Abstract
Since Korean mistletoe (Viscum album) has been used for alleviating metabolic diseases, it may also prevent the impairment of energy, glucose, lipid, and bone metabolisms in an estrogen-deficient animal model. We determined that long-term consumption of Korean mistletoe water extract (KME) can alleviate menopausal symptoms such as hot flush, increased abdominal fat mass, dyslipidemia, hyperglycemia, and decreased bone mineral density in ovariectomized (OVX) rats fed a high-fat diet, and explored the mechanisms of the effects. OVX rats were divided into four groups and fed high-fat diets supplemented with either 0.6% dextrin (control), 0.2% lyophilized KME + 0.4% dextrin (KME-L), or 0.6% lyophilized KME (KME-H). Sham rats were fed with the high-fat diets with 0.6% dextrin as a normal-control without estrogen deficiency. After eight weeks, OVX rats exhibited impaired energy, glucose and lipid metabolism, and decreased uterine and bone masses. KME-L did not alleviate energy dysfunction. However, KME-H lowered serum levels of total-, LDL-cholesterol, and triglycerides and elevated serum HDL-cholesterol levels in OVX rats with dyslipidemia, to similar levels as normal-control rats. Furthermore, KME-H improved HOMA-IR, an indicator of insulin resistance, in OVX rats. Surprisingly, KME-H fed rats had greater lean mass in the abdomen and leg without differences in fat mass but neither dosage of KME altered bone mineral density in the lumbar spine and femur. The increased lean mass was related to greater phosphorylation of mTOR and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) in the quadriceps muscles. Hepatic triglyceride contents were lowered with KME-H in OVX rats by increasing carnitine palmitoyltransferase-1 (CPT-1) expression and decreasing fatty acid synthase (FAS) and sterol regulatory element-binding protein-1c (SREBP-1c) expression. In conclusion, KME may be useful for preventing some menopausal symptoms such as hot flushes, dyslipidemia, hepatic steatosis, and loss of muscle mass in post-menopausal women.
Keywords: Korean mistletoe, menopausal symptoms, hot flush, lean mass, lipid metabolism
Introduction
Estrogen deficiency induces menopausal symptoms (hot flush, urogenital atrophy, and cold sweats), fat redistribution, hyperlipidemia, osteoporosis, and cardiovascular disease.1 Hormone replacement therapy (HRT) uses exogenous hormones to relieve menopausal symptoms, but HRT has adverse effects, including increased risk of ischemic stroke, venous thromboembolism, and breast cancer.2,3 Investigators are actively exploring alternative medicines which have estrogen-like effects on glucose, lipid, and bone metabolisms but antiestrogen-like effects on the uterus and breasts in human and animal studies.4 Selective estrogen receptor modulators (SERMs) possess these characteristics and there is widespread interest finding new SERMS that are safe and effective.5,6 Phytoestrogens are attractive potential candidates that act like SERMs used for treating menopausal syndrome and for minimizing its complications and side effects.6,7 However, there are no natural substances or botanical extracts that fully substitute for HRT.
Mistletoe (Viscum album) is a parasitic plant that grows on a wide range of host trees worldwide. Mistletoe contains various alkaloids, lectins, viscotoxins, phenylpropanoids, tannins, lignans, and polyphenols and its contents are somewhat varied according to the host trees from which they are collected and the area in which they grow.8–10 The major components in Korean mistletoe have been identified as lectins, viscothionin, steroids, triterpenes, sesquiterpene lactones, flavonoids, and alkaloids.11,12 Since these components have different water solubilities, the water and methanol extracts have different active compounds and therefore may have different bioactivities. Mistletoe has been widely used as a folk medicine to treat various diseases. Lectins and viscotoxins in mistletoe are reported to exert anti-tumor properties by inducing cell cycle delay or arrest, increasing apoptosis, inhibiting angiogenesis, and potentiating immune responses.12–14 Furthermore, mistletoe has been demonstrated to possess a number of therapeutic efficacies for treating a wide range of diseases such as diabetes mellitus, chronic cramps, stroke, stomach problems, heart palpitations, blood pressure, and difficulties in breathing.15,16 Therefore, the consumption of mistletoe extracts may modulate energy, glucose, and lipid metabolisms.
We hypothesized that the long-term consumption of mistletoe water extract would alleviate menopausal symptoms such as hot flushes, increased abdominal fat mass, dyslipidemia, hyperglycemia, and decreased bone mineral density (BMD) in estrogen-deficient animals with diet-induced obesity. The present study tested the hypothesis and explored the mechanisms of energy, glucose and lipid metabolism modulation by mistletoe water extract in ovariectomized (OVX) rats fed a high-fat diet (HFD). This model exhibits similar symptoms as post-menopausal women and the symptoms are mostly reversible with estrogen replacement.3–5 Sham rats were fed a HFD as a normal-control without estrogen deficiency.
Materials and methods
Mistletoe extracts and viscothionin contents
Fresh mistletoe growing on oak trees was collected in January 2011 from Taebak mountain in Kangwon-Do, Republic of Korea, and it was identified by Dr BS Ko (Korea Institute of Oriental Medicine, Daejeon, Korea), and a voucher specimen (No. 2012-05) was deposited at the herbarium of Department of Food & Nutrition, Hoseo University. To prepare the mistletoe extract, mistletoe was washed, dried at room temperature, freeze-dried, and powdered. The powder was heated in distilled water at 100℃ for 12 h or in 70% ethanol for 5 h. After centrifugation, both supernatants were lyophilized in freeze-dryer. The contents of total phenolic compounds were measured using Folin–Ciocalteu reagent and expressed as mg gallic acid (97.5% purity, Sigma, St. Louise, MO) equivalents· g−1. The extracts were dissolved in ethanol and the contents of total flavonoids were measured by a colorimetric assay.17 Rutin (>94% purity, Sigma) was used as the standard.
The contents of terpenoids in the 70% ethanol and water extracts were analyzed using an HPLC instrument (Agilent Technologies, USA) with a Luna C18 column (4.6 × 250 mm, 5 µm; Phenomenex, USA). The mobile phase consisted of distilled water and acetonitrile (20:80) under isocratic conditions. The mobile phase flow rate was 0.5 mL/min, the column temperature was 40℃, the injection volume was 10 µL, and UV detection was set at 210 nm. Betulinic acid (≥98% purity), oleanolic acid (≥97% purity), and betulin (≥98% purity, Sigma) were used as standards for quantification.
The isolation of viscothionin from Korean mistletoe extract (KME) was performed using a slight modification of a previously described method.11,18 Briefly, lyophilized powder of the aqueous mistletoe extract was dissolved in distilled water and dialyzed using membrane tubing with a molecular weight cut off of 3.5 kDa against a 25 mmol/L sodium acetate buffer (pH 4.8). The inner dialysate was loaded on a carboxymethyl-sepharose fast flow column (20 × 4.6 cm) and eluted with an NaCl gradient solution (0–0.5 mol/L). After removing NaCl by dialysis, the dialysate was passed through a Sephadex G-50 column (70 × 1.6 cm) and fractions were collected according to size of molecules. The second peak fractions were re-pooled and centrifuged at 4000 r/min for 30 min using a 9 kDa molecular weight cut off centrifugal filtration unit (Thermo Scientific, Rockford, USA). The viscothionin was detected after separation on a 20% SDS-PAGE gel and was identified as viscothionin by the partial sequencing of amino acids and protein blast searching using Pubmed as described previously.11,18 The contents of viscothionin in KME were measured using bovine serum albumin as an external standard.
