Abstract
Nicotine replacement therapy (NRT) improves the long-term success rate of smoking cessation, but induces oxidative stress and inflammatory responses that may delay the restoration of vascular endothelial function (VEF). No studies have examined co-therapy of NRT-assisted smoking abstinence with γ-tocopherol (γ-T), a vitamin E form with antioxidant and anti-inflammatory activities, on improvements in VEF. In a randomized, double-blind, placebo-controlled study, healthy smokers (25 ± 1 y old; mean ± SEM) received NRT and abstained from smoking for 24 h with placebo (n = 12) or oral administration of γ-T-rich mixture of tocopherols (γ-TmT; n = 11) that provided 500 mg γ-T. Brachial artery flow-mediated dilation (FMD), and biomarkers of nitric oxide metabolism, antioxidant status, inflammation, and lipid peroxidation [8-iso-prostaglandin F2α stereoisomers (8-iso-15(R)-PGF2α and 8-iso-15(S)-PGF2α)] were measured prior to and after 24 h of smoking abstinence. Smoking abstinence with NRT regardless of γ-TmT similarly decreased urinary naphthol (P < 0.05) without affecting plasma cotinine. γ-TmT increased plasma γ-T by 4-times and the urinary metabolite of γ-T, γ-carboxyethyl-chromanol, by three times. Smoking abstinence with γ-TmT, but not smoking abstinence alone, increased FMD without affecting plasma nitrate/nitrite or the ratio of asymmetric dimethylarginine/arginine. Urinary 8-iso-15(S)-PGF2α decreased only in those receiving γ-TmT and was inversely correlated to FMD (R = −0.43, P < 0.05). Circulating markers of inflammation were unaffected by smoking abstinence or γ-TmT. Short-term NRT-assisted smoking abstinence with γ-TmT, but not NRT-assisted smoking abstinence alone, improved VEF by decreasing 8-iso-15(S)-PGF2α, a vasoconstrictor that was otherwise unaffected by NRT-assisted smoking abstinence.
Keywords: Vascular endothelial function, vitamin E, smoking cessation, flow-mediated dilation, nicotine replacement therapy
Introduction
Cigarette smoking is the foremost preventable cause of premature mortality and a leading risk factor for cardiovascular disease (CVD).1 Despite the known risks, 22% of Americans continue to smoke. Vascular dysfunction, an early event leading to CVD, occurs in smokers2,3 in an oxidative stress-dependent manner2,4–8 due to reactive oxygen and nitrogen species (ROS/RNS) from cigarette smoke and activated inflammatory cells.9–12 Smoking abstinence decreases morbidity,13 but the success rate for quitting unassisted (i.e. ‘cold-turkey’) is 3–5%, and increases to 24% with the use of pharmacological aids and behavioral support.14 Although nicotine replacement therapy (NRT) curbs tobacco addiction,15 studies in rodents show that nicotine induces oxidative stress and inflammation16 and impairs vascular endothelial function (VEF) independent of cigarette smoking.17 Thus, complementary therapies that attenuate nicotine-induced ROS/RNS and vascular dysfunction are needed to fully realize the benefits of NRT-assisted smoking cessation.
Vitamin E describes eight lipophilic tocopherols (T) and tocotrienols.18 γ-T is the most abundant dietary form, and it and/or its physiologic metabolite γ-carboxyethyl-hydroxychroman (γ-CEHC) has anti-oxidative, anti-nitrative, anti-inflammatory, and vasoprotective activities that have received limited attention compared to α-T, the major circulating form of vitamin E.19–22 Short-term administration of a γ-T-rich mixture of tocopherols (γ-TmT) during smoking cessation in the absence of NRT decreases TNFα and myeloperoxidase (MPO) while improving VEF beyond that of smoking cessation alone.21 We therefore hypothesized that improvements in γ-T status during NRT-assisted smoking abstinence would improve VEF by decreasing oxidative stress and inflammation. We measured brachial artery flow-mediated dilation (FMD), and oxidative stress and inflammatory markers, during a randomized, double-blind, placebo-controlled study examining NRT-assisted smoking abstinence with γ-TmT administration.
