hypothyroidism, for example, is characterized by decreased numbers of parafollicular cells,48 but hypothyroidism in expectant mothers has been found to cause an increase in the size of parafollicular cells in newborns.49 Harmful environmental effects due to microgravity or hypergravity in outer space are known to provoke a decrease in the number of parafollicular cells,50 while low frequency EMFs increase their numbers.45 In the present experiment, exposure of rat thyroid gland to RF at 2.45 GHz and 0.102 ± 12.10–3 SAR increased HSP-90 marking in the parafollicular cells. However, HSP-90 stress immunomarking decreased in the parafollicular cells at 0.429 ± 12.10–3 SAR or with repeated exposure (see Figure 7). HSP-90 in the parafollicular cell is sensitive to the nature and intensity of radiation stimulus, which can modify cellular function and serve as a biomarker for cellular damage.
Conclusions
Thyroid gland exposed to 2.45 GHz radiation in this experimental model of diathermy in rats presented the following visible morphological effects: (a) glandular hypertrophy in relation to the SAR and/or number of exposures; (b) modification of the distribution of HSP-90 associated with membranes and parafollicular cells. These effects might not be exclusively or directly produced by radiation and can be included with other indirect effects from the hypothalamus. However, further research is needed to ascertain whether the continued effect of this physical agent could provoke pathology in the thyroid gland.
Acknowledgements
The authors are grateful to the Ministerio de Economía y Competitividad for funding awarded in Project TEC-2011-24441. We also greatly appreciate the assistance provided by Jose Carlos Santos, Rafael Fuentes, Isabel Tarrío, Eva Dominguez, and Eva García.
Footnotes
Minimum time exposure was tested previously.
Power levels of 3 and 12 W were used in this experiment, as they are multiples of the initial frequency (×2 or ×8); 12 W is the maximum power of the amplifier.
Authors’ contributions
MJM-A performed the research, operations, and did the histology. TJ-M was involved in all experiments. FJJ-B reviewed the histological data. JS-Q reviewed the paper and acted as consultant for the study. EM-P reviewed the electromagnetic part of the research. FJA-P designed the electromagnetic study and analyzed the electromagnetic data. EL-M analyzed all experimental data, wrote the paper, and supervised all experiments.
References
- 1.Pilla A, Fitzsimmons R, Muehsam D, Wu J, Rohde C, Caspe D. Electromagnetic fields as first messenger in biological signaling: application to calmodulin-dependent signaling in tissue repair. Biochim Biophys Acta 2011; 1810: 1236–45. [DOI] [PubMed] [Google Scholar]
- 2.Pilla A. Electromagnetic fields instantaneously modulate nitric oxide signalingin challenged biological systems. Biochem Biophys Res Commun 2012; 426: 330–3. [DOI] [PubMed] [Google Scholar]
- 3.Pall Martin L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med 2013; 17: 958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambucci M, Laudisi F, Nasta F, Pinto R, Lodato R, Altavista P, Lovisolo GA, Marino C, Pioli C. Prenatal exposure to non-ionizing radiation: effects of WiFi signals on pregnancy outcome, peripheral B-cell compartment and antibody production. Radiat Res 2010; 174: 732–40. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Búrdalo M, Martín A, Sanchis A, Villar R. FDTD assessment of human exposure to electromagnetic fields from WiFi and bluetooth devices in some operating situations. Bioelectromagnetics 2009; 30: 142–51. [DOI] [PubMed] [Google Scholar]
- 6.Hansson Mild K, Hand J, Hietanen M, Gowland P, Karpowicz J, Keevil S, Lagroye I, van Rongen E, Scarfi MR, Wilén J. Exposure classification of MRI workers in epidemiological studies. Bioelectromagnetics 2013; 34: 81–84. [DOI] [PubMed] [Google Scholar]
- 7.Sinha RK. Chronic non-thermal exposure of modulated 2450 MHz Microwave radiation alters thyroid hormones and behavior of male rats. Int J Radiat Biol 2008; 84: 505–13. [DOI] [PubMed] [Google Scholar]
- 8.Sinha RK, Aggarwal Y, Upadhyay PK, Dwivedi A, Keshri AK, Das BN. Neural network-based evaluation of chronic non-thermal effects of modulated 2450 MHz microwave radiation on electroencephalogram. Ann Biomed Eng 2008; 36: 839–51. [DOI] [PubMed] [Google Scholar]
