Abstract
Song-Bing H, Hao Z, Jian Z, Guo-Qiang Z, Tuo H, Dai-Wei W, Wen G, Lin G, Yi Z, Xiao-Feng X, Li-Feng Z, Min F, Shui-Qing H, Xiao-Dong Y, Xin-Guo Z, Liang W, De-Chun L. Inhibition of EZH2 expression is associated with the proliferation, apoptosis and migration of SW620 colorectal cancer cells in vitro. Experimental Biology and Medicine July 8, 2014. doi: 10.1177/1535370214542215
A previous Online First version of the article ‘Inhibition of EZH2 expression is associated with the proliferation, apoptosis and migration of SW620 colorectal cancer cells in vitro’, first published on July 8, 2014 as doi: 10.1177/1535370214542215, has been amended in the current version of record as below. Text in red indicates changes made to the original published version, requested because of unattributed duplication. Inhibition of EZH2 expression is associated with the proliferation, apoptosis and migration of SW620 colorectal cancer cells in vitro
Inhibition of EZH2 expression is associated with the proliferation, apoptosis and migration of SW620 colorectal cancer cells in vitro
Song-Bing He1,2, Hao Zhou2, Jian Zhou2, Guo-Qiang Zhou3, Tuo Han2, Dai-Wei Wan4, Wen Gu2, Lin Gao2, Yi Zhang2, Xiao-Feng Xue2, Li-Feng Zhang2, Min Fei5, Shui-Qing Hu6, Xiao-Dong Yang7, Xin-Guo Zhu2, Liang Wang2 and De-Chun Li2
1Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China; 2Department of General Surgery, the First Affiliated Hospital of Soochow University, Suzhou 215006, China; 3Department of gastrointestinal Surgery, Changshu No.2 Hospital, Suzhou 215500, China; 4Department of General Surgery, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510000, China; 5Jiangsu Institute of Hematology, Suzhou 215006, China; 6Department of Clinical Laboratories, Ninth People’s Hospital, Shanghai 200011, China; 7Department of General Surgery, the Second Affiliated Hospital of Soochow University, Suzhou 215006, China
Corresponding authors: Song-Bing He and Xin-Guo Zhu. Emails: captain_hsb@163.com; lidechun1955@163.com
Abstract
Epigenetic changes have been recently recognized as important in many human cancers. Enhancer of zeste homologue 2 (EZH2) gene has shown overexpression in various human cancers, consistent with a straightforward role of EZH2 as an oncogene, but its function in carcinogenesis is partly contradictory. The role of EZH2 in development of human colorectal cancer (CRC) has not yet been clarified. In present study, we observed up-regulation of EZH2 expression in tumor tissues from CRC patients. The expression of EZH2 in CRC cell lines is consistent with the trend in cancer tissues using RT-PCR. We showed that TNM stage and lymph node metastasis in CRC patients are significantly correlated with EZH2 expression levels. EZH2 level of transcription and protein was inhibited by small interfering RNA (siRNA). More importantly, EZH2-siRNA inhibited the proliferation and migration of SW620 cells while promoting their apoptosis, and inducing G0/G1 cell cycle arrest of CRC cells. Collectively, our results suggest that up-regulated EZH2 expression may contribute to the progression of the patients with CRC. A comprehensive study of epigenetic mechanisms and the relevance of EZH2 in CRC is important for fully understanding this disease and as a basis for developing new treatment options in patients with CRC.
Keywords: EZH2, colorectal cancer, small interfering RNA, proliferation, apoptosis, migration
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death in woman and the fourth leading cause of cancer mortality in males.1 Meanwhile, there is a swift growth in CRC morbidity in developing countries such as China where lifestyle has significantly changed.2 Although the therapies of CRC such as chemotherapy and radiation have made great progress, it is regrettably still hard to cure advanced CRC, which has a poor five-year overall survival rates and a 40% overall mortality rate.3,4 Hence, identifying the molecular mechanisms during the development of CRC is important for fully understanding this disease and as a basis for developing new treatment options in patients with CRC.
It has been demonstrated that epigenetic changes including histone modifications are important in many human cancers.5 The enhancer of zeste homologue 2 gene (EZH2), a core component of the poly-comb repressive complex 2 (PRC2), modifies transcription by influencing histone and DNA methylation.6 Recently, many researchers have observed the over-expression of this epigenetic molecule, EZH2, in various human cancers such as breast cancer, prostate cancer, pancreatic cancer, renal cell carcinoma, bladder cancer, and lung cancer.7–12 Meanwhile, experimental evidence from a series of studies demonstrated that EZH2 can contribute to the carcinogenesis by stimulating cell proliferation, blocking apoptosis, activating tumor angiogenesis, and promoting cell invasion.13–16 Moreover, EZH2 also has an important pro-metastatic role in development of cancer by silencing of tumor and metastasis suppressor genes.17,18 However, there is also study suggesting that EZH2 acts as an effect of anti-tumor in certain cancers.19 In this context, there is considerable controversy, over the function of EZH2 in CRC despite the high biomedical significance of this cancer.20
However, the role of EZH2 in CRC and the effects of targeting EZH2 on the biological behavior of CRC are not clear and require elucidation. To address these issues, in this study, we firstly showed the expression of EZH2 in CRC tissues samples and cell lines. Next, we evaluated the effects of transfection of SW620 cells with EZH2-small interfering RNA (EZH2-siRNA) on the expression of EZH2 mRNA and protein level. Finally, we investigated the contribution of inhibition of EZH2 expression to the biological behavior of CRC including the proliferation, apoptosis and migration by RNA interference.