Animals and ethics
Eight-week-old female Sprague–Dawley rats (weighing 236 ± 19 g) were housed individually in stainless steel cages in a controlled environment (23℃ and with a 12-h light/dark cycle). All surgical and experimental procedures were performed according to the guidelines of the Animal Care and Use Review Committee of Hoseo University, Korea (2013-01). Rats underwent ovariectomy or a sham operation under anesthesia induced by intramuscular injection of a mixture of ketamine and xylazine (100 and 10 mg/kg body weight [bw], respectively).
Experimental design
Our preliminary study found that low dosage (0.5–2 µmol/L) treatment with Korean mistletoe water extracts lowered tumor necrosis factor-α expression in RAW 264.7 cells (mouse leukemic monocyte macrophage cell line) activated with lipopolysaccharides. Based on the preliminary study, diets containing 0.2% and 0.6% of the Korean mistletoe water extracts were given to OVX rats. After the ovariectomy or sham operation, 30 OVX rats were randomly assigned to the following three groups according to supplementation of the diets: (1) low dose, 0.2% water extract of Korean mistletoe+0.4% dextrose (KME-L); (2) high dose, 0.6% water extract of Korean mistletoe (KME-H), and (3) OVX placebo control, 0.6% dextrose (OVX-control). Ten sham-operated rats were assigned to the control HFD containing 0.6% dextrose as a normal-control that naturally maintained normal estrogen levels. All experimental animals were given free access to water and a HFD containing KME or dextrose during the eight-week experimental period. The HFD was a modified semi-purified AIN-93 formulation for experimental animals.19 The diet consisted of 40 percent energy (En%) from carbohydrates, 20 En% from protein, and 45 En% from fats. The major carbohydrate, protein, and fat sources were starch plus sugar, casein (milk protein), and lard (CJ Co., Seoul, Korea), respectively.
Overnight-fasted serum glucose levels, food and water intakes, and bws were measured every Tuesday at 10:00. Insulin resistance was determined using the homeostasis model assessment estimate of insulin resistance (HOMA-IR) (HOMA-IR = fasting insulin [µIU/mL] × fasting glucose [mmol/L]/22.5).20 At the end of the study, rats were anesthetized with ketamine and xylazine (100 and 10 mg/kg bw, respectively). Epididymal and retroperitoneal fat mass and uteruses were then removed and weighed. Uterus index was calculated as uterus weight divided by bw. After blood collection by abdominal cardiac puncture, human insulin (5U/kg bw) was injected through the inferior vena cava to determine insulin signaling in the liver. Serum samples were then stored at −70℃ for biochemical analysis.
Tail skin temperature measurement
Tail skin temperature was monitored at the fourth and eighth weeks of the experimental periods during the sleep cycle. Tail skin temperature measurements were conducted using an infrared thermometer (BIO-152-IRB, Bioseb, Chaville, France) designed for small rodents and three measurements were made 10 min apart and the average value for the animal was used as a single data point.21
Energy expenditure analysis by indirect calorimetry
After seven weeks of the assigned treatment, energy expenditure was assessed at the beginning of the dark phase of the light/dark cycle after 6 h of fasting. The rats were placed into metabolic chambers (airflow = 800 mL/min) with a computer-controlled O2 and CO2 measurement system (BIOPAC Systems, Inc., Goleta, CA) to determine their calorimetric parameters. The respiratory quotients (RQs) and resting energy expenditures (REEs) were calculated using previously reported equations.22,23
BMD and lean and fat mass measurements
A densitometer was calibrated daily with a phantom supplied by the manufacturer. After anesthetization with ketamine and xylazine (100 and 10 mg/kg bw, respectively), the animals were laid in a prone position with their hind legs maintained in external rotation with tape. Hip, knee, and ankle articulations were in 90° flexion. Upon completion of scanning, BMD was determined in the designated and equivalent areas of right femur and lumbar spine in all rats by dual-energy X-ray absorptiometry (DEXA) using an absorptiometer (pDEXA Sabre; Norland Medical Systems Inc., Fort Atkinson, WI), which was equipped with the appropriate software for assessment of bone density in small animals.23 Similarly, abdominal fat mass and lean mass were measured by DEXA.
Isolation of liver total RNA and real-time PCR
Livers were collected at the end of treatment period, powdered with a cold steel mortar and pestle, and then mixed with a monophasic solution of phenol and guanidine isothiocyanate (TRIzol reagent; Gibco-BRL, Rockville, MD) for total RNA extraction, according to the manufacturer’s instructions. cDNA was synthesized from equal amounts of total RNA using superscript III reverse transcriptase, and polymerase chain reaction (PCR) was performed with high-fidelity Taq DNA polymerase. Equal amounts of cDNA were added to SYBR Green mix (Bio-Rad, Richmond, CA) and amplified using a real-time PCR instrument (Bio-Rad). The expression levels of the genes of interest were normalized to that of the housekeeping gene β-actin. To assess changes in the expression of genes related to fatty acid synthesis and oxidation in the liver, the expressions of sterol regulatory element-binding protein-1c (SREBP-1c), acetyl CoA carboxylase (ACC), fatty acid synthase (FAS), carnitine palmitoyltransferase-1 (CPT-1), and uncoupling protein (UCP)-1 were measured with corresponding primers as previously described.24
Immunoblot analysis
Quadriceps muscles were dissected after 10 min of insulin stimulation and immediately frozen in liquid nitrogen. Frozen tissues were lysed with a 20 mmol/L Tris buffer (pH 7.4) and after 30 min on ice, the lysates were then centrifuged for 10 min at 12,000 r/min at 4℃. Lysate samples with equivalent protein levels (30–50 µg) were directly resolved by SDS-PAGE. Immunoblotting with specific antibodies against mammalian target of rapamycin (mTOR), eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), phosphorylated mTORSer2448, 4E-BP1Thr37/46, and β-actin (Cell Signaling Technology, Beverly, MA) as previously described.23 The intensity of protein expression was determined using Imagequant TL image analysis software (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
Statistical analysis was performed using SAS, version 7.0 (SAS, Inc. Cary, NC). Results are expressed as means ± standard deviations. The significant differences among dextrin (OVX-control), KME-L, KME-H and normal-control were analyzed by one-way analysis of variance (ANOVA). Multiple comparisons among the groups were identified by Tukey’s tests. If there was no significant difference in ANOVA, the significant differences between the OVX-control and normal-control groups were determined using two-sample t-tests. P < 0.05 was considered statistically significant.