- 9.Mortavazi S, Habib A, Ganj-Karami A, Samimi-Doost R, Pour-Abedi A, Babaie A. Alterations in TSH and thyroid hormones following mobile phone use. Oman Med J 2009; 24: 274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyu A, Cesur G, Ozguner F, Akdogan M, Mollaoglu H, Ozen S. Effects of 900 MHz electromagnetic field on TSH and thyroid hormones in rats. Toxicol Lett 2005; 157: 257–62. [DOI] [PubMed] [Google Scholar]
- 11.Samadi A, Loo P, Mukerji R, O’Donnell G, Tong X, Timmermann BN, Cohen MS. A novel HSP90 modulator with selective activity against thyroid cancers in vitro. Surgery 2009; 146: 1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JW, Yeh MW, Wong MG, Lobo M, Hyun WC, Duh QY, Clark OH. The heat shock protein 90-binding geldanamycin inhibits cancer cell proliferation, down-regulates oncoproteins, and inhibits epidermal growth factor-induced invasion in thyroid cancer cell lines. J Clin Endocrinol Metab 2003; 88: 3346–53. [DOI] [PubMed] [Google Scholar]
- 13.Jorge-Mora T, Misa-Agustiño MJ, Rodríguez-González JA, Jorge-Barreiro FJ, Ares-Pena FJ, López-Martín E. The effects of single and repeated exposure to 2.45 GHz radiofrequency fields on c-Fos protein expression in the paraventricular nucleus of rat hypothalamus. Neurochem Res 2011; 36: 2322–32. [DOI] [PubMed] [Google Scholar]
- 14.Misa Agustiño MJ, Leiro JM, Jorge Mora MT, Rodríguez-González JA, Jorge Barreiro FJ, Ares-Pena FJ, López-Martín E. Electromagnetic fields at 2.45 GHz trigger changes in heat shock proteins 90 and 70 without altering apoptotic activity in rat thyroid gland. Biol Open 2012; 151: 831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 2002; 283: 281–3. [DOI] [PubMed] [Google Scholar]
- 16.Real decreto 1201/2005. 21 Octubre 2005 BOE n 252 pp.34391–2.
- 17.Directiva 20110/63/UE del Parlamento Europeo y del Consejo 22 de septiembre 2010. Relativa a la protección de los animales utilizados con fines científicos. Diario oficial de la Unión Europea 22 de septiembre 2010; 276: 33–79. [Google Scholar]
- 18.Santin AP and Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res 2011;1-7:875125. [DOI] [PMC free article] [PubMed]
- 19.Jorge-Mora T, Alvarez Folgueiras M, Leiro-Vidal JM, Jorge-Barreiro FJ, Ares-Pena FJ, López-Martin E. Exposure to 2.45 GHz microwave radiation provokes cerebral changes in induction of HSP-90 a/b heat shock protein in rat. PIER 2010; 100: 351–79. [Google Scholar]
- 20.Schmid & Partner Engineering AG. Reference manual for the SEMCAD simulation plat-form for electromagnetic compatibility, antenna design and dosimetry, www.semcad.com, 2009.
- 21.Schaffner Electrotest Gmbh. GTEM Test Cells, Datasheet 2005.
- 22.Rajkovic V, Matavulj M, Johansson O. Light and electron microscopic study of the thyroid gland in rats exposed to power-frequency electromagnetic fields. J Exp Biol 2006; 209: 3322–8. [DOI] [PubMed] [Google Scholar]
- 23.Rajkovic V, Matavulj M, Gledic D, Lazetic B. Evaluation of rat thyroid gland morphophysiological status after three months exposure to 50 Hz electromagnetic field. Tissue Cell 2003; 35: 223–31. [DOI] [PubMed] [Google Scholar]
- 24.Adair ER, Black DR. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics 2003Suppl 6): S17–38. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez-Mariscal M, Sánchez E, García-Vázquez A, Rebolledo-Solleiro D, Charli JL, Joseph-Bravo P. Acute response of hypophysiotropic thyrotropin releasing hormone neurons and thyrotropin release to behavioral paradigms producing varying intensities of stress and physical activity. Regulat Pept 2012; 179: 61–70. [DOI] [PubMed] [Google Scholar]
- 26.Novikova NS, Kazakova TB, Rogers V, Korneva A. Expression of c-Fos gene in the rat hypothalamus in electrical pain stimulation and UHF stimulation of the ski. Neurosci Behav Physiol 2008; 38: 415–20. [DOI] [PubMed] [Google Scholar]
- 27.Hartoft-Nielsen ML, Rasmussen ÅKU, Feldt-Rasmussen U, Buschard K, Bock T. Estimation of number of follicles, volume of colloid and inner follicular surface area in the thyroid gland of rats. J Anat 2005; 207: 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eşmekaya MA, Seyhan N, Ömeroğlu S. Pulse modulated 900 MHz radiation induces hypothyroidism and apoptosis in thyroid cells: a light, electron microscopy and immunohistochemical study. Int J Radiat Biol 2010; 86: 1106–16. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Kawashima A, Yoshihara A, Akama T, Sue M, Yoshida A, Kimura HJ. Role of thyroglobulin on negative feedback autoregulation of thyroid follicular function and growth. J Endocrinol 2011; 209: 169–74. [DOI] [PubMed] [Google Scholar]
