Abstract
Although tumor necrosis factor alpha (TNF-α) is known to play a critical role in intervertebral disc (IVD) degeneration, the effect of TNF-α on nucleus pulposus (NP) cells has not yet been elucidated. The aim of this study was to explore the effect of TNF-α on proliferation of human NP cells. NP cells were treated with different concentrations of TNF-α. Cell proliferation was determined by cell counting kit-8 (CCK-8) analysis and Ki67 immunofluorescence staining, and expression of cyclin B1 was studied by quantitative real-time RT-PCR. Cell cycle was measured by flow cytometry and cell apoptosis was analyzed using an Annexin V–fluorescein isothiocyanate (FITC) & propidium iodide (PI) apoptosis detection kit. To identify the mechanism by which TNF-α induced proliferation of NP cells, selective inhibitors of major signaling pathways were used and Western blotting was carried out. Treatment with TNF-α increased cell viability (as determined by CCK-8 analysis) and expression of cyclin B1 and the number of Ki67-positive and S-phase NP cells, indicating enhancement of proliferation. Consistent with this, NP cell apoptosis was suppressed by TNF-α treatment. Moreover, inhibition of NF-κB, c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) blocked TNF-α-stimulated proliferation of NP cells. In conclusion, the current findings suggest that the effect of TNF-α on IVD degeneration involves promotion of the proliferation of human NP cells via the NF-κB, JNK, and p38 MAPK pathways.
Keywords: Tumor necrosis factor alpha, nucleus pulposus cell, proliferation, intervertebral disc degeneration, nuclear factor-κB, mitogen-activated protein kinase
Introduction
Although intervertebral disc (IVD) degeneration has been characterized as a chronic process involving multiple factors, its exact mechanism has not been fully elucidated. Previous studies have demonstrated the presence in the degenerative disc of a number of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α),1–3 interleukin-1α (IL-1α),1 interleukin-1β (IL-1β),1,4 interleukin-6 (IL-6),1,5 granulocyte macrophage colony stimulation factor (GM-CSF),1,5 transforming growth factor-β1 (TGF-β1),6,7 insulin-like growth factor-1 (IGF-1),7 and prostaglandin E2 (PGE2),8 suggesting that inflammation plays an important role in IVD degeneration.
TNF-α, a member of the TNF superfamily, is a potent proinflammatory cytokine that is expressed at high levels in resident nucleus pulposus (NP) and annulus fibrosus (AF) cells during IVD degeneration and herniation.3,9 TNF-α plays important roles in the pathophysiology of IVD degeneration, including increasing levels of other potent cytokines, such as IL-1, IL-6, IL-8, and PGE2, and promoting the development of inflammatory hyperalgesia.3 Since TNF-α stimulates expression of ADAMTS4 (a disintegrin and metalloproteinase with thrombospondin motifs 4), ADAMTS5, and matrix metalloproteinases (MMPs), and inhibits synthesis of collagen and proteoglycan,10–13 cells were treated with TNF-α to model degenerative changes in IVD.11,14 Indeed, treatment with TNF-α for seven days has been shown to induce senescence of disc cells in a bovine organ culture model.10 Although it is reasonable to speculate that TNF-α causes degenerative changes in IVD, the question of whether it initially promotes proliferation of human NP cells is unclear.
TNF-α has been shown to promote cell proliferation,15 a common event in the initiation and promotion of malignant disease.16,17 For example, TNF-α stimulates tumor growth and metastasis in a mouse model of pancreatic cancer, an effect that was reversed by treatment with the TNF-α inhibitors infliximab or etanercept.18 Moreover, TNF-α enhances the proliferation of vascular smooth muscle cells in atherosclerosis,19,20 and promotes the growth of skin, ovarian, and intestinal tumors.21–23
It has been reported that disc cell density increases with age or during degeneration.24–27 A characteristic feature of IVD degeneration is the appearance in degenerative areas of cell clusters,24 conceivably arising from proliferation of disc cells in response to inflammatory damage to the degenerative disc.28 While TNF-α potentially plays a role in the proliferation of cells in the NP, the inner core of the IVD, this has not been studied to date. Accordingly, the purpose of the present study was to assess whether TNF-α promotes the proliferation of human NP cells.