Results
Bioactive components of KME
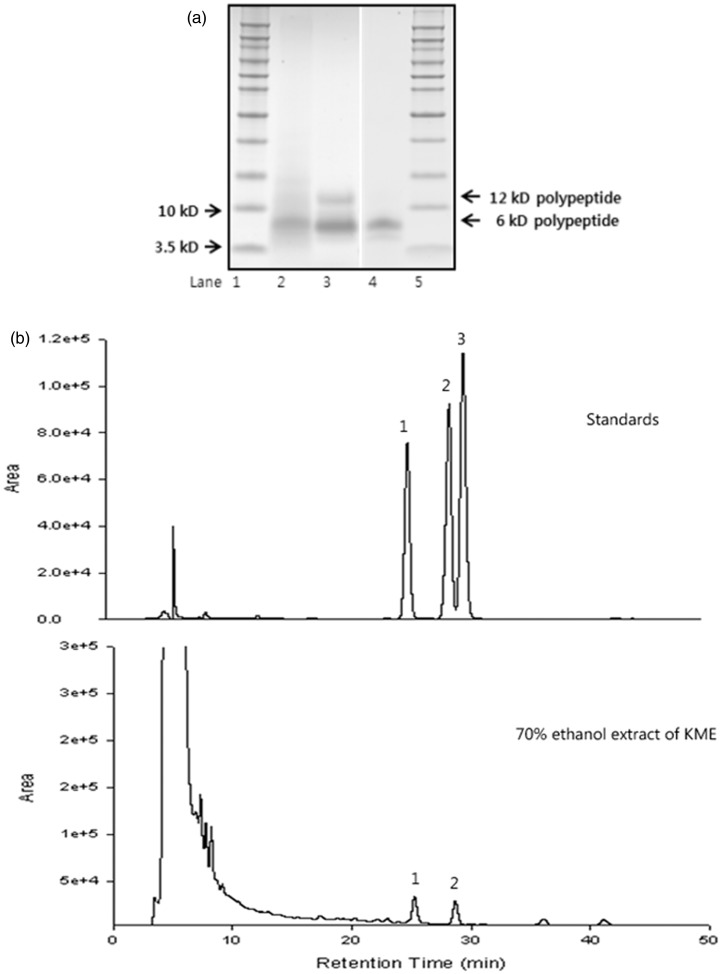
Both water and methanol extracts of mistletoe have been studied for physiological bioactivities. Water extracts of Korean mistletoe contained less total phenolic compounds and flavonoids than 70% methanol extracts, but peptides were mostly found in water extracts (Table 1). The terpenoids, betulinic acid, and oleanolic acid were only present in the 70% methanol extracts (Table 1, Figure 1a). The main peptide in water extracts was the 6 kDa viscothionin (Figure 1b) at about 2.7 mg/g dry weight (Table 1).
Table 1.
The contents of phenolic compounds and flavonoids (unit: mg/g dry weight)
| Water extract of Korean mistletoe | 70% methanol extract of Korean mistletoe | |
|---|---|---|
| Polyphenols | 3.53 ± 0.54 | 5.86 ± 0.63 |
| Flavonoids | 1.32 ± 0.22 | 2.38 ± 0.31 |
| Betulinic acid | ND | 0.51 ± 0.00 |
| Oleanolic acid | ND | 0.89 ± 0.01 |
| Viscothionin | 2.71 ± 0.52 | ND |
ND: non-detectable. Values are means ± SD.
Figure 1.
HPLC chromatogram of 70% ethanol extract (a) and peptide patterns of Korean mistletoe water extract (b). Lanes 1, 5 – molecular weight marker, lane 2 – water extract of Korean mistletoe (KME), lane 3 – semi-purified polypeptide from KME, and lane 4 – purified polypeptide from KME. 1: betulinic acid, 2: oleanolic acid, 3: betulin
Changes in skin temperature, body composition, and energy metabolism by KME
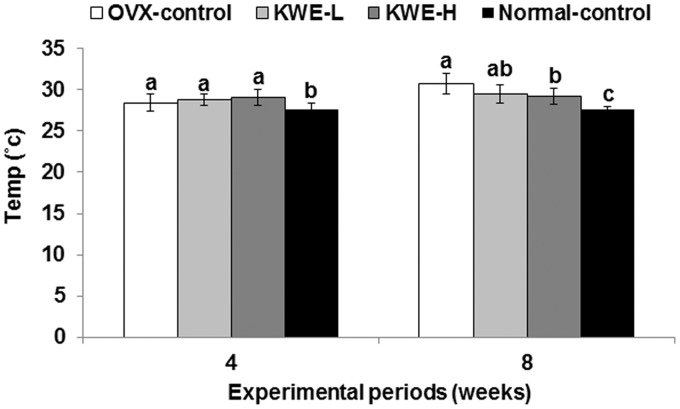
Tail skin temperature was initially only slightly lower in the normal-control group than control group, but at the fourth and eighth weeks it was markedly increased in control rats (30.7 ± 1.3℃) compared to normal-control rats (28.4 ± 1.0℃) (Figure 2), indicating that tail skin temperature increased only in OVX rats over time. KME dose-dependently lowered tail skin temperature by the eighth week of the experimental period, but was still higher than the normal-controls.
Figure 2.
Tail skin temperature at 4th and 8th weeks of the experimental period. OVX-control, OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L, OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H, OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control, Sham rats fed a HFD with 0.6% dextrin with normal estrogen levels. At 4th and 8th weeks, tail skin temperature was measured using infrared thermometer. Each bar represents the mean ± SD (n = 15). a,b,cSignificantly different among all groups at P < 0.05
KME supplementation, regardless of dosage, did not modify bw or peri-uterine and retroperitoneal fat pad weights of OVX-control rats, which were higher than normal-control rats (Table 2). Overnight-fasted leptin levels were significantly higher in OVX-control rats than in normal-control rats in parallel with visceral fat mass; neither KME-L nor KME-H changed serum leptin levels in OVX rats (Table 2). In contrast to visceral fat mass, brown fat mass was lowered in OVX-control rats than normal-control rats, whereas KME increased the brown fat mass in a dose-dependent manner and was slightly higher in KME-H than the normal-controls. UCP-1 expression in brown fat decreased in OVX-control rats in comparison to normal-control rats whereas KME-H prevented the decreased expression in OVX rats (Table 2). Serum estrogen levels were almost completely suppressed in OVX-control rats and they were 3.7 folds lower in OVX rats than normal-control rats, and KME did not prevent the suppression (Table 2). Uterine weight was much lower in OVX-control rats than normal-control rats but KME did not alter the weight (Table 2).
Table 2.
Metabolic parameters at the end of the 12-week treatment
| OVX-control (n = 15) | KME-L (n = 15) | KME-H (n = 15) | Normal-control (n = 15) | |
|---|---|---|---|---|
| Body weight at 4th weeks (g) | 348 ± 38 | 350 ± 36 | 355 ± 37 | 301 ± 32* |
| Body weight at 8th weeks (g) | 379 ± 41 | 380 ± 39 | 383 ± 40 | 323 ± 35* |
| Peri-uterine fat (g) | 7.8 ± 1.2 | 7.7 ± 1.0 | 7.3 ± 0.8 | 4.5 ± 0.8* |
| Retroperitoneum fat (g) | 11.7 ± 1.2 | 11.3 ± 1.4 | 10.7 ± 1.4 | 5.6 ± 0.9* |
| Serum leptin levels (ng/mL) | 4.2 ± 0.6 | 4.0 ± 0.5 | 3.8 ± 0.5 | 3.4 ± 0.5* |
| Brown fat (g) | 0.20 ± 0.04b | 0.24 ± 0.04ab | 0.28 ± 0.05a | 0.25 ± 0.04a |
| UCP-1 expression | 1.32 ± 0.27b | 1.44 ± 0.25ab | 1.71 ± 0.25a | 1.84 ± 0.29a |
| Serum 17β-estradiol levels (pg/mL) | 1.5 ± 0.8 | 1.7 ± 0.7 | 1.6 ± 0.7 | 5.5 ± 1.1* |
| Uterine weight (g) | 0.21 ± 0.04 | 0.22 ± 0.05 | 0.22 ± 0.05 | 0.64 ± 0.13* |
OVX-control: OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L: OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H: OVX rats fed HFD with 0.6% Korean mistletoe water extract; normal-control: sham rats fed a high-fat diet (HFD) with 0.6% dextrin with normal estrogen levels. Values are mean ± SD.