Methods
Study design
This protocol was approved by the Institutional Review Boards at University of Connecticut and The Ohio State University. Healthy male and female cigarette smokers (≥10 cigarettes/day; ≥1 year) were randomized to abstain from smoking with the use of NRT while taking placebo (n = 12) or γ-TmT (n = 11) for 24 h. Transdermal nicotine patches (NicoDerm CQ Step 1; GlaxoSmithKline, Philadelphia, PA, USA) provided 20 mg nicotine for 24 h. Placebo was a corn oil capsule (0.07 mg α-T and 0.18 mg γ-T) and γ-TmT contained 500 mg γ-T, 62 mg α-T, 24 mg β-T and 6 mg δ-T (provided by Dr. Jose Llobrera, Designs for Health, Inc., Suffield, CT, USA). Participants visited the study center in the fasted state (10–12 h) prior to (pre) and after 24 h of smoking abstinence (post). They ingested placebo or γ-TmT capsules with dinner the night prior to their post-visit to achieve near-maximal plasma γ-T concentrations during their visit.23 FMD assessment, and plasma and 24 h urine collection were performed as described.21 Lastly, participants completed four-day food records for three days prior to their pre-visit and 24 h prior to their post-visit. These were analyzed using the 2010 Nutrition Data System for Research (University of Minnesota, Minneapolis, MN, USA).
Flow-mediated dilation and carotid artery intima media thickness
Brachial artery FMD was assessed by high-frequency ultrasonography.21 Brachial artery FMD is expressed as change from baseline (Δ mm; post-occlusion peak diameter – baseline diameter) and percent change from baseline (%; Δ mm/baseline diameter (mm) × 100). Statistical analysis for absolute (Δ mm) and relative (%) FMD responses was performed in parallel and the results did not differ qualitatively. For simplicity, only results for FMD (%) are reported for regression analyses. To control for possible confounding factors, FMD measurements for each participant were performed at the same time of day during the pre- and post-visits, and participants were required to avoid exercise, caffeine, and alcohol for 24 h prior to any FMD measurements. Additionally, FMD measurements for female participants were completed between days 7 and 14 following the completion of menses to account for changes in vascular reactivity that occur throughout the menstrual cycle.24 Finally, to define atherosclerotic risk, carotid intima media thickness (cIMT) was assessed at pre-visit as described.21
Nitric oxide (NO•) metabolism
Plasma arginine, the substrate for NO• synthase (NOS)-mediated NO• synthesis, and asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of NOS, were measured by HPLC-FL.25 Plasma nitrate and nitrite (NOx), the end-metabolites of NO•, were measured by colorimetric assay (Cayman Chemical, Ann Arbor, MI, USA).
Plasma cotinine and urinary naphthol
Plasma cotinine21 and urinary 2-naphthol,26 the metabolite of naphthalene, a polycyclic aromatic hydrocarbon found in cigarette smoke,27 were measured by HPLC-FL.
Antioxidants, oxidative stress, and inflammation
Plasma vitamin E (as α- and γ-T) and urinary α- and γ-CEHC were measured by UHPLC-MS methods, and plasma vitamin C and uric acid by HPLC-Coularray.21 CEHCs were normalized to urinary creatinine, which was measured by clinical assay (Pointe Scientific, Canton, MI, USA). Urinary 8-iso-prostaglandin F2α stereoisomers (8-iso-15(R)-PGF2α and 8-iso-15(S)-PGF2α) and the metabolites of 8-iso-15(S)-PGF2α, 2,3-dinor-5,6-dihydro-8-iso-PGF2α (2,3-dinor-F1) and 2,3-dinor-8-iso-PGF2α (2,3-dinor-F2) were measured by LC-MS/MS.28 Plasma malondialdehyde (MDA) was measured by HPLC-FL.21 Plasma-oxidized LDL (oxLDL; Mercodia, Winston Salem, NC, USA), MPO, C-reactive protein (CRP; BioCheck, Foster City, CA, USA), soluble intracellular adhesion molecule-1 (sICAM-1), and monocyte chemoattractant protein-1 (MCP-1; R&D Systems, Minneapolis, MN, USA) were measured by ELISA.
Statistical analysis
A power calculation was performed using FMD responses to determine appropriate sample sizes (Power and Sample Size Calculation Version 3.0.43). In the absence of studies examining γ-T during NRT-mediated smoking cessation, we estimated an additional 1% increase in FMD responses following γ-TmT supplementation in combination with smoking cessation. This increase is of physiological relevance because a 1% increase in FMD corresponds to a 14% decrease in future CVD events.24 Based on a power calculation of previously published data of FMD responses during smoking cessation,29 we estimated that a minimum of eight participants per group would be needed to detect differences with 80% power (P < 0.05).
Data (means ± SEM) were analyzed by SPSS Version 15.0 (IBM, Armonk, NY, USA). Student’s independent t-tests were used to evaluate pre-data between placebo and γ-TmT groups. Initial analysis was performed using three-way repeated measures ANOVA to define main effects for gender, time, γ-TmT, and their interaction. Because no gender effects were observed, two-way repeated measures ANOVA with Bonferroni correction was performed to assess effects due to time, γ-TmT, and their interaction. Pearson correlation coefficients (r) for study variables at pre-visit were determined by linear regression. Multiple linear regression, controlling for within-subject repeated measures, was used to calculate correlation coefficients (R) as described.30 P ≤ 0.05 indicates statistical significance for all analyses.