Materials and methods
Cells and reagents
Human NP cells, NP cell medium, and trypsin/ethylenediaminetetraacetic acid (trypsin/EDTA) solution were purchased from Sciencell Research Laboratories (Carlsbad, CA). Human NP cells were isolated from human IVD and characterized by immunofluorescence analysis using antibodies against fibronectin and vimentin by the manufacturer. Recombinant human TNF-α was obtained from Peprotech (Rocky Hill, NJ). The cell counting kit-8 (CCK-8) was purchased from Dojindo (Tokyo, Japan). Rabbit anti-human Ki67 polyclonal antibody was purchased from Abcam (Cambridge, UK). Alexa Fluor 594 donkey anti-rabbit IgG antibody was obtained from Molecular Probes (Carlsbad, CA). The Annexin V–fluorescein isothiocyanate (FITC) & propidium iodide (PI) apoptosis detection kit was purchased from Jiamay Biotech (Beijing, China), and PI/RNase Staining Buffer was purchased from BD Biosciences (San Diego, CA). Antibodies against phospho-p65 (p-p65), p65, p-p38, p38, p-c-Jun N-terminal kinase (JNK), JNK, and horseradish peroxidase (HRP)-linked anti-rabbit IgG antibody and U0126 were obtained from Cell Signaling Technology (Danvers, MA). SP60025, SB203580, and pyrrolidinedithiocarbamate ammonium were obtained from Tocris (Bristol, UK).
Cell culture and TNF-α treatment
NP cells were cultured with NP cell medium containing 2% fetal bovine serum (FBS), 1% NP cell growth supplement, and 1% penicillin/streptomycin solution. Cells were seeded into T-25 flasks (Corning Life Sciences, NY) at a cell density of 5000 cells/cm2. Cultures were kept at 37℃ in a 5% CO2–95% air atmosphere and medium was changed every three days until the culture was approximately 70% confluent, after which medium was changed every other day. When 90% confluence was reached, cells were subcultured with trypsin-EDTA (0.25% trypsin, 0.5 mmol/L EDTA). NP cells were used within the first five passages. To investigate the effect of TNF-α, different final concentrations (10–120 ng/mL) of TNF-α were added to NP cell medium after attachment of NP cells to flasks or plates for 24 h or 48 h.
CCK-8 analysis
NP cells were transferred to 96-well plates at a cell density of 4000 cells/well. Cells were treated with TNF-α (0, 20, 40, 80, 120 ng/mL) and cultured at 37℃ in a 5% CO2–95% air atmosphere for 48 h. Cell proliferation was assessed using CCK-8 according to the manufacturer’s protocol. For inhibitor treatment experiments, cells were pretreated with inhibitors for NF-κB (pyrrolidinedithiocarbamate ammonium, 100 µmol/L), p38 (SB203580, 10 µmol/L), Erk (U0126, 10 µmol/L) or JNK (SP60025, 10 µmol/L) for 20 min prior to TNF-α treatment (100 ng/mL).
Ki67 immunofluorescence staining
NP cells were seeded onto 12 mm glass coverslips. Cells were treated with TNF-α (0, 20, 40, 80, 120 ng/mL) and cultured at 37℃ in a 5% CO2–95% air atmosphere for 48 h. Then, cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 25 min at room temperature. Cells were washed three times with PBS and permeabilized with 0.2% Triton X-100/PBS for 15 min. Nonspecific binding was blocked by incubating cells in 5% bovine serum albumin (BSA)/PBS for 60 min. Cells were incubated overnight at 4℃ with rabbit polyclonal anti-Ki67 antibody (1:400 dilution) in 1% BSA/PBS. After thorough washing of the cells, the bound primary antibody was detected by incubating the cells for 2 h at room temperature in the dark with an Alexa Fluor 594 conjugated donkey anti-rabbit IgG. Nuclei were counterstained with 4’6-diamidino-2-phenylindole (DAPI) at 37℃ for 2 min. Fluorescence was visualized using a laser scanning confocal microscope (FV2000, Olympus, Tokyo, Japan). Negative controls were prepared without primary antibody (anti-Ki67). The total numbers of nuclei (blue) and the number of Ki67-stained nuclei (pink) were counted. Proliferation was quantified by determining the percentage of Ki67 positive cells compared to the total number of cells counted.
Quantitative real-time RT-PCR
Gene expression of cyclin B1 was analyzed by real-time RT-PCR. Briefly, cells were treated with TNF-α (0, 120 ng/mL) and cultured at 37℃ in a 5% CO2–95% air atmosphere for two days. After treatment, cells were harvested and washed with PBS. Total RNA was prepared, measured, and cDNA synthesis was performed. Specific primers were designed for the different target genes based on published gene sequences (NCBI Nucleotide database). PCR reactions were performed in a StepOnePlus real-time PCR system (Applied Biosystems) according to the manufacturer’s instructions. Transcript levels were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Melting curves are analyzed to verify the specificity of the RT-PCR reaction and the absence of primer dimer formation. Real-time PCR primer sequences are as follows: human cyclin B1 (F: 5’ ACATGGTGCACTTTCCTCCT 3’, R: 5’ AGGTAATGTTGTAGAGTTGGTGTCC 3’), human GAPDH (F: 5’ ATGGGGAAGGTGAAGGTCG 3’, R: 5’ TAAAAGCAGCCCTGGTGACC 3’).
