Abstract
Synchronization of oocyte maturation in vitro has been shown to produce higher in vitro fertilization (IVF) rates than those observed in oocytes matured in vitro without synchronization. However, the increased IVF rates never exceeded those observed in oocytes matured in vivo without synchronization. This study was therefore designed to define the effect of in vivo synchronization of oocyte maturation on IVF rates. Mice were superovulated and orally treated with 7.5 mg cilostazol (CLZ), a phosphodiesterase 3A (PDE3A) inhibitor, to induce ovulation of immature oocytes at different stages depending on frequency and time of administration of CLZ. Mice treated with CLZ ovulated germinal vesicle (GV) or metaphase I (MI) oocytes that underwent maturation in vitro or in vivo (i.e. in the oviduct) followed by IVF. Superovulated control mice ovulated mature oocytes that underwent IVF directly upon collection. Ovulated MI oocytes matured in vitro or in vivo had similar maturation rates but significantly higher IVF rates, 2–4 cell embryos, than those observed in control oocytes. Ovulated GV oocytes matured in vitro showed similar maturation rates but significantly higher IVF rates than those observed in control oocytes. However, ovulated GV oocytes matured in vivo had significantly lower IVF rates than those noted in control oocytes. It is concluded that CLZ is able to synchronize oocyte maturation and improve IVF rates in superovulated mice. CLZ may be capable of showing similar effects in humans, especially since temporal arrest of human oocyte maturation with other PDE3A inhibitors in vitro was found to improve oocyte competence level. The capability of a clinically approved PDE3A inhibitor to improve oocyte fertilization rates in mice at doses extrapolated from human therapeutic doses suggests the potential scenario of the inclusion of CLZ in superovulation programs. This may improve IVF outcomes in infertile patients.
Keywords: Cilostazol, superovulation, synchronization, immature oocytes, in vitro maturation, in vivo maturation, in vitro fertilization, fertility
Introduction
Oocyte maturation involves cytoplasmic and meiotic maturation. Oocyte cytoplasmic maturation refers to events that occur during two distinct phases of oocyte development: follicle growth and meiotic maturation. Oocyte cytoplasmic maturation during follicle growth includes accumulation and storage of maternal mRNA, proteins, substrates, and nutrients. This maturation phase is essential for early embryonic survival, especially before maternal-zygotic gene transition, which is around 2 and 4/8-cell stages in mouse and human embryos, respectively.1–3 The higher developmental capacity observed in large oocytes over that of small oocytes in many species such as bovine,4 canine,5 and feline6 suggests more accumulation of nutrients and transcripts at this phase of cytoplasmic maturation, and consequently larger oocyte sizes that efficiently support early embryonic development. This cytoplasmic maturation at follicle growth was found to cease as the fully grown oocyte at the prophase I stage started to enter meiotic maturation. However, oocytes matured in vitro may initiate meiotic maturation even though their cytoplasmic maturation is not yet completed, resulting in oocytes with low competence level.7,8 Studies have shown that temporal arrest of oocyte meiotic maturation in vitro, using phosphodiesterase 3A (PDE3A) inhibitors, was able to improve oocyte quality and in vitro fertilization (IVF) rates. This suggests that temporal arrest of oocyte meiotic maturation in vitro results in oocytes with higher cytoplasmic maturation and competence levels.9–13
The second phase of oocyte cytoplasmic maturation, which occurs during meiotic maturation, includes morphological and biochemical events such as cortical granule migration,14 microfilament relocation,15 mitogen-activated protein kinase phosphorylation,16 cyclin B synthesis,17 and p34cdc2 kinase activation.18 This phase of maturation is also believed to fall behind meiotic maturation when oocytes are matured in vitro. Temporal arrest of oocyte meiotic maturation in vitro can synchronize both cytoplasmic and meiotic maturation and result in oocytes of higher quality.9–13
Meiotic maturation refers to the 1st meiotic division and transition of an immature oocyte at the prophase I stage with a germinal vesicle (GV) to a mature oocyte at the metaphase II (MII) stage with a 1st polar body. Nonsynchronized maturation of oocytes can not only occur in vitro but also in vivo upon the administration of exogenous gonadotropins in superovulation programs.19,20 However, the beneficial effect of in vivo synchronization of oocyte maturation on IVF rates in superovulation programs has not yet been addressed.