Materials and methods
Patients and specimens
Paraffin-embedded CRC tissue samples were collected at the First Affiliated Hospital of Soochow University from 2011 to 2013. There were also 42 paired non-tumor tissue samples (used as controls). These patients had not received any preoperative treatment. Tumors were staged according to the American Joint Committee on Cancer pathologic tumor-lymph node-metastasis classification. The study protocol was approved by the Ethics Committee at the First Affiliated Hospital of Soochow University.
Cell culture
All human CRC cell lines were purchased from the Chinese Academy of Sciences (Shanghai, China). These cell lines were routinely maintained as described previously21 and used in the next experiments.
Immunohistochemical (IHC) staining and evaluation of the results
Sections were subjected to routine IHC staining as described previously.21,22 Two pathologists who were unaware of these patients’ outcome operated IHC staining independently. Evaluation of IHC results was as described previously.21
Transfection of SW620 cells with EZH2-siRNA
EZH2-siRNA (Genepharma, Shanghai, China), which included one 25-nucleotide stealth RNAi targeting EZH2, and a fluorescently labeled siRNA oligos segment which was used to detect the transfection efficacy by flow-assisted cell sorting (FACS). There are three groups in this section: (a) EZH2-siRNA1, (b) scrambled-siRNA, and (c) non-siRNA. The sequence of EZH2-siRNA was 5′-AAGACTCTGAATGCAGTTGCT-3′. In brief, SW620 cells were transfected using Lipofectamine 2000 (Invitrogen) on the basis of the manufacturer’s protocol. SW620 cells were exposed to siRNA in DMEM, and cells were continued to be incubated for two days. In the preliminary experiment, the maximal transfection efficacy was obtained when the ratio of Lipofectamine 2000 to siRNA was 4 µL:4 µL.
RT-PCR analysis
The mRNA expression of EZH2 in CRC cell lines and SW620 cells after EZH2-siRNA transfection was routinely quantified by RT-PCR as described previously.21 The primer sequences of EZH2 were 5′-AGGAGGACGAGGTAGATGCTTG-3′ (forward) and 5′- CATTGTTCCCTTGGTCGTAGTT-3′ (reverse); and the primer sequences of β-actin were 5′-AACTCCATCATGAAGGGTTGTGA-3′ (forward) and 5′-ACTCCTGCTTGCTGATCCAC-3′ (reverse).
Western blot analysis
Following a 72 h transfection, protein was extracted from SW620 cells performed as previously described and then subjected to SDS-PAGE.23 In brief, protein concentrations were transferred onto PVDF membranes (Merck KGaA, Darmstadt, Germany), and then membranes were incubated with rabbit anti-human EZH2 antibody (1:1000) at 4°C overnight. The membranes underwent hybridization with a goat anti-rabbit IgG secondary antibody (1:1000) at 37°C for 1 h after three washes with TBS-T solution. Finally, we use an ECL chemiluminescence kit (Merck KGaA, Darmstadt, Germany) to detect EZH2 level.
MTT assay
SW620 cells were subjected to routine MTT assay as described previously.24 The reaction product was quantified by measuring the optical density (OD) using test wave length for 490 nm at room temperature.
Flow cytometry analysis
Transfection efficiency was estimated with the use of fluoresein phosphoramidite (FAM)-antisense oligodeoxynucleotides by FACS, as previously described.20 SW620 cells were transfected with the mixture of Lipofectamine™2000 and FAM-NC-siRNA according to preset mixing ratio. In addition, SW620 cells were analyzed for cell cycle by FACS as described previously.24
Cell migration assay
Cell migration assay was performed by the wound-healing method. Cells (1 × 105) of each group were plated in 6-well plates and grown to confluence. The wound-healing method was carried out as described previously.25 In brief, SW620 cells were wounded by scratching with a sterile pipette tip lengthwise along the chamber and were washed with PBS and cultured at 37°C for 1 day. After cell wounding for 0 h and 24 h, images were captured promptly, and OpenLab software was used to detect the wound width (µm). Wound healing rate = (0 h scratch width–24 h scratch width)/0 h scratches width × 100%
Statistical analysis
Differences were evaluated using Statistical Package for Social Science software (SPSS, Version 17.0, Chicago, IL, USA). All measurement data are presented as mean ± standard deviation (SD). Statistical significance was evaluated by the Student’s t-test, and F test was used for correlation analyses. Values of P < 0.05 were considered to be statistically significant.