Cell cycle analysis
Cells were treated with TNF-α (0, 10, 100 ng/mL) and cultured at 37℃ in a 5% CO2–95% air atmosphere for 24 h. Cells were harvested, washed with PBS, and centrifuged at 1000 r/min for 10 min, after which the supernatant was carefully aspirated. Five milliliters of cold 75% ethanol were added in a dropwise manner to the centrifuge tubes, which were then incubated at −20℃ overnight. Cells were washed once with PBS and once with stain buffer, after which they were centrifuged for 10 min at 1500 r/min, and the supernatant was aspirated. Cells were resuspended in 0.5 mL of PI/RNase staining buffer, incubated for 15 min at room temperature, and analyzed on a flow cytometer (BD Biosciences) immediately thereafter. Data were analyzed using FlowJo software. Results are expressed as the percentage of cells in S phase in each group.
Apoptosis analysis
Cells were treated with TNF-α (0, 10, 100 ng/mL) and cultured at 37℃ in a 5% CO2–95% air atmosphere for two days. Cell apoptosis analysis was performed using an Annexin V-FITC & PI apoptosis detection kit. Briefly, cells were harvested and washed with PBS. After centrifuging, cells were resuspended in 300 µL 1 ×binding buffer and incubated with 5 µL Annexin V-FITC and 5 µL PI for 5 min at room temperature in the dark. Cells were analyzed on a flow cytometer. The results are expressed as the percentage of apoptotic cells in each group.
Protein extraction and Western blotting
NP cells (8 × 104 cells/well) were transferred to a six-well plate. After TNF-α (100 ng/mL) treatment for different periods of time (0, 5, 15, 30, 60 min, 6 h, 30 h), plates were placed on ice immediately and washed with ice-cold PBS. All wash buffers and the final resuspension buffer included 1 ×protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), 5 mmol/L NaF, and 200 µmol/L Na3VO4. Proteins were resolved on 10% SDS-PAGE gels and transferred by electroblotting onto polyvinylidene difluoride membranes (EMD Millipore Corporation, Billerica, MA). The membranes were blocked with 5% BSA in Tris-buffered saline-Tween (50 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 0.1% Tween-20) and incubated overnight at 4℃ in 5% BSA in Tris-buffered saline-Tween with specific antibodies at a dilution of 1:1000. Proteins were visualized using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
Statistical analysis
Data are presented as mean ± standard error of mean (SEM). Differences between groups were analyzed by one-way analysis of variance (ANOVA) and Student’s t-test (SPSS for Windows version 17.0, Chicago, IL). P < 0.05 was considered statistically significant. Significant differences compared with appropriate controls are denoted with asterisks: *P < 0.05; **P < 0.01; ***P < 0.001. All experiments were repeated at least three times independently.
Results
TNF-α induces proliferation of human NP cells
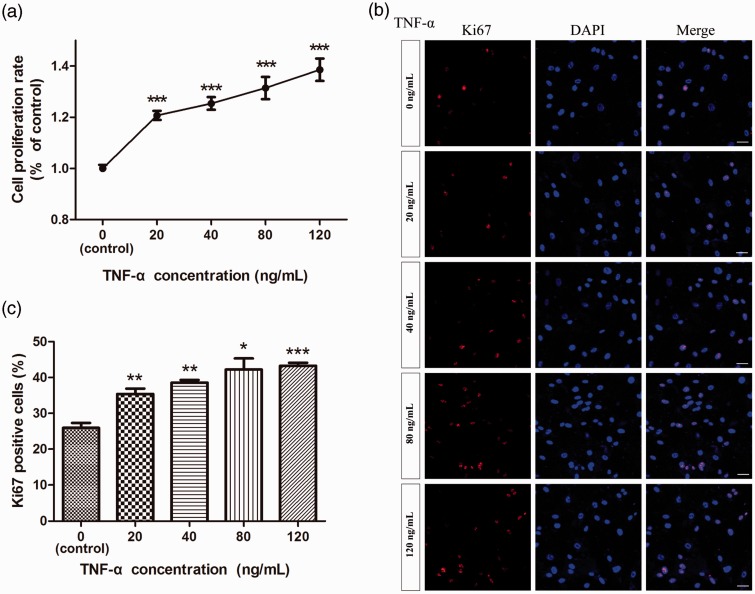
The effect of TNF-α on the proliferation of human NP cells was evaluated by CCK-8 analysis. As shown in Figure 1a, the proliferation of human NP cells was significantly stimulated by TNF-α in a dose-dependent manner over a dose range of 20–120 ng/mL. To further validate the role of TNF-α on proliferation of human NP cells, we next carried out Ki67 immunofluorescence staining and cell cycle analysis. There was a trend towards a positive correlation between the percentage of Ki67 positive NP cells and the concentration of TNF-α, although this failed to reach statistical significance. Compared with control cells, treatment with TNF-α increased the percentage of Ki67 positive NP cells (Figure 1b and c), indicating a positive effect of TNF-α on NP cell proliferation.