Significantly different among all groups at P < 0.05.
Significant difference between OVX-control and normal-control rats at P < 0.05.
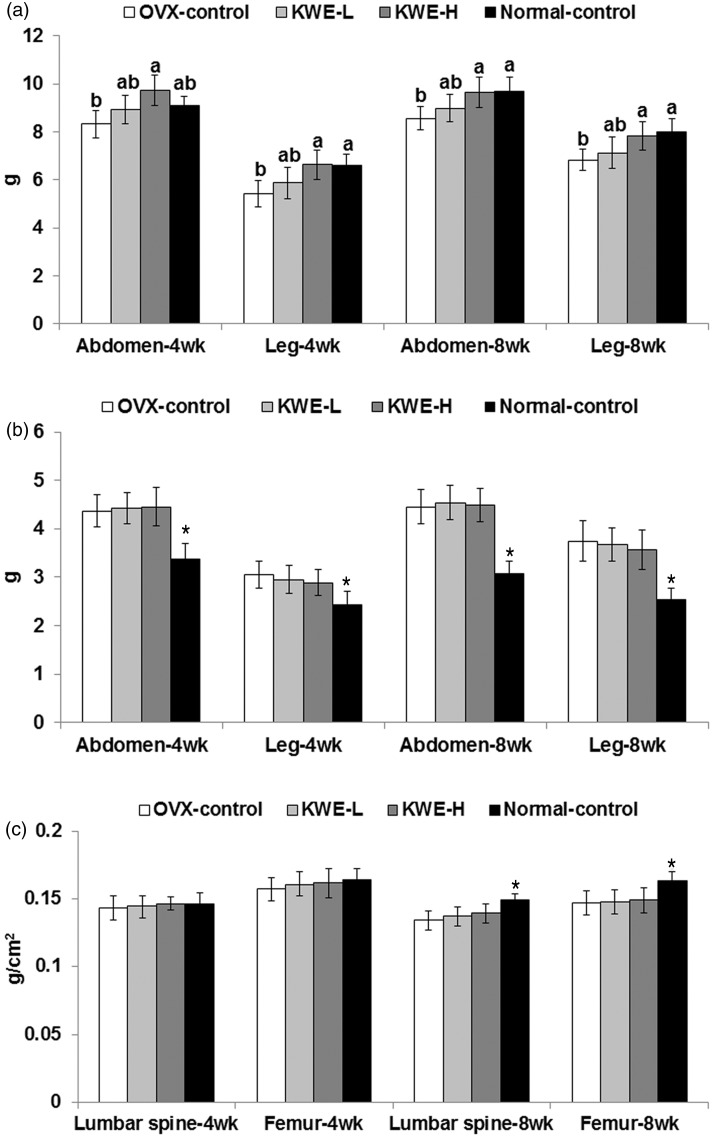
As determined by DEXA, lean mass in the abdominal and leg regions was lowered in OVX-control rats than normal-control rats and the decrease was greater at eighth week than the fourth week. Therefore, estrogen deficiency decreased lean mass and increased fat mass. Surprisingly, KME-H prevented the decreased lean mass by OVX (Figure 3a). However, fat mass in the abdomen and leg regions, like visceral fat, was not changed by KME treatments (Figure 3b). The BMD was lower in OVX rats than normal-control rats at week 8, but KME did not alter BMD (Figure 3c).
Figure 3.
Body composition at 4th and 8th weeks of the experimental period measured by DEXA. OVX-control, OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L, OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H, OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control, sham rats fed a HFD with 0.6% dextrin with normal estrogen levels. At 4th and 8th week, fat mass and lean mass were measured in the abdomen and leg by DEXA whereas BMDs of the femur and lumbar spine were also measured. a, Lean body mass in the abdomen and right leg. b, Fat mass in the abdomen and right leg. c, Bone mineral density in lumbar spine and femur. Each bar represents the mean ± SD (n = 15). a,bSignificantly different among all groups at P < 0.05. *Significantly different between the OVX-control and normal-control groups at P < 0.05
Energy intake did not differ between OVX-control rats and normal-control rats and KME did not affect energy intake regardless of dosage (Table 3). However, daily energy expenditure, measured by indirect calorimetry, was lower in OVX-control rats than in normal-control rats and KME did not change energy expenditure. RQ, calculated by the ratio of CO2 and O2, appeared to be higher in OVX-control rats than in normal-control rats, but the difference was not significant. KME did not affect RQ in OVX rats (Table 3). Carbohydrate oxidation was higher and fat oxidation was lower in OVX-control rats than in normal-control rats. KME-H did not alter carbohydrate oxidation but fat oxidation was greater in KME-H than OVX-control (Table 3). Thus, overall, KME did not improve the energy dysfunction but fat metabolism was partially normalized in OVX-control rats.
Table 3.
Energy intake and energy expenditure measured by indirect calorimetry at the end of the 12-week treatment
| OVX-control (n = 15) | KME-L (n = 15) | KME-H (n = 15) | Normal-control (n = 15) | |
|---|---|---|---|---|
| Food intake at 4th week (g/day) | 14.3 ± 1.6 | 14.2 ± 1.5 | 14.0 ± 1.5 | 13.3 ± 1.4 |
| Food intake at 8th week (g/day) | 14.2 ± 1.4 | 13.8 ± 1.3 | 14.4 ± 1.5 | 13.5 ± 1.4 |
| Energy expenditure (kcal/kg0.75/day) | 103 ± 11 | 106 ± 12 | 110 ± 11 | 130 ± 13* |
| Respiratory quotient | 0.84 ± 0.11 | 0.84 ± 0.10 | 0.81 ± 0.10 | 0.79 ± 0.09 |
| VO2 (mL/kg0.75/min) | 14.7 ± 1.8 | 14.9 ± 1.8 | 15.2 ± 2.0 | 18.5 ± 2.5* |
| VCO2 (mL/kg0.75/min) | 12.7 ± 1.7 | 12.9 ± 1.8 | 13.0 ± 2.0 | 15.0 ± 2.1 |
| Carbohydrate oxidation (mg/kg0.75/min) | 5.8 ± 0.7 | 5.8 ± 0.7 | 5.4 ± 0.7 | 4.9 ± 0.7* |
| Fat oxidation (mg/kg0.75/min) | 5.2 ± 0.6c | 5.4 ± 0.7bc | 6.0 ± 0.7b | 9.0 ± 1.1a |
OVX-control: OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L: OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H: OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control: sham rats fed a high-fat diet (HFD) with 0.6% dextrin with normal estrogen levels. Values are mean ± SD.