Results
Participants and compliance
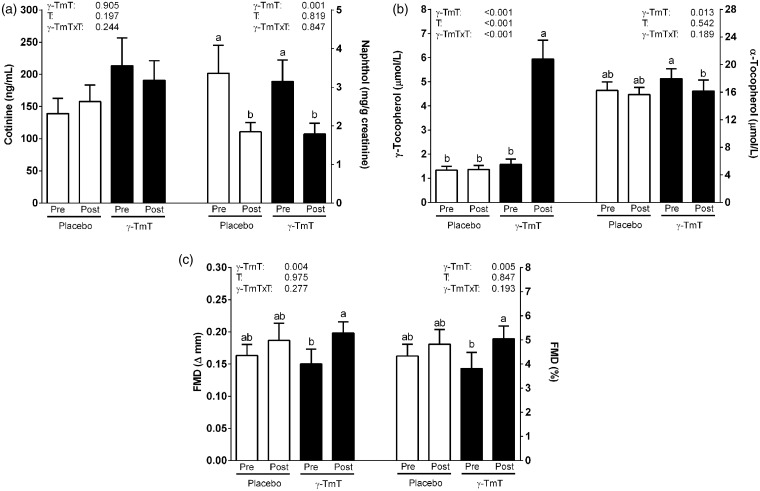
Participants were generally healthy despite their smoking history (Table 1) and cIMT indicated no evidence of plaque or increased CVD risk.31 Baseline brachial artery diameter, FMD, smoking frequency, and smoking burden did not differ between groups (Table 1). Baseline plasma cotinine and urinary naphthol were also similar between groups and correlated to smoking burden (P < 0.05; r = 0.45–0.53). Cotinine was unaffected by smoking abstinence consistent with NRT administration, whereas naphthol decreased regardless of γ-TmT administration (Figure 1a). No group differences occurred in dietary intakes (Table 2).
Table 1.
Participant characteristics
| Placebo | γ-TmT | |
|---|---|---|
| M/F | 8/4 | 7/4 |
| Age (y) | 23.2 ± 1.4 | 26.5 ± 2.4 |
| Smoking frequency (cigarettes/day) | 15.2 ± 1.5 | 12.5 ± 1.5 |
| Smoking burden (pack-y) | 5.55 ± 1.42 | 5.95 ± 2.31 |
| Cotinine (nmol/L) | 139 ± 24 | 214 ± 43 |
| Naphthol (µmol/L) | 3.36 ± 0.73 | 3.15 ± 0.55 |
| Body mass index (kg/m2) | 25.6 ± 1.5 | 27.5 ± 1.1 |
| Systolic blood pressure (mm Hg) | 123 ± 3 | 125 ± 3 |
| Diastolic blood pressure (mm Hg) | 72 ± 2 | 74 ± 2.6 |
| Total cholesterol (mg/dL) | 156 ± 10 | 144 ± 8 |
| Triglyceride (mg/dL) | 79.9 ± 11.4 | 94.7 ± 17.6 |
| Glucose (mg/dL) | 89.4 ± 3.4 | 89.5 ± 2.3 |
| Blood urea nitrogen (mg/dL) | 12.8 ± 1.1 | 13.2 ± 0.7 |
| Creatinine (mg/dL) | 0.84 ± 0.04 | 0.85 ± 0.05 |
| Sodium (mEq/L) | 140 ± 0.4 | 141 ± 1 |
| Potassium (mEq/L) | 4.72 ± 0.10 | 4.47 ± 0.15 |
| Chloride (mEq/L) | 104 ± 1 | 104 ± 1 |
| Bicarbonate (mEq/L) | 20.6 ± 0.8 | 21.8 ± 0.6 |
| Calcium (mg/dL) | 9.45 ± 0.12 | 9.30 ± 0.11 |
| Albumin/globulin | 1.98 ± 0.08 | 2.11 ± 0.09 |
| Total bilirubin (mg/dL) | 0.97 ± 0.18 | 0.63 ± 0.09 |
| Alkaline phosphatase (U/L) | 69.5 ± 6.0 | 62.3 ± 5.2 |
| Aspartate aminotransaminase (U/L) | 20.0 ± 1.8 | 25.2 ± 3.1 |
| Alanine aminotransaminase (U/L) | 20.4 ± 5.5 | 31.7 ± 7.8 |
| Lactate dehydrogenase (U/L) | 187 ± 20 | 205 ± 24 |
| Baseline brachial artery diameter (mm) | 3.80 ± 0.14 | 4.06 ± 0.29 |
| Average left cIMT (mm) | 0.50 ± 0.02 | 0.49 ± 0.03 |
| Average right cIMT (mm) | 0.50 ± 0.03 | 0.50 ± 0.02 |
Note: No significant differences (P > 0.05) were observed between groups, and all participants had blood chemistry values that were within the normal clinical reference range. Values expressed as means ± SEM.
cIMT: carotid intima media thickness, γ-TmT: γ-tocopherol-rich mixture of tocopherols.