Figure 1.
Effect of TNF-α on proliferation of human nucleus pulposus cells. The proliferation of NP cells was measured by CCK-8 analysis (a) and Ki67 immunofluorescence staining (b, c). TNF-α (20, 40, 80, 120 ng/mL) increased NP cell proliferation. n = 27. Bar = 100 µm. (A color version of this figure is available in the online journal.)
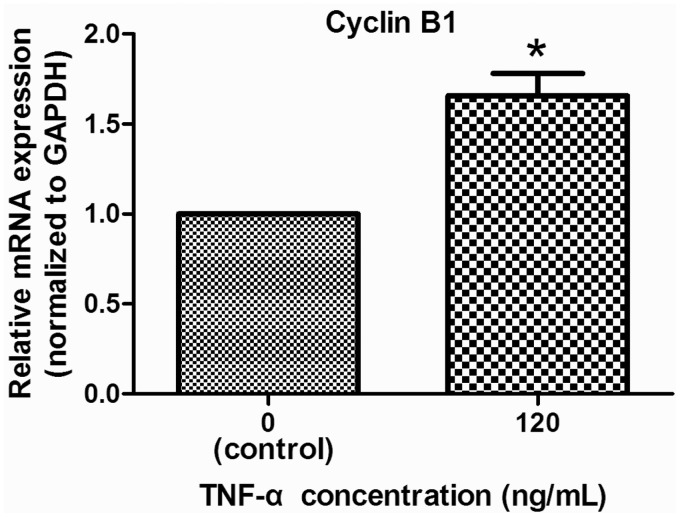
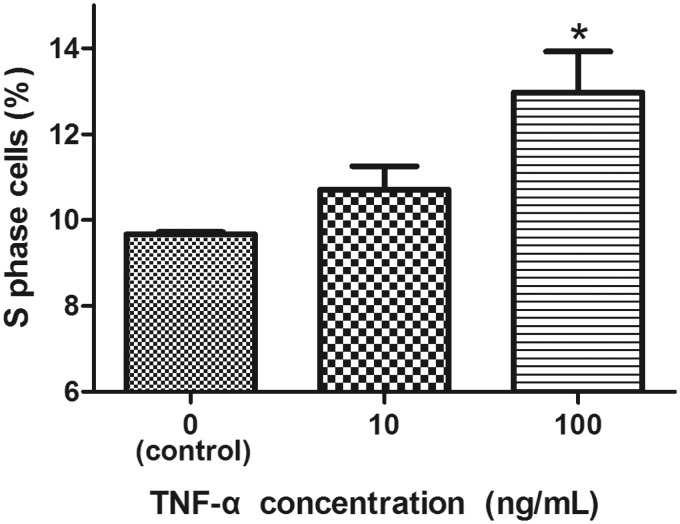
To further confirm the TNF-α induction of NP cell proliferation, we studied expression of cyclin B1 and cell cycle distribution in TNF-α-treated cells. As shown in Figure 2, TNF-α treatment induced a significant increase in cyclin B1 mRNA levels. In addition, relative to untreated cells, treatment with 100 ng/mL TNF-α effected a significant increase in the S phase NP cell population (Figure 3), again validating the stimulation by TNF-α of NP cell proliferation.
Figure 2.
Effect of TNF-α on cyclin B1 expression in NP cells. Expression of cyclin B1 was studied using quantitative real-time RT-PCR. Treatment with TNF-α (100 ng/mL) for two days resulted in a significant increase in cyclin B1 mRNA levels. n = 3
Figure 3.