- 30.Kmieć Z, Kotlarz G, Smiechowska B, Myśliwski A. The effect of fasting and refeeding on thyroid follicle structure and thyroid hormone levels in young and old rats. Arch Gerontol Geriatr 1998; 26: 161–75. [DOI] [PubMed] [Google Scholar]
- 31.Ohye H, Sugawara M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med 2010; 235: 424–33. [DOI] [PubMed] [Google Scholar]
- 32.Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol 2010; 79: 939–47. [DOI] [PubMed] [Google Scholar]
- 33.Lyn Patrick ND. Thyroid disruption: mechanisms and clinical implications in human health. Altern Med Rev 2009; 14: 326–46. [PubMed] [Google Scholar]
- 34.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. Int J Obstet Gynaecol 2013; 120: 681–94. [DOI] [PubMed] [Google Scholar]
- 35.Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, Carter WH, Jr, DeVito MJ. Thyroid-hormone-disrupting chemicals: evidence for dose-dependent additivity or synergism. Environ Health Perspect 2005; 113: 1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilber ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 2012; 33: 842–52. [DOI] [PubMed] [Google Scholar]
- 37.Lu YS, Huang BT, Huang YX. Reactive oxygen species formation and apoptosis in human peripheral blood mononuclear cell induced by 900 MHz mobile phone radiation. Oxid Med Cell Longev 2012;1--8:740280. [DOI] [PMC free article] [PubMed]
- 38.Jiang DP, Li J, Zhang J, Xu SL, Kuang F, Lang HY, Wang YF, An GZ, Li JH, Guo GZ. Electromagnetic pulse exposure induces overexpression of Beta amyloid protein in rats. Arch Med Res 2013; 44: 178–84. [DOI] [PubMed] [Google Scholar]
- 39.De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One 2009; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiray A, Tayefi H, Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Ozogul C. The effects of exposure to electromagnetic field on rat myocardium. Toxicol Ind Health 2013; 29: 418–25. [DOI] [PubMed] [Google Scholar]
- 41.Karbownik-Lewińska M, Kokoszko-Bilska A. Oxidative damage to macromolecules in the thyroid – experimental evidence. Thyroid Res 2012; 5: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lope V, Pérez-Gómez B, Aragonés N, López-Abente G, Gustavsson P, Floderus B, Dosemeci M, Silva A, Pollán M. Occupational exposure to ionizing radiation and electromagnetic fields in relation to the risk of thyroid cancer in Sweden. Scand J Work Environ Health 2006; 32: 276–84. [DOI] [PubMed] [Google Scholar]
- 43.Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif 2000; 33: 341–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sreedhar AS, Soti C, Csermely P. Inhibition of Hsp90: a new strategy for inhibiting protein kinases. Biochim Biophys Acta 2004; 1697: 233–42. [DOI] [PubMed] [Google Scholar]
- 45.Rajkovic V, Matavulj M, Johansson O. Histological characteristics of cutaneous and thyroid mast cell populations in male rats exposed to power-frequency electromagnetic fields. Int J Radiat Biol 2005; 81: 491–9. [DOI] [PubMed] [Google Scholar]
- 46.Kostoff RN, Lau CGY. Combined biological and health effects of electromagnetic fields and other agents in the published literature. Technol Forecast Soc Change 2013;80:1331--49.
- 47.Martín-Lacave I, Borrero MJ, Utrilla JC, Fernández-Santos JM, de Miguel M. C cells evolve at the same rhythm as follicular cells when thyroidal status changes in rats. J Anat 2009; 214: 301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zbucki RL, Winnicka MM, Sawicki B, Szynaka B, Andrzejewska A. Alteration of parafollicular (C) cells activity in the experimental model of hypothyroidism in rats. Folia Histochem Cytobiol 2007; 45: 115–12. [PubMed] [Google Scholar]
- 49.Usenko VS, Lepekhin EA, Lyzogubov VV, Kornilovska IN, Apostolov EO. The influence of maternal hypothyroidism and radioactive iodine on rat embryonal development: thyroid C-cells. Anat Rec 1999; 256: 7–13. [DOI] [PubMed] [Google Scholar]
- 50.Albi E, Curcio F, Spelat R, Lazzarini A, Lazzarini R, Cataldi S, Loreti E, Ferri I, Ambesi-Impiombato FS. Loss of parafollicular cells during gravitational changes (microgravity, hypergravity) and the secret effect of pleiotrophin. PLoS One 2012; 7: e48518–e48518. [DOI] [PMC free article] [PubMed] [Google Scholar]