Figure 1.
Plasma cotinine and urinary naphthol, plasma tocopherols, and flow-mediated dilation (FMD) responses in healthy smokers following 24 h smoking abstinence with (n = 11) or without (n = 12) γ-tocopherol-rich mixture of tocopherols (γ-TmT). (a) Plasma α- and γ-tocopherol concentrations, (b) plasma cotinine and urinary naphthol concentrations, and (c) FMD responses expressed as absolute change and percent change from baseline diameter in smokers following smoking abstinence with or without oral administration of γ-TmT. T: time; γ-TmT × T: γ-TmT × time interaction. Bars are means ± SEM. Means not sharing a common superscript are significantly different (P < 0.05)
Table 2.
Dietary intakes from 4-day dietary records of smokers who abstained from smoking with (n = 11) and without (n = 12) oral administration of γ-tocopherol-rich mixture of tocopherols (γ-TmT) prior to the start of the study
| Placebo | γ-TmT | |
|---|---|---|
| Total energy (kcal) | 2217 ± 174 | 2268 ± 262 |
| Energy from carbohydrate (%) | 44.4 ± 2.5 | 44.8 ± 2.9 |
| Energy from protein (%) | 14.8 ± 0.9 | 14.7 ± 1.2 |
| Energy from fat (%) | 37.0 ± 2.2 | 39.9 ± 2.2 |
| Total saturated fat (g) | 31.2 ± 2.2 | 36.4 ± 5.0 |
| Monounsaturated fat (g) | 32.1 ± 2.5 | 34.3 ± 4.2 |
| Polyunsaturated fat (g) | 19.2 ± 1.5 | 23.5 ± 3.7 |
| Cholesterol (mg) | 251 ± 19 | 251 ± 35 |
| Vitamin A (RAE) | 5466 ± 2133 | 4930 ± 1744 |
| β-Carotene (µg) | 2511 ± 1308 | 2015 ± 941 |
| Vitamin C (mg) | 98.2 ± 21.5 | 76.8 ± 21.9 |
| Vitamin D (µg) | 3.4 ± 0.5 | 3.2 ± 0.7 |
| α-Tocopherol (mg) | 8.0 ± 0.9 | 10.1 ± 1.7 |
| γ -Tocopherol (mg) | 13.5 ± 1.5 | 15.9 ± 2.5 |
| Vitamin K (µg) | 64.4 ± 8.3 | 76.6 ± 13.2 |
| Magnesium (mg) | 256 ± 23 | 242 ± 24 |
| Selenium (µg) | 116 ± 9 | 102 ± 10 |
| Zinc (mg) | 11.1 ± 1.2 | 12.1 ± 2.0 |
| Sodium (mg) | 4307 ± 503 | 3788 ± 473 |
Note: Values are means ± SEM. No significant differences (P > 0.05) were observed between groups.
Plasma γ- and α-T, and urinary γ- and α-CEHC, were unaffected in participants receiving placebo, whereas γ-TmT administration increased γ-T (Figure 1b) and γ-CEHC (Table 3) by approximately 3–4 times. γ-TmT administration decreased α-T by 10% (Figure 1b), while increasing α-CEHC by 62% (Table 3).
Table 3.