Effect of TNF-α on cell cycle distribution of NP cells. Treatment with TNF-α (10 or 100 ng/mL) for 24 h increases the S phase population. n = 3
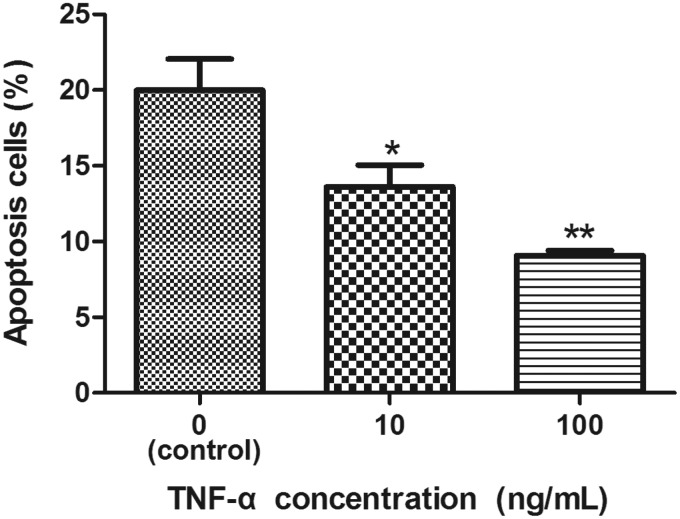
TNF-α inhibits apoptosis in human NP cells
We next determined the effect of TNF-α on NP cell apoptosis using an Annexin V-FITC & PI apoptosis detection kit. As shown in Figure 4, relative to control cells, treatment with TNF-α at 10 and 100 ng/mL effected a significant dose-dependent decrease in the number of apoptotic NP cells.
Figure 4.
Effect of TNF-α on apoptosis in NP cells. Cell apoptosis was demonstrated by flow cytometry using annexin V-FITC/PI. Treatment with TNF-α (10 or 100 ng/mL) for 24 h reduced apoptosis in NP cells. n = 3
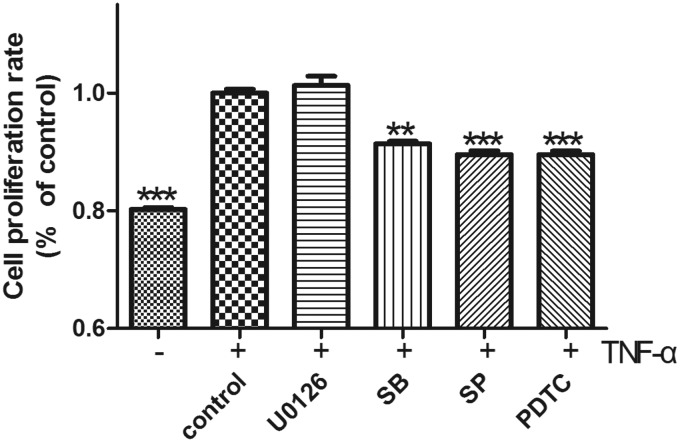
TNF-α promotes proliferation of human NP cells by activating NF-κB and MAPK signaling
We next used selective inhibitors of major signaling pathways to identify the mechanism by which TNF-α induced NP cell proliferation. We found that NP cell proliferation induced by 100 ng/mL TNF-α was significantly inhibited by the NF-κB inhibitor pyrrolidinedithiocarbamate ammonium, the p38 inhibitor SB203580, and the JNK inhibitor SP60025, but not by the Erk inhibitor U0126 (Figure 5).
Figure 5.
Effect of signaling pathway inhibitors on TNF-α induction of NP cell proliferation. CCK-8 analysis showed that treatment with inhibitors of NF-κB (pyrrolidinedithiocarbamate ammonium [PDTC]; 100 µmol/L), p38 (SB203580 [SB]; 10 µmol/L), and JNK (SP60025 [SB]; 10 µmol/L) blocked TNF-α-induced proliferation of NP cells, with the exception of Erk1/2 (U0126; 10 µmol/L) inhibition, which had no effect on the TNF-α-induced proliferation of NP cells. n = 4
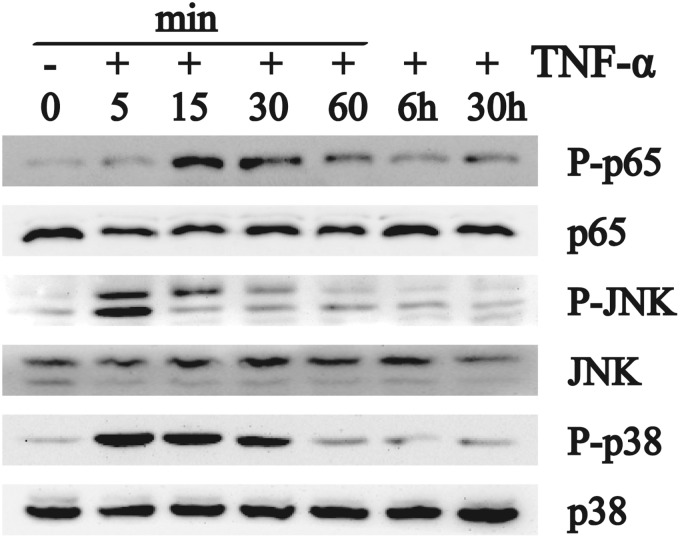
TNF-α activates NF-κB and MAPK in human NP cells
To further validate the role of NF-κB, JNK, and p38 mitogen-activated protein kinase (MAPK) signaling in TNF-α induction of NP cell proliferation, we next evaluated the extent to which these signaling pathways were activated by treatment with TNF-α. As shown in Figure 6, 100 ng/mL TNF-α rapidly induced p-JNK (<5 min treatment), and p-p65 and p-38 MAPK (<15 min treatment), after which levels of these proteins declined (1 h treatment). TNF-α had no effect on total cellular levels of p65, JNK, or p38 MAPK. These results confirm that TNF-α activates the p65, JNK, and p38 MAPK signaling pathways in NP cells.