Significantly different among all groups at P < 0.05.
Significant difference between OVX-control and normal-control rats at P < 0.05.
Changes in lipid metabolism by KME
Triglyceride contents in the gastrocnemius and quadriceps muscles were higher in OVX-control rats than normal-control rats, but were significantly lowered by KME-H in comparison to the OVX-control (Table 4). The triglyceride storage in the quadriceps muscles was dose-dependently decreased by KME in the OVX rats (Table 4), but did not decrease to the levels of the normal-control group.
Table 4.
Triglyceride storage in the liver, gastrocnemius muscle, and quadriceps muscles
| OVX-control (n = 15) | KME-L (n = 15) | KME-H (n = 15) | Normal-control (n = 15) | |
|---|---|---|---|---|
| Liver (mg/g tissues) | 0.61 ± 0.08a | 0.55 ± 0.08ab | 0.49 ± 0.07b | 0.45 ± 0.06b |
| Gastrocnemius muscles (mg/g tissues) | 1.21 ± 0.26a | 1.03 ± 0.24ab | 0.91 ± 0.18b | 0.67 ± 0.18c |
| Quadriceps muscles (mg/g tissues) | 2.57 ± 0.38a | 2.36 ± 0.32b | 2.04 ± 0.24c | 1.21 ± 0.22c |
OVX-control: OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L: OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H: OVX rats fed HFD with 0.6% Korean mistletoe water extract; normal-control: sham rats fed a high-fat diet (HFD) with 0.6% dextrin with normal estrogen levels. Values are mean ± SD.
Significantly different among all groups at P < 0.05.
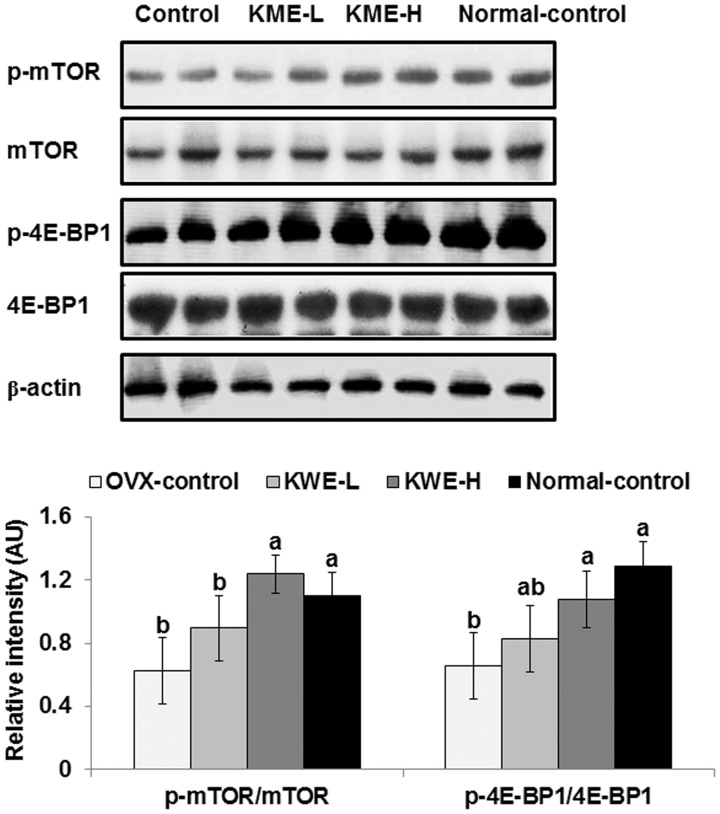
Since lean mass in the abdomen and legs was affected by OVX and KME, the mTOR → 4E-BP1 signaling, a regulator of muscle mass, was examined in the gastrocnemius muscles. The phosphorylation of mTORSer2448 and 4E-BP1Thr37/46 was lower in OVX-control rats than normal-control rats, but KME normalized the phosphorylation to levels of the normal-control group (Figure 4).
Figure 4.
The phosphorylation of mTOR and 4E-BP1Thr37 in the quadriceps muscle. OVX-control, OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L, OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H, OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control, sham rats fed a HFD with 0.6% dextrin with normal estrogen levels. At end of the experimental period, the phosphorylation of mTORSer2448, mTOR, and 4E-BP1Thr37/46 in the quadriceps muscle was measured by immunoblotting. Each bar represents the mean ± SD (n = 6). a,bSignificantly different among all groups at P < 0.05
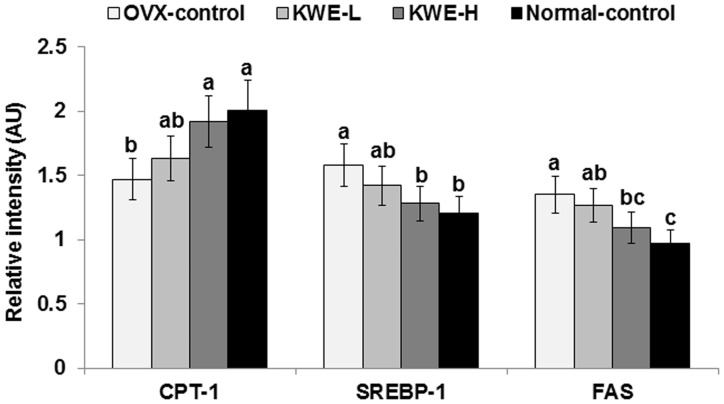
Hepatic triglyceride contents were higher in OVX-control rats than in normal-control rats, but were normalized by KME-H (Table 4). Expression of CPT-1, a key regulator of fatty acid oxidation, was lower in OVX-control rats, but KME-H rats had similar expression levels as normal-control rats (Figure 5). SREBP-1c and FAS, which are involved in the regulation of fatty acid synthesis, were higher in OVX-control rats, but KME-H maintained their expression at similar levels as normal-control rats (Figure 5).
Figure 5.
mRNA expression of CPT-1, SREBP-1c, and FAS in the liver. OVX-control, OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L, OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H, OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control, sham rats fed a HFD with 0.6% dextrin with normal estrogen levels. At end of the experimental period, mRNA levels of hepatic genes involved in fatty acid oxidation (CPT-1) and synthesis (SREBP-1c and FAS) were measured by real-time PCR. Each bar represents the mean ± SD (n = 5). a,b,cSignificantly different among all groups at P < 0.05
Total and low-density lipoprotein (LDL) cholesterol and triacylglycerol concentrations were significantly higher and high-density lipoprotein (HDL) cholesterol levels were lower in OVX-control rats than normal-control rats (Table 5). KME-H decreased the serum levels of total and LDL cholesterol, but not to the normal-control levels. Serum triglyceride and HDL cholesterol levels were lower in a dose-dependent manner in OVX rats, but in KME-H rats they were similar to those of normal-control rats (Table 5).
Table 5.