Antioxidant status, oxidative stress and inflammation markers of smokers who abstain from smoking with (n = 11) and without (n = 12) oral administration of γ-tocopherol-rich mixture of tocopherols (γ-TmT) prior to (pre) and after 24 h smoking abstinence (post)
| Placebo |
γ-TmT |
T | γ-TmT | T × γ-TmT | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Arginine, ADMA, and NOx | |||||||
| Arginine (µmol/L) | 79.7 ± 4.2 | 81.8 ± 4.4 | 74.7 ± 3.7 | 73.0 ± 3.4 | 0.893 | 0.219 | 0.221 |
| ADMA (nmol/L) | 744 ± 49 | 742 ± 42 | 864 ± 52 | 874 ± 54 | 0.753 | 0.079 | 0.681 |
| ADMA/Arginine (nmol/µmol) | 11.5 ± 0.8 | 11.1 ± 0.7 | 11.3 ± 0.8 | 11.0 ± 0.8 | 0.166 | 0.896 | 0.787 |
| NOx (µmol/L) | 17.5 ± 2.5 | 14.2 ± 1.2 | 22.2 ± 3.0 | 18.3 ± 2.2 | 0.017 | 0.140 | 0.840 |
| Antioxidants and oxidative stress | |||||||
| α-CEHC (nmol/L) | 13.6 ± 3.0 | 12.1 ± 2.2 | 15.4 ± 3.3 | 25.0 ± 4.7* | 0.117 | 0.078 | 0.037 |
| γ-CEHC (nmol/L) | 69 ± 10 | 76 ± 10 | 106 ± 16 | 296 ± 61* | 0.012 | <0.01 | 0.018 |
| Vitamin C (µmol/L) | 44.2 ± 3.7 | 44.6 ± 3.7 | 43.0 ± 4.6 | 45.8 ± 5.0 | 0.391 | 0.999 | 0.502 |
| Uric Acid (µmol/L) | 306 ± 19 | 304 ± 20 | 289 ± 18 | 291 ± 17 | 0.993 | 0.573 | 0.757 |
| Malondialdehyde (µmol/L) | 1.08 ± 0.04 | 1.08 ± 0.05 | 1.06 ± 0.03 | 1.08 ± 0.04 | 0.794 | 0.798 | 0.551 |
| Oxidized LDL (U/L) | 48.8 ± 3.7 | 50.1 ± 3.9 | 61.9 ± 7.4 | 58.1 ± 5.3 | 0.486 | 0.153 | 0.152 |
| Total 8-iso-PGF2α (ng/g creatinine) | 523 ± 37 | 479 ± 37 | 676 ± 59 | 639 ± 71 | 0.181 | 0.030 | 0.905 |
| 8-iso-15(R)-PGF2α (ng/g creatinine) | 267 ± 26 | 250 ± 25 | 367 ± 41 | 396 ± 63 | 0.808 | 0.026 | 0.367 |
| 8-iso-15(S)-PGF2α (ng/g creatinine) | 256 ± 22 | 229 ± 20 | 309 ± 26 | 242 ± 24* | 0.005 | 0.257 | 0.200 |
| 2,3-dinor-F1 (ng/g creatinine) | 1431 ± 178 | 1275 ± 134 | 1507 ± 168 | 1551 ± 226 | 0.636 | 0.437 | 0.407 |
| 2,3-dinor-F2 (ng/g creatinine) | 2162 ± 166 | 2015 ± 174 | 2158 ± 339 | 2416 ± 322 | 0.663 | 0.566 | 0.124 |
| Inflammation | |||||||
| MPO (ng/mL) | 19.8 ± 2.5 | 15.9 ± 1.6 | 18.6 ± 1.9 | 17.8 ± 1.9 | 0.101 | 0.880 | 0.260 |
| CRP (mg/L) | 1.25 ± 0.46 | 1.28 ± 0.52 | 1.71 ± 0.64 | 1.79 ± 0.68 | 0.682 | 0.560 | 0.855 |
| MCP-1 (pg/mL) | 58.1 ± 4.0 | 53.7 ± 4.8 | 54.9 ± 3.7 | 53.0 ± 2.3 | 0.167 | 0.700 | 0.580 |
| sICAM-1 (pg/mL) | 10.7 ± 0.6 | 10.8 ± 0.7 | 11.5 ± 1.1 | 11.3 ± 1.2 | 0.630 | 0.622 | 0.342 |
Note: Values expressed as means ± SEM.
MPO: myeloperoxidase; CRP: C-reactive protein; MCP-1: monocyte chemoattractant protein-1; sICAM-1: soluble intracellular adhesion molecule-1; T: main effect of time; T × γ-TmT: time × γ-TmT interaction effect.
Significantly different from pre (P < 0.05).
Brachial artery flow-mediated dilation and NO• metabolism
Pre-occlusion brachial artery diameters at pre and post were unaffected by smoking abstinence and γ-TmT (placebo: 3.80 ± 0.14 vs. 3.82 ± 0.15 mm; γ-TmT: 4.06 ± 0.29 vs. 4.06 ± 0.28 mm). FMD increased only in participants receiving γ-TmT (Figure 1c). FMD was correlated to γ-T and γ-CEHC (R = 0.43–0.55, P < 0.05). Plasma arginine, ADMA, and ADMA/arginine, an index of NO• bioavailability,32 and plasma NOx were unaffected by smoking abstinence or γ-TmT (Table 3).