Results
Up-regulated expression of EZH2 in CRC tissues and cell lines
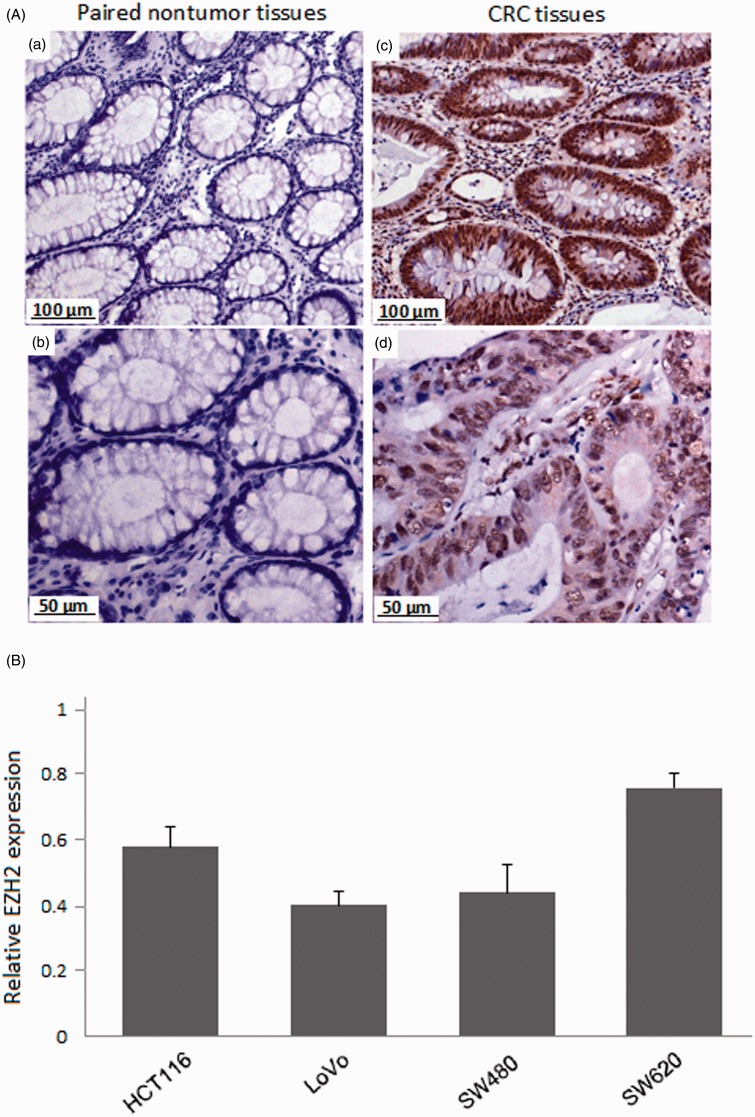
To obtain the insights into the role of EZH2 in colorectal tumorigenesis, we firstly investigate the expression of EZH2 in primary CRC tissues by IHC staining. In Figure 1(A), we observed that EZH2 staining was mainly presented in the nuclei of cells in tumor tissues, while there is almost no EZH2 staining observed in the paired non-tumor tissues. Among 42 CRC patients, a higher percentage (78.6%) of tumor tissues expressed EZH2. However, expression of EZH2 was absent or weakly present in the paired non-tumor tissues (P < 0.01, Table 1). This indicated that EZH2 might facilitate the progression in CRC.
Figure 1.
EZH2 Expression in CRC tissues and cell lines. (A) Expression of EZH2 in tumor tissues and the paired non-tumor tissues was detected by IHC. Negative expression of EZH2 in the paired non-tumor tissues (a and b); EZH2 was up-regulated expressed in tumor tissues (c and d). Original magnification ×200 (a and c), ×400 (b and d). (B) Relative expression of EZH2 mRNA in CRC cell lines (HCT116, LoVo, SW480, and SW620) detected by RT-PCR. (A color version of this figure is available in the online journal)
Table 1.
Immunohistochemical staining of EZH2 in CRC tissues
| EZH2 expression | CRC tissues (n) | Paired nontumor tissues (n) |
|---|---|---|
| – | 5 | 22 |
| + | 4 | 13 |
| ++ | 12 | 7 |
| +++ | 21 | 0 |
| total | 42 | 42 |
| P-value | <0.01 |
CRC, colorectal cancer. –, negative; +, weakly positive; ++, positive; and +++, strongly positive.
Based on the EZH2 expression in tumor tissues, we are interested to explore the biology of EZH2 in human CRC. In order to investigate the expression of EZH2 in CRC cells, we detected a panel of CRC cell lines (HCT116, LoVo, SW480, and SW620) by RT-PCR. As shown in Figure 1(B), there were relatively high expression in these cell lines and the SW620 cells showed the highest elevation among them, which were used for further studies.
Correlations between expression of EZH2 in CRC tissues and clinicopathological features
To pursue the clinical significance of EZH2 expression in CRC, the association between the expression of EZH2 in CRC tissues and clinicopathological features of patients was analyzed. As given in Table 2, EZH2 expression was associated with TNM stage and lymph node metastasis of the patients (P < 0.01), but it was not associated with other clinicopathological features (P > 0.05). These results demonstrated that up-regulated expression levels of EZH2 might contribute to the development of CRC. Moreover, EZH2 might be a potential prognostic biomarker in CRC patients.
Table 2.