Figure 6.
Effect of TNF-α on levels of activated NF-κB, JNK, and p38 in NP cells. Western analysis indicated that treatment of NP cells with TNF-α for less than 15 min induced phosphorylation of p65, JNK, and p38, with no appreciable effect on the overall cellular levels of p65, JNK, and p38
Discussion
Our study demonstrates for the first time that acute exposure of TNF-α enhances survival of human NP cells by promoting proliferation and inhibiting apoptosis. Moreover, TNF-α stimulation of NP cell proliferation is dependent upon the integrity of the NF-κB, JNK, and p38 signaling pathways. These results point to a proliferative role of TNF-α in IVD at the very beginning of TNF-α exposure.
TNF-α was initially identified as an inducer of necrosis in mouse solid tumors,29 and was subsequently characterized as potent proinflammatory cytokine with important roles in many pathophysiological processes. TNF-α has multiple context specific effects on fundamental cellular processes, including induction of cell senescence,30,31 apoptosis32,33 as well as proliferation.20,22,34 A variety of studies have pointed to a role for TNF-α in the pathogenesis of IVD herniation, degeneration, and sciatic pain. Elevated levels of TNF-α are present in herniated discs,1 and in the serum of patients with chronic low back pain due to herniated disc.35 Moreover, TNF-α expression in IVDs has been shown to be associated with the degree of disc degeneration,3 and TNF-α is known to play important roles in disc cell apoptosis, disc herniation, and nerve irritation.36 Consistent with these observations, TNF-α has been shown to stimulate expression of catabolic MMPs, ADAMTS-4, and ADAMTS-5.11,37,38 Despite this wealth of evidence implicating TNF-α in degenerative disc conditions, however, the effect of TNF-α on human NP cells has to date been unclear.
Our results show that TNF-α promotes proliferation and suppresses apoptosis in NP cells. CCK-8 assay and Ki67 immunofluorescence analysis demonstrated that TNF-α effected proliferation of NP cells in a dose-dependent manner, observations that were supported by the increased expression of cyclin B1 and increased number of S phase cells after TNF-α treatment. A recent study showed that treatment of human NP cells with TNF-α for 24 h enhances Notch signaling,39 which has in turn been shown to be a critical component of NP cell proliferation.40 In contrast however, treatment of whole bovine disc culture with TNF-α for seven days resulted in the appearance of senescent NP cells.10 These contrasting results may be attributed to the differing durations of TNF-α treatment, such that a brief exposure to TNF-α induces an acute inflammatory reaction and promotes NP cell proliferation, while a longer treatment time results in a subacute or chronic inflammatory reaction, ultimately effecting senescence in NP cells.
A number of studies have demonstrated that TNF-α alternately induces survival or apoptosis in a variety of cell types.41–45 In this study, we found that TNF-α treatment for two days significantly reduced NP cell apoptosis in a dose-dependent manner. Together with the promotion by TNF-α of NP cell proliferation, these data indicate that NP cells may be involved in the promotion of tissue repair under acute inflammatory conditions. Increased levels of TNF-α in IVD results in induction of NP cell proliferation, subsequently leading to inflammatory hyperplasia. Given the scarcity and uneven availability of cellular nutrients and oxygen in degenerative discs, TNF-α induction of NP cell proliferation would be anticipated to occur only in locations with elevated levels of nutrients and oxygen, leading to the formation of NP cell clusters. The excessive proliferation of NP cells that would ensue would have the likely effect of further compromising the nutritional environment of the disc, and exacerbating disc degeneration.