Serum lipid and glucose profiles in overnight-fasted rats at the end of the 12-week treatment
| OVX-control (n = 15) | KME-L (n = 15) | KME-H (n = 15) | Normal-control (n = 15) | |
|---|---|---|---|---|
| Serum total cholesterol (mg/dL) | 112 ± 10a | 107 ± 10ab | 101 ± 8ab | 92.1 ± 9c |
| Serum LDL cholesterol (mg/dL) | 58.0 ± 6.9a | 51.7 ± 6.7a | 42.8 ± 6.2b | 36.1 ± 4.8c |
| Serum HDL cholesterol (mg/dL) | 36.4 ± 4.1b | 38.9 ± 3.9ab | 42.4 ± 4.3a | 41.1 ± 3.3a |
| Serum triglyceride (mg/dL) | 87.4 ± 6.9a | 82.3 ± 5.6ab | 79.2 ± 6.0b | 74.6 ± 6.2b |
| Serum glucose (mg/dL) | 108 ± 12 | 104 ± 12 | 101 ± 13 | 88.0 ± 10* |
| Serum insulin (ng/mL) | 1.33 ± 0.18 | 1.30 ± 0.17 | 1.27 ± 0.16 | 0.94 ± 0.13* |
| HOMA-IR | 8.0 ± 1.0a | 7.5 ± 0.9ab | 7.1 ± 0.9b | 4.6 ± 0.7c |
OVX-control: OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L: OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H: OVX rats fed HFD with 0.6% Korean mistletoe water extract; normal-control: sham rats fed a high-fat diet (HFD) with 0.6% dextrin with normal estrogen levels. Values are mean ± SD.
Significantly different among all groups at P < 0.05.
Significant difference between OVX-control and normal-control rats at P < 0.05.
Changes in glucose metabolism by KME
OVX-control rats exhibited impaired glucose homeostasis compared to normal-controls: serum levels of both glucose and insulin were higher in OVX-control than in normal-control rats (Table 5). KME did not alter overnight-fasted serum glucose and insulin levels, but HOMA-IR was markedly increased in OVX rats, compared to normal-control rats and was partially restored by KME-H supplementation (Table 5).
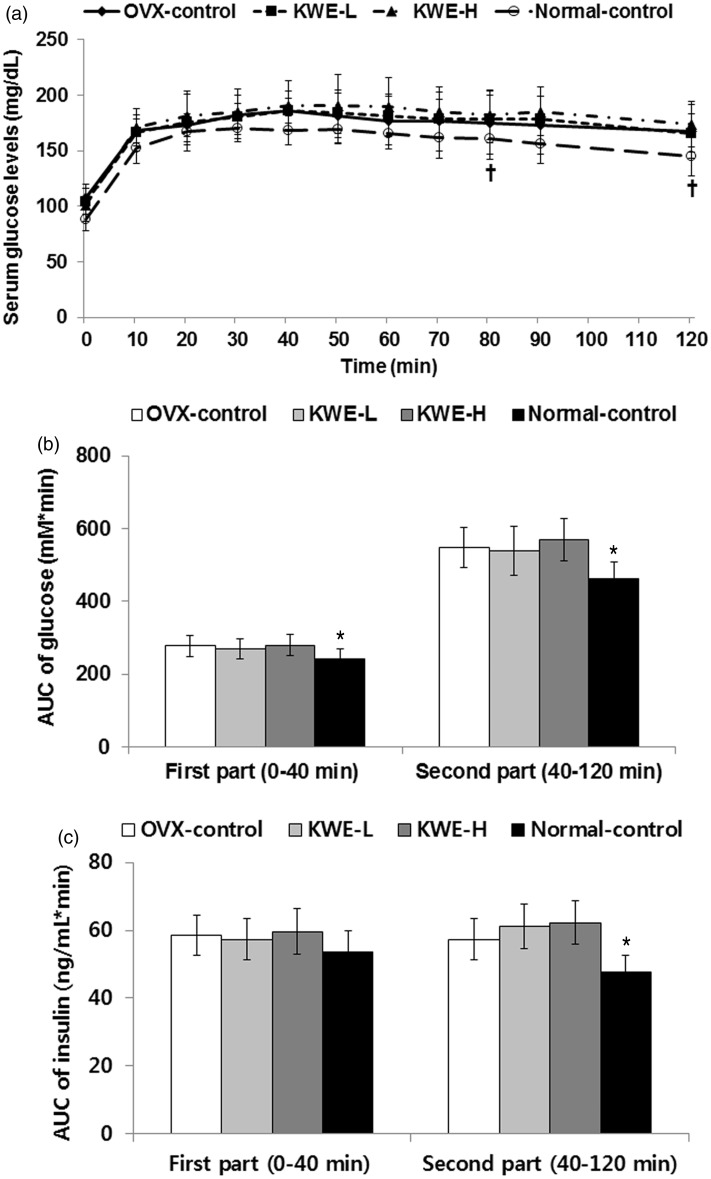
During oral glucose tolerance test (OGTT), serum glucose levels at peak were higher and they decreased more slowly in OVX-control rats than normal-control rats (Figure 6a), indicating that OVX induced glucose intolerance, but KME did not affect serum glucose levels during OGTT. Area under the curve (AUC) of glucose at both first phase (0–40 min) and second phase (40–120 min) during OGTT was higher in OVX-control rats than normal-control rats (Figure 6b). The AUC of insulin during the second phase was higher by 20.3% in OVX-control rats than normal-control rats, but was not affected at either phase by KME (Figure 6c), suggesting that KME did not alter glucose tolerance although it did reduce insulin resistance.
Figure 6.
Serum glucose levels and area under the curve (AUC) of glucose and insulin during oral glucose tolerance test (OGTT). OVX-control, OVX rats fed a high-fat diet (HFD) with 0.6% dextrin; KME-L, OVX rats fed HFD with 0.2% Korean mistletoe water extract + 0.4% dextrin; KME-H, OVX rats fed HFD with 0.6% Korean mistletoe water extract. Normal-control, sham rats fed a HFD with 0.6% dextrin with normal estrogen levels. a. Changes of serum glucose levels during OGTT. b. The average of the AUC of glucose during the first part (0–40 min) and second part (40–120 min) of OGTT. c. The average of the AUC of insulin during the first part (0–40 min) and second part (40–120 min) of OGTT. Each dot and bar represents the mean ± SD (n = 15). *Significantly different between the OVX-control and normal-control groups at P < 0.05
Discussion
Menopause results in disturbances in energy, glucose, lipid, and bone metabolism due to low estrogen levels.1,3 These dysregulations are reversed by estrogen treatment.1,3 However, estrogen treatment has adverse side effects such as potential risk of ischemic stroke, breast cancer, and endometrial cancer.2,3 Estrogen works differently when binding to receptor-α versus -β in different target tissues, and some phytoestrogens and drugs selectively activate estrogen receptor-β in liver, brain, and skeletal muscles, but not in the α receptors in breast and uterus and are considered SERMs.4,5 These SERMs alleviate metabolic dysregulation but minimize adverse effects. In addition, phytoestrogens have varied activities for alleviating menopausal symptoms and metabolic disturbances. Thus, all menopausal symptoms need to be measured when herbal extracts and/or single compounds are studied for the alleviation of menopausal symptoms. The present study found that KME-H suppressed serum levels of total and LDL cholesterol and increased HDL cholesterol in OVX rats that exhibited dyslipidemia. Furthermore, KME-H improved insulin sensitivity in OVX rats. Surprisingly, KME increased lean body mass in the abdomen and leg without changing fat mass, but KME did not alter BMD in the lumbar spine and femur. The increase in lean mass was related to increased phosphorylation of mTOR and 4E-BP1 in the skeletal muscles. Hepatic triglyceride contents were lowered by KME-H in OVX rats by increasing CPT-1 expression and decreasing FAS and SREBP-1c expression, which indicates an upregulation of fatty acid oxidation and downregulation of fatty acid synthesis. Therefore, KME may have efficacy for improving post-menopausal hepatic lipid metabolism and suppressing the loss of skeletal muscle mass.