Correlation of EZH2 expression with clinicopathological features in CRC
| EZH2 expression |
||||
|---|---|---|---|---|
| Clinicopathological parameters | Case no. | Low (%) | High (%) | P value |
| Total cases | 42 | 9 (21.4) | 33 (78.6) | |
| Age | >0.05 | |||
| ≤60 years | 19 | 4 (21.1) | 9 (78.9) | |
| >60 years | 23 | 7 (30.0) | 16 (70.0) | |
| Gender | >0.05 | |||
| Male | 26 | 7 (27.0) | 19 (73.0) | |
| Female | 16 | 6 (37.5) | 10 (72.5) | |
| BMI | >0.05 | |||
| ≥30 kg/m2 | 8 | 2 (25.0) | 6 (75.0) | |
| <30 kg/m2 | 34 | 7 (20.6) | 27 (79.4) | |
| Tumor size | >0.05 | |||
| ≤5 cm | 31 | 9 (29.0) | 22 (71.0) | |
| >5 cm | 11 | 2 (18.2) | 9 (81.8) | |
| Histological grade | >0.05 | |||
| Well differentiated | 10 | 3 (30.0) | 7 (70.0) | |
| Moderate differentiated | 18 | 5 (27.8) | 13 (72.2) | |
| Poor differentiated | 14 | 3 (21.4) | 11 (78.6) | |
| TNM stage | <0.01 | |||
| I | 8 | 7 (85.7) | 1 (14.3) | |
| II | 17 | 6 (35.3) | 11 (64.7) | |
| III | 12 | 2 (16.7) | 10 (83.3) | |
| IV | 5 | 2 (40.0) | 3 (60.0) | |
| Lymph node metastasis | <0.01 | |||
| Positive | 29 | 4 (13.8) | 25 (86.2) | |
| Negative | 13 | 6 (46.2) | 7 (53.8) | |
| Distant metastasis | ||||
| Positive | 5 | 2 (40.0) | 3 (60.0) | >0.05 |
| Negative | 37 | 12 (32.4) | 25 (67.6) | |
CRC, colorectal cancer; BMI, body mass index; TNM, tumor-lymph node-metastasis.
Inhibited expression of EZH2 mRNA and protein after transfection of SW620 cells with EZH2-siRNA
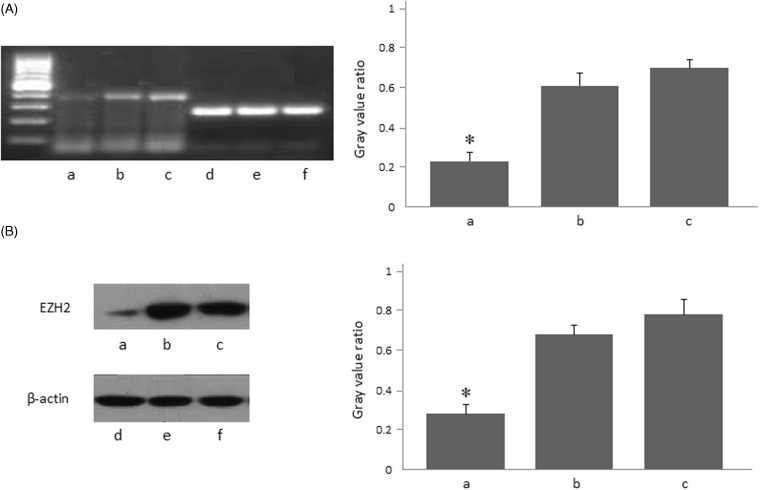
In order to assess the efficacy of EZH2-siRNA in down-regulation of expression of EZH2, we investigated the EZH2 expression in transfected SW620 cells at transcript and protein levels, respectively. As shown in Figure 2(A), we observed that mRNA expression of EZH2 was significantly higher in the control groups than in the EZH2-siRNA group (P < 0.05, respectively). Likewise in Figure 2(B), EZH2 expression of protein level was remarkably higher in the control groups than in the EZH2-siRNA group (P < 0.05, respectively).
Figure 2.
EZH2 expression in SW620 cells after EZH2-siRNA transfection. (A) EZH2 mRNA expression in SW620 cells 48 h after EZH2-siRNA transfection detected by RT-PCR. (B) EZH2 protein expression in SW620 cells 48 h after EZH2-siRNA transfection detected by Western blot. (a) EZH2-siRNA; (b) scrambled-siRNA; (c) non-siRNA; (d, e, f) internal reference. *Compared with scrambled-siRNA group and non-siRNA group, P < 0.05. The results shown are representative of three independent experiments
Influence of EZH2-siRNA transfection on the growth of SW620 cells
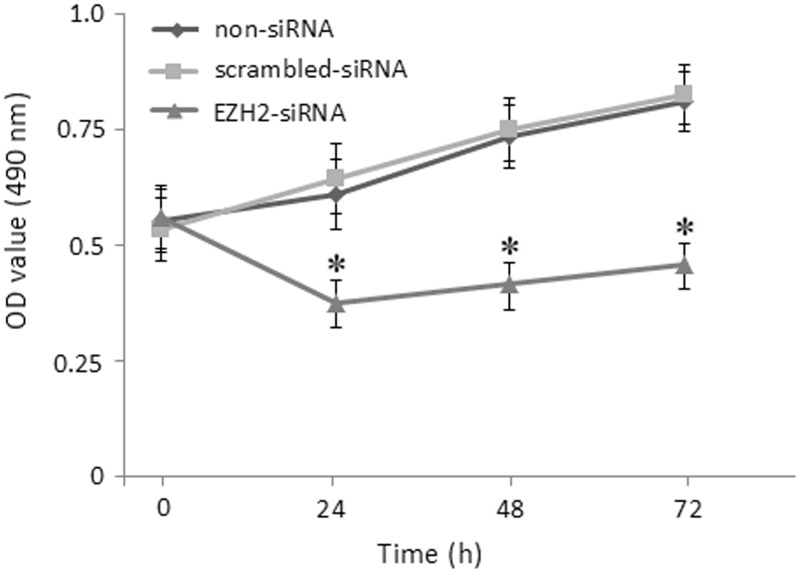
We sought to investigate its influence on cell proliferation in vitro. The results showed that the number of viable cells in the EZH2-siRNA group was markedly decreased compared to other two groups at 24, 48, and 72 h after the treatment, respectively (P < 0.05, Figure 3). The results demonstrated that RNA interference mediated specific down-regulation of EZH2 induced strong inhibition of CRC cell growth.