Antioxidants, oxidative stress, and inflammation
Plasma vitamin C, uric acid, MDA, oxLDL, and inflammatory markers (MPO, CRP, MCP-1, sICAM-1) were unaffected by smoking abstinence and γ-TmT (Table 3). Urinary 8-iso-15(S)-PGF2α, but not urinary 8-iso-15(R)-PGF2α, total 8-iso-PGF2α (sum of 8-iso-15(R)- and 8-iso-15(S)-PGF2α), or metabolites of 8-iso-15(S)-PGF2α (i.e. 2,3-dinor-F1 and 2,3-dinor-F2) decreased following smoking abstinence only in the γ-TmT group (Table 3). Urinary 8-iso-15(S)-PGF2α was also inversely correlated to FMD (R = -0.43, P < 0.05).
Discussion
This study demonstrated that short-term NRT-assisted smoking abstinence with γ-TmT, but not NRT-assisted smoking abstinence alone, improved FMD in association with lowered 8-iso-15(S)-PGF2α. γ-TmT administration during NRT-assisted smoking abstinence decreased urinary 8-iso-15(S)-PGF2α, suggesting that γ-TmT increases VEF by attenuating activities of this vasoconstrictor.33 γ-TmT increased plasma γ-T and urinary γ-CEHC without affecting pro-inflammatory proteins, suggesting that γ-TmT improves VEF independent of inflammation. These findings provide the first evidence that greater γ-T status improves VEF during smoking abstinence by specifically decreasing 8-iso-15(S)-PGF2α that was otherwise unaffected by smoking abstinence.
Nicotine, independent of cigarette smoking, impairs VEF.17 We showed that smoking cessation without NRT or γ-TmT increases FMD.21 In the present study, NRT was provided to maintain participants’ nicotine levels, and had no effect on FMD, suggesting that its short-term use limits smoking abstinence-mediated restoration of VEF. In contrast, γ-TmT use during NRT-assisted smoking abstinence improves VEF.
NRT-assisted smoking abstinence in combination with γ-TmT, but not NRT-assisted smoking abstinence alone, decreased urinary 8-iso-15(S)-PGF2α. This F2-isoprostane is generated by non-enzymatic free radical peroxidation of arachidonic acid, and is recognized as an oxidative stress biomarker and vasoconstrictor.33 Antagonism of thromboxane A2 (TXA2)/prostaglandin H2 (TP) receptors inhibit 8-iso-15(S)-PGF2α-induced vasoconstriction in vitro.33 Likewise, 8-iso-15(S)-PGF2α induces vasoconstriction in wild-type mice but not in TP-receptor-deficient mice.34 In CVD patients, circulating 8-iso-15(S)-PGF2α is increased35 and TP receptor antagonists improve their FMD responses.36,37 Although 8-iso-15(S)-PGF2α binds to TP receptors, the mechanism by which it induces vasoconstriction is unclear. 8-iso-15(S)-PGF2α is suggested to mediate endothelium-dependent vasoconstriction in porcine periventricular and retinal vessels by stimulating TXA2 release.38,39 Furthermore, inhibition of TXA2 synthase abolishes 8-iso-15(S)-PGF2α-induced vasoconstriction.38 In turn, TXA2 induces vasoconstriction by activating TP receptors on vascular smooth muscle cells (i.e. endothelium-independent) or by decreasing eNOS-mediated NO• synthesis.40 We showed that γ-TmT decreased 8-iso-15(S)-PGF2α without affecting ADMA/arginine or NOx, suggesting that γ-TmT likely improved VEF in a NO•-independent manner, although further study is needed to define the mechanisms involved. In addition, future studies should specifically measure the stereoisomer 8-iso-15(S)-PGF2α, because monitoring total 8-iso-PGF2α, which is more common, would have precluded any observation of γ-TmT-mediated decreases in 8-iso-15(S)-PGF2α as a possible explanation for improved VEF.
The mechanism by which γ-TmT decreased 8-iso-15(S)-PGF2α is unknown, but is likely independent of oxidative stress in the present study, consistent with the lack of decrease in oxLDL, MDA, 8-iso-15(R)-PGF2α, 2,3-dinor-F1, and 2,3-dinor-F2. Activity of 15-prostaglandin dehydrogenase, an enzyme that hydroxylates isoprostanes prior to β-oxidation,41 is upregulated in α-T-deficient rabbits.42 This suggests that γ-TmT-mediated decreases in α-T may have contributed to oxidative degradation of 8-iso-15(S)-PGF2α. Alternatively, decreases in 8-iso-15(S)-PGF2α may reflect γ-T-mediated inhibition of phospholipase A2, which hydrolyzes F2-isoprostanes from phospholipids.43,44 Regardless of the mechanism, which requires additional study, lowering of this vasoconstrictor would be expected to improve VEF, consistent with our observations.