Although recent evidence indicates that the NF-κB and MAPK signaling pathways are master regulators of inflammation and catabolism in IVD degeneration,12,14,46 they also play critical roles in regulation of cell proliferation.47,48 Although the mechanism is unknown, several reports indicate that TNF-α induction of NP cell proliferation is mediated by NF-κB and MAPK signaling pathways.39,43,45 NF-κB has been implicated in TNF-α enhancement of 17β-estradiol-induced cell proliferation in estrogen-dependent breast tumor cells,45 and in TNF-α induction of proliferation in mouse colon cancer cells.43 Moreover, TNF-α induction of Notch pathway genes involved in regulation of cell proliferation occurs via NF-κB and MAPK signaling.39 Here, we demonstrated that inhibitors of NF-κB, JNK, and p38 MAPK block TNF-α-stimulated NP cell proliferation, supporting the notion that the NF-κB, JNK, and p38 MAPK signaling pathways play key roles in the TNF-α-dependent cell proliferation. The fact, however, that inhibition of Erk1/2 had no effect on TNF-α stimulation of NP cell proliferation, indicates that distinct signal pathways are involved in TNF-α-induced cell proliferation in different cell types.
Although the relationship between TNF-α and intervertebral disk disease (IVDD) has been well characterized, the exact effect of TNF-α on NP cells has until now not been fully investigated. The current study has shown that a brief exposure of TNF-α promotes proliferation of human NP cells through NF-κB, JNK, and p38 MAPK, but not Erk1/2. Our findings suggest that after short-term exposure of TNF-α, NP cells are proliferative rather than senescent and necrotic.
ACKNOWLEDGMENTS
We thank Dr Jian-Qiong Zhang, Dr You-Ji He, and members of their lab for careful guidance. This work was supported by grants from: the National Natural Science Foundation of China (No. 81071493, No. 31070876, No. 81272035, and No. 81201423).
Author contributions
X-HW designed and conducted experiments and wrote the manuscript; XH, LZ, Y-TW, J-PB, LL, and FW performed part of the experiments; and X-TW directed the designation of the experiments, evaluated the results, and supervised this study.
References
- 1.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine 1996; 21: 218–24. [DOI] [PubMed] [Google Scholar]
- 2.Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001; 26: 1522–7. [DOI] [PubMed] [Google Scholar]
- 3.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine 2005; 30: 44–53. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto H, Saura R, Harada T, Doita M, Mizuno K. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci 2000; 46: 13–28. [PubMed] [Google Scholar]
- 5.Rand N, Reichert F, Floman Y, Rotshenker S. Murine nucleus pulposus-derived cells secrete interleukins-1-β,-6, and-10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine 1997; 22: 2598–601. [DOI] [PubMed] [Google Scholar]
- 6.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-β and receptors in cells of intervertebral discs. J Neurosurg Spine 2003; 98: 63–7. [DOI] [PubMed] [Google Scholar]
- 7.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J 2002; 11: 145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, III, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine 1996; 21: 271–7. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Moon CS, Sul D, Lee J, Bae M, Hong Y, Lee M, Choi S, Derby R, Kim B-J. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem 2009; 42: 1504–11. [DOI] [PubMed] [Google Scholar]
- 10.Purmessur D, Walter B, Roughley P, Laudier D, Hecht A, Iatridis J. A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem Biophys Res Commun 2013; 433: 151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factorα modulates matrix production and catabolism in nucleus pulposus tissue. Spine 2005; 30: 1940–8. [DOI] [PubMed] [Google Scholar]
- 12.Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am J Pathol 2013; 182: 2310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008; 33: 356–65. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-nuclear factor kappaB axis: implications in inflammatory disc disease. Am J Pathol. Epub ahead of print 2014. [DOI] [PMC free article] [PubMed]
- 15.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001; 4: 1116–22. [DOI] [PubMed] [Google Scholar]
- 16.Cordingley FT, Bianchi A, Hoffbrand AV, Reittie JE, Heslop HE, Vyakarnam A, Turner M, Meager A, Brenner MK. Tumour necrosis factor as an autocrine tumour growth factor for chronic B-cell malignancies. Lancet 1988; 1: 969–71. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7: 211–7. [DOI] [PubMed] [Google Scholar]
- 18.Egberts J-H, Cloosters V, Noack A, Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C, Tepel J. Anti–tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res 2008; 68: 1443–50. [DOI] [PubMed] [Google Scholar]
- 19.Selzman CH, Shames BD, McIntyre RC, Jr, Banerjee A, Harken AH. The NFκB inhibitory peptide, IκBα, prevents human vascular smooth muscle proliferation. Ann Thorac Surg 1999; 67: 1227–31. [DOI] [PubMed] [Google Scholar]