Figure 3.
Influence of SW620 cells growth after EZH2-siRNA transfection by MTT assay. Curves of SW620 cells growth were showed 24, 48 and 72 h after EZH2-siRNA transfection by MTT assay. OD: optical density. *Compared with scrambled-siRNA and non-siRNA group, P < 0.05. The results shown are representative of three independent experiments
Influence of EZH2-siRNA transfection on the apoptosis and cell cycle of SW620 cells
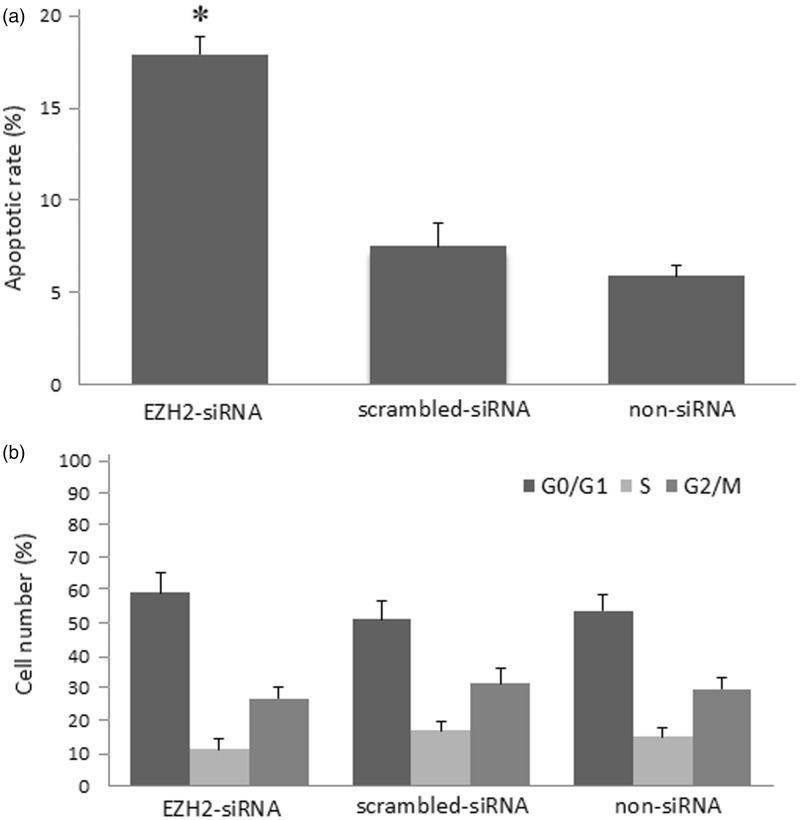
To determine whether down-regulation of EZH2 has influence on the apoptosis and cell cycle distribution of SW620 cells, we further carried out the experiments by PI staining. The results showed that the rate of apoptosis in the EZH2-siRNA group (17.9 ± 3.55)% was remarkably higher than that of the scrambled-siRNA (7.5 ± 1.58)% and non-siRNA groups (5.9 ± 1.29)% (P < 0.05, respectively, Figure 4(a)). In addition, we also found that the cell cycle analysis in the EZH2-siRNA group showed increased G0/G1 phase cells (59.25 ± 4.95)% and decreased percentage of S (11.28 ± 2.78)% and G2/M (26.95 ± 4.50)% phase cells although there are no significant differences between the two control groups (Figure 4(b)).
Figure 4.
Influence of apoptosis and cell cycle in SW620 cells after EZH2-siRNA transfection. (a) Influence of apoptotic rate in SW620 cells 48 h after EZH2-siRNA transfection determined by FACS. *Compared with scrambled-siRNA group and non-siRNA group, P < 0.05. (b) Influence of cell cycle in SW620 cells after EZH2-siRNA transfection: 48 h after transfection, cell cycle was determined by FACS. The results shown are representative of three independent experiments
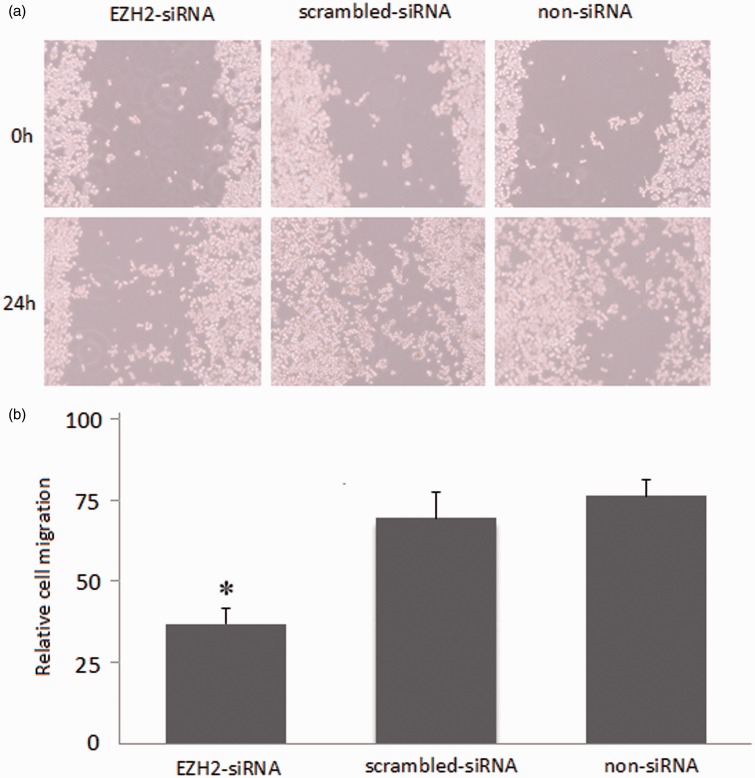
Influence of EZH2-siRNA transfection on the migration of SW620 cells
In Figure 5(a), the result showed that the wound-healing rate of EZH2-siRNA transfected group was (31.66 ± 4.27)%, which was remarkably lower than that of scrambled siRNA (71.55 ± 6.25)% and non-siRNA group (73.28 ± 5.11)% (P < 0.05, Figure 5b). The results suggested that SW620 cells after transfection with EZH2-siRNA were sufficient to restore the migratory capability.