The increasing use of brachial artery FMD to assess vascular function in clinical studies is attributed to its accuracy and sensitivity,45 and prognostic value for predicting future CVD events.24 However, subtle variations in FMD protocols critically impact FMD responses.46 To ensure accuracy and reliability of our FMD measurements, numerous variables known to affect FMD were standardized according to current guidelines (i.e. occlusion duration, cuff placement, and positioning of the ultrasound probe, diurnal variations, dietary supplements, medication, exercise, and menstrual phase).46 Although dietary intakes did not differ between treatment groups or visits, we cannot exclude possible effects of other vasoactive dietary components (e.g. nitrates and flavonoids) that are not included in food composition tables. Improvements in FMD in our study cannot be fully attributed to either γ-T or γ-CEHC alone because concentrations of both were increased by γ-TmT. We also cannot conclude whether improvements in FMD resulted from a synergistic or additive interaction between γ-TmT, smoking abstinence, and NRT. This study was specifically limited to young and healthy smokers to control for confounding factors affecting VEF, thus precluding any extrapolations to those with existing co-morbidities.
In conclusion, short-term NRT-assisted smoking abstinence with oral administration of γ-TmT, but not NRT-assisted smoking abstinence alone, improved VEF in association with decreases in the vasoconstrictor 8-iso-15(S)-PGF2α. Although NRT precluded the restoration of VEF by smoking abstinence at 24 h, FMD increases similarly following 1 year of smoking cessation with or without NRT,47 suggesting that long-term smoking cessation improves VEF, despite NRT use. Chronic γ-TmT administration with NRT has not been studied and warrants investigation to better define γ-TmT as a complementary strategy to restore cardiovascular health in former smokers.
ACKNOWLEDGEMENTS
The authors thank Ciara Beck, Natalie Faella, Stephanie Richards, Sarah Kranz, and Katherine Lainas for dietary assessment assistance, Lei Cao for recruitment assistance, and Scott Leonard for LC-MS technical assistance. This study was supported by the Connecticut Department of Public Health. Isoprostane analysis was supported by a training fellowship to EM from the Society for Free Radical Biology and Medicine. This trial was registered at www.clinicaltrials.gov as NCT01314443.
Authors’ contributions
RSB, JSV, EM, and CM were responsible for the study design. RSB, EM, RP, YG, CM, and KDB were responsible for collecting and analyzing data. KDB and JSV reviewed the FMD analyses. BAP assisted with cIMT analysis and AWT and MGT assisted with analysis of urinary isoprostanes. EM and RSB wrote the initial draft of the manuscript and all authors contributed to the editing and review of this manuscript.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the Surgeon General. In: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, eds. Atlanta, GA, 2014.
- 2.Amato M, Frigerio B, Castelnuovo S, Ravani A, Sansaro D, Tremoli E, Squellerio I, Cavalca V, Veglia F, Sirtori CR, Werba JP, Baldassarre D. Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis 2013; 228: 153–60. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 1996; 334: 150–4. [DOI] [PubMed] [Google Scholar]
- 4.Solak ZA, Kabaroglu C, Cok G, Parildar Z, Bayindir U, Ozmen D, Bayindir O. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin Exp Med 2005; 5: 99–105. [DOI] [PubMed] [Google Scholar]
- 5.Pilz H, Oguogho A, Chehne F, Lupattelli G, Palumbo B, Sinzinger H. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thromb Res 2000; 99: 209–21. [DOI] [PubMed] [Google Scholar]
- 6.Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 1992; 339: 883–7. [DOI] [PubMed] [Google Scholar]
- 7.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 1995; 332: 1198–203. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 2000; 86: E36–41. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J 2005; 26: 1765–73. [DOI] [PubMed] [Google Scholar]
- 10.Jefferis BJ, Lowe GD, Welsh P, Rumley A, Lawlor DA, Ebrahim S, Carson C, Doig M, Feyerabend C, McMeekin L, Wannamethee SG, Cook DG, Whincup PH. Secondhand smoke (SHS) exposure is associated with circulating markers of inflammation and endothelial function in adult men and women. Atherosclerosis 2010; 208: 550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri SS, Zacchi E, Amadio P, Gianellini S, Mussoni L, Weksler BB, Tremoli E. Cytokines present in smokers' serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res 2011; 90: 475–83. [DOI] [PubMed] [Google Scholar]
- 12.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation 2007; 115: 2621–7. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation 2013; 127: e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laniado-Laborin R. Smoking cessation intervention: an evidence-based approach. Postgrad Med 2010; 122: 74–82. [DOI] [PubMed] [Google Scholar]