- 20.Davis R, Pillai S, Lawrence N, Sebti S, Chellappan SP. TNFα-mediated proliferation of vascular smooth muscle cells involves Raf-1-mediated inactivation of Rb and transcription of E2F1-regulated genes. Cell Cycle 2012; 11: 109–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR. An anti-tumor necrosis factor-α antibody inhibits the development of experimental skin tumors. Mol Cancer Ther 2003; 2: 445–51. [PubMed] [Google Scholar]
- 22.Wu S, Boyer C, Whitaker R, Berchuck A, Wiener J, Weinberg J, Bast R. Tumor necrosis factor α as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor α expression. Cancer Res 1993; 53: 1939–44. [PubMed] [Google Scholar]
- 23.Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell–derived tumor necrosis factor-α mediates the tumor growth–promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res 2007; 67: 1038–45. [DOI] [PubMed] [Google Scholar]
- 24.Ishii T, Tsuji H, Sano A, Katoh Y, Matsui H, Terahata N. Histochemical and ultrastructural observations on brown degeneration of human intervertebral disc. J Orthop Res 1991; 9: 78–90. [DOI] [PubMed] [Google Scholar]
- 25.Hastreiter D, Ozuna RM, Spector M. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of α-smooth muscle actin. J Orthop Res 2001; 19: 597–604. [DOI] [PubMed] [Google Scholar]
- 26.Maroudas A, Stockwell R, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat 1975; 120: 113–113. [PMC free article] [PubMed] [Google Scholar]
- 27.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies: Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine 1997; 22: 2781–95. [DOI] [PubMed] [Google Scholar]
- 28.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res 2001; 42: 197–207. [DOI] [PubMed] [Google Scholar]
- 29.Larrick J, Wright S. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J 1990; 4: 3215–23. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Herbert B-S, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-α via the p38 mitogen-activated protein kinase pathway. FASEB J 2009; 23: 1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res 2008; 42: 625–32. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases. J Immunol 1999; 162: 2154–61. [PubMed] [Google Scholar]
- 33.Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-α-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J 2000; 14: 1705–14. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama M, Hideshima T, Hayashi T, Tai Y-T, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Nuclear factor-κB p65 mediates tumor necrosis factor α-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res 2003; 63: 18–21. [PubMed] [Google Scholar]
- 35.Kraychete DC, Sakata RK, Issy AM, Bacellar O, Santos-Jesus R, Carvalho EM. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J 2010; 128: 259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000; 25: 2975–80. [DOI] [PubMed] [Google Scholar]
- 37.Séguin CA, Bojarski M, Pilliar RM, Roughley PJ, Kandel RA. Differential regulation of matrix degrading enzymes in a TNFα-induced model of nucleus pulposus tissue degeneration. Matrix Biol 2006; 25: 409–18. [DOI] [PubMed] [Google Scholar]
- 38.Liacini A, Sylvester J, Qing Li W, Huang W, Dehnade F, Ahmad M, Zafarullah M. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp Cell Res 2003; 288: 208–17. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Tian Y, Wang J, Phillips KL, Binch AL, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM. Inflammatory cytokines induce notch signaling in nucleus pulposus cells implications in intervertebral disc degeneration. J Biol Chem 2013; 288: 16761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiyama A, Skubutyte R, Markova D, Anderson DG, Yadla S, Sakai D, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum 2011; 63: 1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallipoli P, Pellicano F, Morrison H, Laidlaw K, Allan EK, Bhatia R, Copland M, Jorgensen HG, Holyoake TL. Autocrine TNF-alpha production supports CML stem and progenitor cell survival and enhances their proliferation. Blood 2013; 122: 3335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am J Physiol-Lung Cell Mol Physiol 2000; 278: L312–9. [DOI] [PubMed] [Google Scholar]
- 43.Luo J-L, Maeda S, Hsu L-C, Yagita H, Karin M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004; 6: 297–305. [DOI] [PubMed] [Google Scholar]
- 44.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science 1996; 274: 787–9. [DOI] [PubMed] [Google Scholar]
- 45.Rubio M, Werbajh S, Cafferata E, Quaglino A, Colo G, Nojek I, Kordon E, Nahmod V, Costas M. TNF-α enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene 2006; 25: 1367–77. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Tian Y, Phillips KL, Chiverton N, Haddock G, Bunning RA, Cross AK, Shapiro IM, Le Maitre CL, Risbud MV. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum 2013; 65: 832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 2001; 159: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18. [DOI] [PubMed] [Google Scholar]