Figure 5.
Influence of migration in SW620 cells after EZH2-siRNA transfection. (a) The SW620 cells were subjected to the migration assay 24 h after transfection of EZH2-siRNA. (b) The histograms represent the quantification of cells having wounding remaining width. *Compared with scrambled-siRNA group and non-siRNA group, P < 0.05. The results shown are representative of three independent experiments
Discussion
In the present study, we have demonstrated up-regulated expression of EZH2 in CRC tissues compared with the paired non-tumor tissues, which was related the clinicopathological significance of the patients. The expression of EZH2 in CRC cell lines was consistent with the trend in cancer tissues. We also observed that the EZH2 level of transcription and protein was remarkably reduced by RNA interference. More importantly, we explored the role of EZH2 in the biological behavior of CRC through silencing EZH2. Collectively, these data indicate a significant role for targeting EZH2 in the influences of the development and progression in CRC.
As a transcriptional repressor, EZH2 plays a critical role in the control of cell proliferation, determination of stem cell fate, and carcinogenesis.26,27 Recently, over-expressions of EZH2 in a variety of malignancies were observed in several studies.7–12 Moreover, up-regulated expression of EZH2 in cancers is related with poor prognosis or increased cancer risk from several epithelial cell-derived tumors, consistent with a straightforward role of EZH2 as an oncogene.28–30 Intriguingly, Simon et al.31 found that deregulation of EZH2 and associated genes is sufficient to induce aggressive T-acute lymphoblastic leukemia (T-ALL) in mouse and human indicating EZH2 as a tumor suppressor in T-ALL. Mutations about PRC2 and its mark, H3K27me3, were found recently, which maybe related this potential reason leading to the conflicting views in cancer progression.32 Moreover, no direct results were shown to address whether the abnormalities of EZH2 in CRC have a potential role in the pathogenesis of CRC.
The expression of EZH2 in CRC remains a critical first step in furthering the understanding and characterization of this molecule in CRC patients. Herein, we observed an increase in EZH2 expression in 42 CRC tumor tissues and several CRC cell lines. Moreover, up-regulated expression levels of EZH2 were remarkably associated with TNM stage and lymph node metastasis. Likewise, in prostate cancer, EZH2 overexpression is associated with aggressive and metastatic disease as well as a poor clinical outcome.8,20 A remarkable relevance has been described between PRC2 occupancy and the aberrant methylation of CpG islands at promoters seen in some cancers, although the precise contexts in which this occurs is still unclear. Therefore, our results impelled us to comprehensively study the potential function of EZH2 in the progression of CRC.
To determine the influence of EZH2 on the biological behavior of CRC, the RNA interference technique was used to down-regulate EZH2 expression in SW620 cells. In present study, our results showed that silencing EZH2 can markedly inhibit the cell proliferation in SW620 cells. Apoptosis constitutes a system for the removal of aged or damaged cells, which plays a specific role in the process of carcinogenesis.33 Notably, FACS analysis found that the cell apoptotic rate in EZH2 inhibitor group was significantly higher than the control groups. Similarly, the data of cell cycle also revealed that the cells of S and G2/M phase in the EZH2 inhibitor group were lower than that of the control groups, while the cells of G0/G1 phase were also increased. Migration of tumor cells is one of the most important prerequisites or tumor progression and metastasis. Consistent with our hypothesis, the migration of tumor cells was inhibited in varying degrees after silencing EZH2. Moreover, it would be of great interest to reveal the mechanisms of migratory capability of the tumor cells. The therapeutic modulation of epigenetic marks has led to improvements in treatments of some cancers, which has been proposed as a therapeutic goal and our study also provides the evidence for this concept.34,35 Targeting of down-regulated epigenetic proteins has been an attractive strategy in cancer treatment, and eradicating tumor-initiating HCC cells in nude mice and inducing apoptosis in breast cancer cells have been observed in previous studies.36,37
There are still several limitations in our present study. First, other CRC cell lines should be also investigated to support the relevance of EZH2 in CRC. Second, the regulation effect of silencing EZH2 in vivo should be further studied. Though the precise molecular mechanisms of EZH2 on apoptosis and cell death in CRC have not been clearly clarified, the regulation effects of malignant phenotypes by silencing EZH2 expression in CRC have not been reported to date. Finally, future studies are warranted to further elucidate the comprehensive mechanisms for the influence of EZH2 in the development of CRC, and we thought that it is very important for the forming of targeted treatment strategy in patients with CRC. It has been increasingly recognized that dysregulation of autophagy pathway is related to different types of tumor progression.38 Autophagy has also been reported to serve a particular effect in CRC cells as autophagy can improve the aggressiveness of CRC cells and increase the ability to adapt to apoptotic stimuli.39 Meanwhile, our results have shown that Ambra1, a new autophagy-related protein, took a pro-survival role of the balance between autophagy and apoptosis in CRC cells in our previous study.40 In this regard, a crosstalk between apoptosis and autophagy on interfering EZH2 could be further investigated. On the other hand, emerging evidence including our previous study demonstrated that miRNAs play significant roles in cancer progression by regulating expression of their downstream target genes including EZH2.41–43 We thought that it will be interesting to explore the inter-relationship between EZH2 and its target miRNA and biological relevance in the process of CRC.