As mistletoe contains polyphenols, phenolic acids, flavonoids, terpenoids, and lectins, it has been studied as both water and methanol extracts.9 Methanol and ethanol extracts contain more flavonoids and terpenoids but do not have peptides. However, water extracts include peptides and proteins. In the present study, water extract of Korean mistletoe contained less polyphenols, and did not contain terpenoids such as betulinic acid and oleanolic acid. However, the water extract of Korean mistletoe contained viscothionins. Thus, the bioactivities of Korean mistletoe water and methanol extracts are expected to be different and should be studied separately. Furthermore, the toxicity of mistletoe has raised concern. Korean mistletoe is known to be relatively non-toxic, compared to European and North American mistletoe, but it was shown to be somewhat toxic if not properly prepared.25 We conducted a preliminary mouse study examining the toxicity of Korean mistletoe water extracts heated for 12 h at 80℃, and it exhibited no toxicity, including liver damage, when 5 g KME/kg bw was given for one month. Therefore, we used the properly prepared water extract to determine its effect on menopausal symptoms in this study.
Hot flushes are one of the common symptoms experienced by menopausal women and are characterized by a transient increase in skin temperature.1 This symptom is also induced in OVX rats and can be monitored by measuring tail skin temperature.21 It is related to vasomotor dysfunction due to estrogen deficiency.26,27 Shuto et al.26 demonstrated that tail skin temperatures are increased in OVX rats after forced exercise and it is related to exacerbated vasomotor dysfunction via decreasing the production and release of nitric oxide. In addition, serotonin reuptake inhibitors are the first-line therapy for hot flushes since serotonin in the brain ameliorates it.28,29 In the present study, skin temperature was higher in OVX-control rats than normal-control rats and it increased over time only in OVX-control rats. KME consumption significantly ameliorated rat skin temperature at the eighth week of the experimental period in comparison to OVX-control rats. Thus, KME may be beneficial for alleviating hot flushes.
Estrogen deficiency is known to disturb energy metabolism, reallocate fat distribution, decrease lean body mass, and as a result, it markedly increases visceral fat mass and decreases muscle mass in humans and animals.24 Energy dysregulation in the estrogen deficient state is mainly due to decreased energy expenditure and not energy intake. In the present study, OVX-control rats increased bw and visceral fat mass and decreased brown adipose tissues. Surprisingly, KME prevented the decrease in lean body mass in the abdomen and leg during the experimental periods, probably due to the potentiation of mTOR → 4 E-BP1 signaling. Jung et al.30 found that water extract of Korean mistletoe enhances exercise endurance by increasing mitochondrial activity, possibly by activating peroxisome proliferator-activated receptor gamma coactivator 1-α and sirtuin-1 in mice. They suggested that the water extract has great potential as a novel mitochondria-activating agent. However, it may also be due to increasing lean muscle mass as shown in the present study. However, KME did not alter bw and visceral fat mass in OVX rats in the present study. In addition, KME did not affect energy intake or energy expenditure in OVX-control rats. However, since daily energy expenditure was slightly higher in the KME-H group than the OVX-control group (P = 0.08), the accumulation of energy expenditure during total experimental periods would be expected to be higher in KME-H group. KME did result in a small but significant increase in fat oxidation in comparison to OVX control rats, but was still much less than normal-control rats. Furthermore, the amount of brown adipose tissues was greater in the KME group than the OVX-control group whereas UCP-1 in brown adipose tissues tended to be greater in the KME group but it was not significantly different. The lack of change in bw might be related to increased muscle mass. In a study of the effects of KME on energy metabolism, Jung et al.30 demonstrated that 12-week supplementation of 0.4% water extract of Korean mistletoe lowered bw and epididymal fat mass in C57BL/6 male mice fed a HFD without changing food intake. In addition, the supplementation increased UCP-1 expression in brown adipose tissues during cold exposure. Thus, KME may increase fat oxidation in some tissues independent of the estrogen pathway.
Post-menopausal dyslipidemia and hepatic steatosis are common in humans and experimental animals.31,32 The anti-dyslipidemia effect and mechanism of mistletoe extracts have not been thoroughly studied although Korean mistletoe protects against hepatic steatosis in mice with high fat induced obesity.33 The present study showed that KME-H prevented the dysregulation of lipid metabolism in OVX rats and the circulating and hepatic lipid levels of KME-H treated OVX rats were similar to those of normal-control rats. Several studies have evaluated the effects of mistletoe on lipid metabolism: ethanol extract of African mistletoe normalized the streptozotocin-induced dyslipidemia in male mice34 and ethanol extract of European mistletoe prevented high cholesterol diet-induced dyslipidemia in male mice, but water extract only decreased serum triglyceride levels.35 A survey of traditional healers in the African country of Cameroon found that the African mistletoe (Loranthaceae species) is used for treating menopausal symptoms, but no scientific evidence of efficacy was provided.36 Therefore, this is the first study we are aware of that investigated the use of mistletoe for treating symptoms and metabolic abnormalities associated with menopause.
In conclusion, KME alleviated hot flushes and ameliorated dyslipidemia and hepatic steatosis by decreasing cholesterol biosynthesis due to suppressed SREBP-1c expression and by increasing fatty acid utilization to normal levels in OVX rats. In addition, KME improved insulin sensitivity and increased lean body mass by potentiating phosphorylation of mTOR and 4E-BP1, to levels similar to those of normal-control rats. These results suggested that KME is beneficial for alleviating some menopausal symptoms and may be useful in combination with other herbs for alleviating energy dysregulation and maintaining lean body mass.
ACKNOWLEDGMENT
This work was supported by “Food Functionality Evaluation program” under the Ministry of Food, Agriculture, Forestry and Fisheries and the Korea Science and Engineering Foundation in Korea in 2013 and the Nclear Research & Development Program of the Korea Science and Engineering Foundation Grant funded by the Government of the Republic of Korea.
Author contributions
MJ-K, JH-P, S-P, and DY-K participated in the experimental design; MJ-K, HJ-Y, DS-K, S-K, BK-S, NR-M, BS-S, and JH-K conducted the experiments; S-P, MJ-K, and JH-P wrote the manuscript; all authors participated in analysis of the data and review of the manuscript.