Cilostazol (CLZ) is a safe PDE3A inhibitor that is prescribed to patients with intermittent claudication disease in Europe, USA, and Japan. This compound was recently reported to inhibit oocyte meiotic maturation in superovulated mice and in a reversible manner.21 This study was designed to evaluate the ability of CLZ to synchronize oocyte maturation in vivo and to improve IVF success rates and development to the 2–4 embryo cell stage in superovulated mice.
Materials and methods
Mice and ethical approval
Swiss Webster mice, 8–10 weeks old, were purchased from Harlan Laboratories (Houston, TX). Mice were maintained under controlled temperature (23℃) and a light/dark (14/10 h) cycle. Food and water were provided ad libitum. All experiments were approved by the Texas A&M University Institutional Animal Care and Use Committee.
Reagents and drug administrations
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Superovulated mice were intraperitoneally injected with 5 IU pregnant mare serum gonadotropin (PMSG) followed in 47 h with 5 IU of human chorionic gonadotropin (hCG). Both PMSG and hCG were purchased from Intervet Inc., (Summit, NJ, USA). CLZ (LKT Laboratories Inc., St. Paul, MN, USA) doses of 7.5 mg were dissolved in aliquots of 0.1 mL dimethyl sulfoxide (DMSO) immediately before oral administration (gavage). This CLZ dose was extrapolated from the pharmacokinetics of CLZ in humans.21 The human tubal fluid (HTF) complete culture medium (Zenith Biotech, Guilford, CT, USA) supplemented with 4.5% fetal bovine serum with or without HEPES was used for oocyte and sperm collection or oocyte incubation and IVF, respectively.
Treated mice ovulating metaphase I oocytes
To study the ability of ovulated metaphase I (MI) oocytes to undergo synchronized in vitro or in vivo maturation and IVF, superovulated mice were treated with 7.5 mg CLZ 7 h pre-hCG to induce ovulation of MI oocytes.21 These mice were designated as Group I. Group I was divided into three subgroups. In the first subgroup, oocytes were retrieved 14 h post-hCG. For the second subgroup, ovulated oocytes were retrieved 14 h post-hCG followed by in vitro maturation (IVM) for 3 h to allow for MI oocytes to mature into MII oocytes. For the third subgroup, oocytes were retrieved 17 h post-hCG to allow for ovulated MI oocytes to undergo in vivo oocyte maturation (IVOM), in the oviduct, for 3 h. The ovulation was monitored and determined to occur in this mouse strain 14 h post-hCG. Oocytes from all of these subgroups were then denuded of cumulus cells, using 0.6% bovine hyaluronidase enzyme, and scored for maturational status. In other experiments, ovulated cumulus enclosed oocytes were collected as described in subgroups 2 and 3 followed by IVF 17 h post-hCG without denudation.
Other mice were treated with 7.5 mg CLZ 4 h pre-hCG to induce ovulation of MI oocytes.21 The latter mice were designated as Group II. Group II was divided into three subgroups. Ovulated oocytes from the first subgroup were retrieved 14 h post-hCG. For the second subgroup, ovulated oocytes were retrieved 14 h post-hCG followed by IVM for 6 h to allow for MI oocytes to mature into MII oocytes. For the third subgroup, oocytes were retrieved 20 h post-hCG to allow for ovulated MI oocytes to undergo IVOM in the oviduct for 6 h. Ovulated oocytes from all of these subgroups were then denuded and scored for maturational status. In other experiments, ovulated oocytes were collected as described in subgroups 2 and 3 followed by IVF 20 h post-hCG without denudation.