In conclusion, our findings indicated that epigenetic molecule EZH2 may not only play a significant role in the development of CRC but also serve as a potential biomarker for diagnosis and prognostic indicator in CRC. Moreover, inhibition of EZH2 expression has a significant influence on the proliferation, apoptosis and migration of CRC cells. These results suggest that elevated EZH2 level might contribute to the development and progression of CRC. A comprehensive study of epigenetic mechanisms and the relevance of EZH2 in CRC are important for fully understanding this disease and as a basis for developing new treatment options in patients with CRC.
Authors’ contributions
S-BH, HZ, and JZ contributed equally to this work. HSB and ZH designed the research; HSB, ZH, ZJ, HT, WDW, and GW performed the research; HSB, ZGQ, GL, ZY, XXF, ZLF, FM, HSQ, YXD, ZXG, WL and LDC contributed to the reagents/analytic tools; HSB, ZH, ZJ, HT and ZXG analyzed the data; HSB wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by China Postdoctoral Science Foundation (2013M540374), Shanghai Postdoctoral Scientific Program of China (13R21415200), Project of Nature Science Foundation of China (81201905, 81302145), Nature Science Research Grants in University of Jiangsu Province of China (12KJB320009), Project of Jiangsu Province Department of Health, China (YG201406), Science and Technology Research Project in Suzhou City of China (SYS201220, SYS201330), and Post-Graduate Scientific Research Innovation Project of Education Department of Jiangsu Province of China (CXZZ12_0842), and sponsored by Government Overseas Scholarship from the Department of Education of Jiangsu Province.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2013; S0140-6736: 61649–9. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology 2013; 98: 180–7. [DOI] [PubMed] [Google Scholar]
- 4.Wan D, Gu W, Xu G, Shen C, Ding D, Shen S, Wang S, Gong X, He S, Zhi Q. Effects of common polymorphisms rs2910164 in miR-146a and rs11614913 in miR-196a2 on susceptibility to colorectal cancer: a systematic review meta-analysis. Clin Transl Oncol. Epub ahead of print 8 January 2014, DOI:10.1007/s12094-013-1150-x. [DOI] [PubMed]
- 5.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012; 150: 12–27. [DOI] [PubMed] [Google Scholar]
- 6.He S, Xie F, Liu Y, Tong Q, Mochizuki K, Lapinski PE, Mani RS, Reddy P, Mochizuki I, Chinnaiyan AM, Mineishi S, King PD, Zhang Y. The histone methyltransferase Ezh2 is a crucial epigenetic regulator of allogeneic T-cell responses mediating graft-versus-host disease. Blood 2013; 122: 4119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 2003; 100: 11606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419: 624–9. [DOI] [PubMed] [Google Scholar]
- 9.Ougolkov AV, Bilim VN, Billadeau DD. Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin Cancer Res 2008, pp. 6790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagener N, Holland D, Bulkescher J, Crnković-Mertens I, Hoppe-Seyler K, Zentgraf H, Pritsch M, Buse S, Pfitzenmaier J, Haferkamp A, Hohenfellner M, Hoppe-Seyler F. The enhancer of zeste homolog 2 gene contributes to cell proliferation and apoptosis resistance in renal cell carcinoma cells. Int J Cancer 2008; 123: 1545–50. [DOI] [PubMed] [Google Scholar]
- 11.Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 2005; 11: 8570–6. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H, Soejima K, Yasuda H, Kawada I, Nakachi I, Yoda S, Naoki K, Ishizaka A. Deregulation of histone lysine methyltransferases contributes to oncogenic transformation of human bronchoepithelial cells. Cancer Cell Int 2008; 8: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 2007; 21: 1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, Ravoori MK, Shahzad MM, Lee JW, Mora E, Langley RR, Carroll AR, Matsuo K, Spannuth WA, Schmandt R, Jennings NB, Goodman BW, Jaffe RB, Nick AM, Kim HS, Guven EO, Chen YH, Li LY, Hsu MC, Coleman RL, Calin GA, Denkbas EB, Lim JY, Lee JS, Kundra V, Birrer MJ, Hung MC, Lopez-Berestein G, Sood AK. Regulation of tumor angiogenesis by EZH2. Cancer Cell 2010; 18: 185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fussbroich B, Wagener N, Macher-Goeppinger S, Benner A, Fälth M, Sültmann H, Holzer A, Hoppe-Seyler K, Hoppe-Seyler F. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS One 2011; 6: e21651–e21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao ZY, Cai MY, Yang GF, He LR, Mai SJ, Hua WF, Liao YJ, Deng HX, Chen YC, Guan XY, Zeng YX, Kung HF, Xie D. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis 2010; 31: 1576–83. [DOI] [PubMed] [Google Scholar]