REFERENCES
- 1.Al-Safi ZA, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertil Steril 2014; 101: 905–15. [DOI] [PubMed] [Google Scholar]
- 2.Renoux C, Suissa S. Hormone therapy administration in postmenopausal women and risk of stroke. Womens Health (Lond Engl) 2011; 7: 355–61. [DOI] [PubMed] [Google Scholar]
- 3.Rozenberg S, Vandromme J, Antoine C. Postmenopausal hormone therapy: risks and benefits. Nat Rev Endocrinol 2013; 9: 216–27. [DOI] [PubMed] [Google Scholar]
- 4.Moreira AC, Silva AM, Santos MS, Sardão VA. Phytoestrogens as alternative hormone replacement therapy in menopause: what is real, what is unknown. J Steroid Biochem Mol Biol 2014; 143C: 61–71. [DOI] [PubMed] [Google Scholar]
- 5.Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol 2014; 143C: 207–22. [DOI] [PubMed] [Google Scholar]
- 6.Ma D, Taku K, Zhang Y, Jia M, Wang Y, Wang P. Serum lipid-improving effect of soyabean β-conglycinin in hyperlipidaemic menopausal women. Br J Nutr 2013; 110: 1680–4. [DOI] [PubMed] [Google Scholar]
- 7.Hajirahimkhan A, Dietz BM, Bolton JL. Botanical modulation of menopausal symptoms: mechanisms of action? Planta Med 2013; 79: 538–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agbo MO, Lai D, Okoye FB, Osadebe PO, Proksch P. Antioxidative polyphenols from Nigerian mistletoe Loranthus micranthus (Linn.) parasitizing on Hevea brasiliensis. Fitoterapia 2013; 86: 78–83. [DOI] [PubMed] [Google Scholar]
- 9.Vicas SI, Rugina D, Socaciu A. Antioxidant Activity of European Mistletoe (Viscum album). In: Rao V. (ed). Phytochemicals as nutraceuticals - global approaches to their role in nutrition and health, Rijeka, Croatia: InTech, 2012. [Google Scholar]
- 10.Choudhary MI, Maher S, Begum A, Abbaskhan A, Ali S, Khan A, Shafique-ur-Rehman, Atta-ur-Rahman. Characterization and antiglycation activity of phenolic constituents from Viscum album (European Mistletoe). Chem Pharm Bull (Tokyo) 2010; 58: 980–2. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH. Viscothionin extract of Korean Mistletoe (Viscum album coloratum) regulates lipid metabolism through activation of AMP-activated protein kinase. Dissertation, Chonbuk National University, 2014.
- 12.Lee CH, Kim JK, Kim HY, Park SM, Lee SM. Immunomodulating effects of Korean mistletoe lectin in vitro and in vivo. Int Immunopharmacol 2009; 9: 1555–61. [DOI] [PubMed] [Google Scholar]
- 13.Seifert G, Jesse P, Laengler A, Reindl T, Luth M, Lobitz S, Henze G, Prokop A, Lode HN. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett 2008; 14: 218–28. [DOI] [PubMed] [Google Scholar]
- 14.Heinzerling L, von Baehr V, Liebenthal C, von Baehr R, Volk HD. Immunologic effector mechanisms of a standardized mistletoe extract on the function of human monocytes and lymphocytes in vitro, ex vivo, and in vivo. J Clin Immunol 2006; 14: 347–59. [DOI] [PubMed] [Google Scholar]
- 15.Orhan DD, Aslan M, Sendogdu N, Ergun F, Yesilada E. Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J Ethnopharmacol 2005; 98: 95–102. [DOI] [PubMed] [Google Scholar]
- 16.Onay-Uçar E, Karagöz A, Arda N. Antioxidant activity of Viscum album ssp. album. Fitoterapia 2006; 77: 556–60. [DOI] [PubMed] [Google Scholar]
- 17.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555–9. [Google Scholar]
- 18.Park JH, Hyun CK, Shin HK. Cytotoxic effects of the components in heat-treated mistletoe. Cancer Lett 1999; 139: 207–13. [DOI] [PubMed] [Google Scholar]
- 19.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 1997; 127: 838S–41S. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–19. [DOI] [PubMed] [Google Scholar]
- 21.Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer's. PLoS One 2013; 8: e59825–e59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lusk G. Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 1924; 59: 41–41. [Google Scholar]
- 23.Ko BS, Kim DS, Kan S, Lee NR, Ryuk JA, Park S. Wnt-signaling-mediated antiosteoporotic activity of porcine placenta hydrolysates in ovariectomized rats. Evid Based Complement Alternat Med 2012; 2012: 367698–367698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko BS, Kim da S, Kang S, Ryuk JA, Park S. Prunus mume and Lithospermum erythrorhizon extracts synergistically prevent visceral adiposity by improving energy metabolism through potentiating hypothalamic leptin and insulin signaling in ovariectomized rats. Evid Based Complement Alternat Med 2013; 2013: 750986–750986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung NY, Byun EB, Song DS, Jin YB, Kim JK, Park JH, Song BS, Jung PM, Byun MW, Lee JW, Park SH, Kim JH. Effect of gamma irradiation on mistletoe (Viscum album) lectin-mediated toxicity and immunomodulatory activity. FEBS Open Bio 2013; 3: 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuto H, Tominaga K, Yamauchi A, Ikeda M, Kusaba K, Mitsunaga D, Hirabara Y, Egawa T, Takano Y, Kataoka Y. The statins fluvastatin and pravastatin exert anti-flushing effects by improving vasomotor dysfunction through nitric oxide-mediated mechanisms in ovariectomized animals. Eur J Pharmacol 2011; 651: 234–9. [DOI] [PubMed] [Google Scholar]
- 27.Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev 2013; 12: CD001395–CD001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 2002; 23: 665–86. [DOI] [PubMed] [Google Scholar]
- 29.Egawa M, Yamauchi T, Sohda A, Koga Y, Tominaga A, Shuto HK, Kataoka Y. Selective serotonin reuptake inhibitors fluvoxamine and paroxetine restore forced exercise-induced temperature dysregulation in ovariectomized mice. Eur J Pharmacol 2008; 579: 439–44. [DOI] [PubMed] [Google Scholar]
- 30.Jung HY, Lee AN, Song TJ, An HS, Kim YH, Kim KD, Kim IB, Kim KS, Han BS, Kim CH, Kim KS, Kim JB. Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. J Med Food 2012; 15: 621–8. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond Engl) 2009; 5: 191–203. [DOI] [PubMed] [Google Scholar]
- 32.Crandall CJ, Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am 2013; 42: 227–53. [DOI] [PubMed] [Google Scholar]
- 33.Jung HY, Kim YH, Kim IB, Jeong JS, Lee JH, Do MS, Jung SP, Kim KS, Kim KT, Kim JB. The Korean mistletoe (Viscum album coloratum) extract has an antiobesity effect and protects against hepatic steatosis in mice with high-fat diet-induced obesity. Evid Based Complement Alternat Med 2013; 2013: 168207–168207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adaramoye O, Amanlou M, Habibi-Rezaei M, Pasalar P, Ali MM. Methanolic extract of African mistletoe (Viscum album) improves carbohydrate metabolism and hyperlipidemia in streptozotocin-induced diabetic rats. Asian Pac J Trop Med 2012; 5: 427–33. [DOI] [PubMed] [Google Scholar]
- 35.Avci G, Kupeli E, Eryavuz A, Yesilada E, Kucukkurt I. Antihypercholesterolaemic and antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. J Ethnopharmacol 2006; 107: 418–23. [DOI] [PubMed] [Google Scholar]
- 36.Didier DS, Laurier EON, Din N, Jules PR, Victor T, Henri F, Georges S, Didier MA, Joseph BI, Akoa A. A assessment on the uses of Loranthaceae in ethno pharmacology in Cameroon: A case study made in Logbessoui, North of Douala. J Med Plant Res 2009; 3: 592–5. [Google Scholar]