- 15.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5: CD009329–CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol 2008; 35: 458–63. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 1997; 29: 1422–31. [DOI] [PubMed] [Google Scholar]
- 18.Bruno RS, Traber MG. Cigarette smoke alters human vitamin E requirements. J Nutr 2005; 135: 671–4. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 2001; 74: 714–22. [DOI] [PubMed] [Google Scholar]
- 20.Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS. Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem 2013; 24: 196–203. [DOI] [PubMed] [Google Scholar]
- 21.Mah E, Pei R, Guo Y, Ballard KD, Barker T, Rogers VE, Parker BA, Taylor AW, Traber MG, Volek JS, Bruno RS. gamma-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med 2013; 65: 1291–9. [DOI] [PubMed] [Google Scholar]
- 22.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med 2007; 28: 668–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med 2006; 40: 689–97. [DOI] [PubMed] [Google Scholar]
- 24.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013; 168: 344–51. [DOI] [PubMed] [Google Scholar]
- 25.Mah E, Noh SK, Ballard KD, Matos ME, Volek JS, Bruno RS. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr 2011; 141: 1961–8. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Kim YD, Lee H, Kawamoto T, Yang M, Katoh T. Assay of 2-naphthol in human urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1999; 734: 211–7. [DOI] [PubMed] [Google Scholar]
- 27.Ichiba M, Matsumoto A, Kondoh T, Horita M, Tomokuni K. Decreasing urinary PAH metabolites and 7-methylguanine after smoking cessation. Int Arch Occup Environ Health 2006; 79: 545–9. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AW, Bruno RS, Traber MG. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids 2008; 43: 925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yugar-Toledo JC, Ferreira-Melo SE, Sabha M, Nogueira EA, Coelho OR, Consolin Colombo FM, Irigoyen MC, Moreno H., Jr Blood pressure circadian rhythm and endothelial function in heavy smokers: acute effects of transdermal nicotine. J Clin Hypertens (Greenwich) 2005; 7: 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1–Correlation within subjects. BMJ 1995; 310: 446–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task F. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21: 93–111;quiz 89–90. [DOI] [PubMed] [Google Scholar]
- 32.Bode-Boger SM, Scalera F, Ignarro LJ. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 2007; 114: 295–306. [DOI] [PubMed] [Google Scholar]
- 33.Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res 2001; 38: 93–103. [DOI] [PubMed] [Google Scholar]
- 34.Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation 2000; 101: 2833–40. [DOI] [PubMed] [Google Scholar]
- 35.Cracowski JL, Durand T. Cardiovascular pharmacology and physiology of the isoprostanes. Fundam Clin Pharmacol 2006; 20: 417–27. [DOI] [PubMed] [Google Scholar]
- 36.Belhassen L, Pelle G, Dubois-Rande JL, Adnot S. Improved endothelial function by the thromboxane A2 receptor antagonist S 18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol 2003; 41: 1198–204. [DOI] [PubMed] [Google Scholar]
- 37.Lesault PF, Boyer L, Pelle G, Covali-Noroc A, Rideau D, Akakpo S, Teiger E, Dubois-Rande JL, Adnot S. Daily administration of the TP receptor antagonist terutroban improved endothelial function in high-cardiovascular-risk patients with atherosclerosis. Br J Clin Pharmacol 2011; 71: 844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou X, Gobeil F, Jr, Peri K, Speranza G, Marrache AM, Lachapelle P, Roberts J, 2nd, Varma DR, Chemtob S, Ellis EF. Augmented vasoconstriction and thromboxane formation by 15-F(2t)-isoprostane (8-iso-prostaglandin F(2alpha)) in immature pig periventricular brain microvessels. Stroke 2000; 31: 516–24. [DOI] [PubMed] [Google Scholar]
- 39.Lahaie I, Hardy P, Hou X, Hassessian H, Asselin P, Lachapelle P, Almazan G, Varma DR, Morrow JD, Roberts LJ, 2nd, Chemtob S. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2 alpha on retinal vessels. Am J Physiol 1998; 274: R1406–16. [DOI] [PubMed] [Google Scholar]
- 40.Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. Interactions between thromboxane A(2), thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res 2014; 102: 9–16. [DOI] [PubMed] [Google Scholar]
- 41.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal 2008; 10: 1405–34. [DOI] [PubMed] [Google Scholar]
- 42.Chan AC, Hegarty PV, Allen CE. The effects of vitamin E depletion and repletion on prostaglandin dehydrogenase activity in tissues of young rabbits. J Nutr 1980; 110: 74–81. [DOI] [PubMed] [Google Scholar]
- 43.Grau A, Ortiz A. Dissimilar protection of tocopherol isomers against membrane hydrolysis by phospholipase A2. Chem Phys Lipids 1998; 91: 109–18. [DOI] [PubMed] [Google Scholar]
- 44.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ., 2nd Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem 2006; 281: 4616–23. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J 1995; 74: 247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 2010; 55: 1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]