- 17.Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE, Beroukhim R, Bronson RT, Ryeom S, Hahn WC, Loda M, Cichowski K. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med 2010; 16: 286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE, Shinomura Y, Imai K, Esteller M. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene 2012; 31: 1988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010; 42: 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodach LL, Jacobs RJ, Heijmans J, van Noesel CJ, Langers AM, Verspaget HW, Hommes DW, Offerhaus GJ, van den Brink GR, Hardwick JC. The role of EZH2 and DNA methylation in the silencing of the tumour suppressor RUNX3 in colorectal cancer. Carcinogenesis 2010; 31: 1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He S, Zhou H, Zhu X, Hu S, Fei M, Wan D, Gu W, Yang X, Shi D, Zhou J, Zhou J, Zhu Z, Wang L, Li D, Zhang Y. Expression of Lgr5, a marker of intestinal stem cells, in colorectal cancer and its clinicopathological significance. Biomed Pharmacother 2014; 68: 507–13. [DOI] [PubMed] [Google Scholar]
- 22.He SB, Zhao H, Fei M, Wu YG, Wang L, Zhu XG, Li DC. Expression of co-signaling molecules CD40-CD40L and their growth inhibitory effect on pancreatic cancer in vitro. Oncol Rep 2012; 28: 262–8. [DOI] [PubMed] [Google Scholar]
- 23.Liu PF, Wu YY, Hu Y, Wang L, He SB, Zhu YB, Zhu XG. PAUF silencing by RNA interference inhibit the malignant phenotypes in human colorectal cancer cell. Oncol Rep 2013; 30: 213–20. [DOI] [PubMed] [Google Scholar]
- 24.He S, Yuan Y, Wang L, Yu M, Zhu Y, Zhu X. Effects of cyclin-dependent kinase 8 specific siRNA on the proliferation and apoptosis of colon cancer cells. J Exp Clin Cancer Res 2011; 30: 109–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J, Li Y, Yang J, Liu Q, Shi M, Zhang R, Shi H, Ren Q, Ma J, Guo H, Tao Y, Xue Y, Jiang N, Yao L, Liu W. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC Cancer 2011; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 2008; 647: 21–9. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa T, Masaki T, Hirai T, Doi S, Kuratsune M, Arihiro K, Kohno N, Yorioka N. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant 2008; 23: 3418–26. [DOI] [PubMed] [Google Scholar]
- 28.Yamada A, Fujii S, Daiko H, Nishimura M, Chiba T, Ochiai A. Aberrant expression of EZH2 is associated with a poor outcome and P53 alteration in squamous cell carcinoma of the esophagus. Int J Oncol 2011, pp. 345–53. [DOI] [PubMed] [Google Scholar]
- 29.Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, Woodward WA, Hsu JM, Hortobagyi GN, Hung MC. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-β-catenin signaling. Cancer Cell 2011; 19: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res 2011; 17: 2613–18. [DOI] [PubMed] [Google Scholar]
- 31.Simon C, Chagraoui J, Krosl J, Gendron P, Wilhelm B, Lemieux S, Boucher G, Chagnon P, Drouin S, Lambert R, Rondeau C, Bilodeau A, Lavallée S, Sauvageau M, Hébert J, Sauvageau G. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev 2012; 26: 651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hock H. A complex Polycomb issue: the two faces of EZH2 in cancer. Genes Dev 2012; 26: 751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol 1998; 8: 324–30. [DOI] [PubMed] [Google Scholar]
- 34.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman, LR; International Vidaza High-Risk MDS, Survival Study Group Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghadjar P, Coupland SE, Na IK, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C, Keilholz U. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol 2006; 24: 1910–6. [DOI] [PubMed] [Google Scholar]
- 36.Chiba T, Suzuki E, Negishi M, Saraya A, Miyagi S, Konuma T, Tanaka S, Tada M, Kanai F, Imazeki F, Iwama A, Yokosuka O. 3-Deazaneplanocin A is a promising therapeutic agent for the eradication of tumor-initiating hepatocellular carcinoma cells. Int J Cancer 2012; 130: 2557–67. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 2007; 21: 1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotze MT, Maranchie J, Appleman L. Inhibiting antophagy: a novel approach for the treatment of renal cell cancinoma. Cancer 2013, pp. 341–7. [DOI] [PubMed] [Google Scholar]
- 39.Zheng HY, Zhang XY, Wang XF, Sun BC. Autophagy enhances the aggressiveness of human colorectal cancer cells and their ability to adapt to apptotic stimulus. Cacer Biol Med 2012; 9: 105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu W, Wan DW, Qian QY, Yi B, Gu YL, He ZL, Wang L, He SB. Ambra1 is an essential regulator of autophagy and apoptosis in SW620 cells: Pro-survival role of Ambra1. Plos One 2014; 9: e90151–e90151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 2008; 322: 1695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan DW, He SB, Xie BH, Xu GH, Gu W, Shen CL, Hu Y, Wang Xinsheng, Zhi QM, Wang L. Aberrant expression of miR-199a-3p and its clinical significance in colorectal cancers. Med Oncol 2013; 30: 378–378. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 2011; 71: 225–33. [DOI] [PubMed] [Google Scholar]