Treated mice ovulating GV oocytes
To study the ability of ovulated GV oocytes to undergo synchronized in vitro or in vivo maturation and IVF, superovulated mice were treated with 7.5 mg CLZ 4 h pre-hCG and 2 h post-hCG to induce ovulation of GV oocytes.21 These mice were designated as Group III. Group III was divided into five subgroups. In the first subgroup, oocytes were retrieved 14 h post-hCG. For the second subgroup, ovulated GV oocytes were collected 15.5 h post-hCG to allow for ovulated GV oocytes to undergo germinal vesicle breakdown (GVBD) in the oviduct for 1.5 h (IVOM). For the third subgroup, oocytes were retrieved 14 h post-hCG followed by IVM for 1.5 h to allow for ovulated GV oocytes to undergo GVBD in vitro. For the fourth subgroup, oocytes were retrieved 14 h post-hCG followed by IVM for 10 h to allow for ovulated GV oocytes to mature into MII oocytes in vitro. For the fifth subgroup, oocytes were retrieved 24 h post-hCG to allow for ovulated oocytes to undergo IVOM in the oviduct for 10 h and to mature into MII oocytes. All oocytes were then denuded and scored for maturational status. In other experiments, ovulated cumulus enclosed oocytes were collected as described in subgroups 4 and 5 followed by IVF 24 h post-hCG without denudation.
Control mice ovulating MII oocytes
The control group corresponding to Group I mice received 0.1 mL DMSO 7 h pre-hCG. The control group corresponding to Group II mice received 0.1 mL DMOS 4 h pre-hCG whereas the control group corresponding to Group III mice received 0.1 mL DMSO 4 h pre-hCG and 2 h post-hCG. All control mice were sacrificed 14 h post-hCG. Ovulated control oocytes were denuded from cumulus cells and scored for maturational status or in vitro fertilized directly upon collection without denudation. These oocytes were designated as control oocytes.
Determination of the timing of oocyte maturation in vivo
To define oocyte maturation in superovulated mice not treated with CLZ, mice were superovulated, and oocytes were collected 1.5, 3, 6, or 10 h post-hCG from large preovulatory follicles. Oocytes were also collected 14 h post-hCG from the oviduct after the ovulation. Oocytes were then denuded and scored for maturation status. These oocytes were designated as control ovarian GV oocytes that underwent IVOM.
Determination of the timing of oocyte maturation in vitro
To define oocyte maturation in vitro, mice not treated with CLZ were sacrificed 47 h post-PMSG, and cumulus enclosed GV oocytes were collected from large antral follicles. The oocytes were incubated for 1.5, 3, 6, 10, or 14 h in the culture medium and then denuded and scored for maturation status. These oocytes were designated as control ovarian GV oocytes that underwent IVM.
IVF
Vasa deferentia attached to thick posterior epididymis tails were isolated from two mature males and flushed in 1.5 mL of HTF medium. Spermatozoa were capacitated for 15 min at 37℃. Final concentrations of 2 × 106 spermatozoa/mL of IVF media containing oocytes collected from three females were prepared. Oocytes were incubated with spermatozoa for 6 h followed by three washes. Oocytes were then incubated for 24 h and scored for 2–4 cell stage embryos. IVM and fertilization of oocyte were conducted at 37℃ with 5% CO2 in humidified air.
Statistics
The Pearson's chi-square test of independence (contingency table of 2 × 2) was used to determine all statistical differences. Two variables, each of two levels, were considered. These variables were the maturation or IVF rates at low or high rates and CLZ treatments at the two doses of 0 or 7.5 mg. The response of low or high rates of oocyte maturation or IVF to the different CLZ treatments was considered dependent when the alpha level of P value was less than 0.05.22 The SPSS 14.0 software (SPSS Inc., Chicago, IL) was used to carry out all the statistical analyses.
Results
MI oocytes
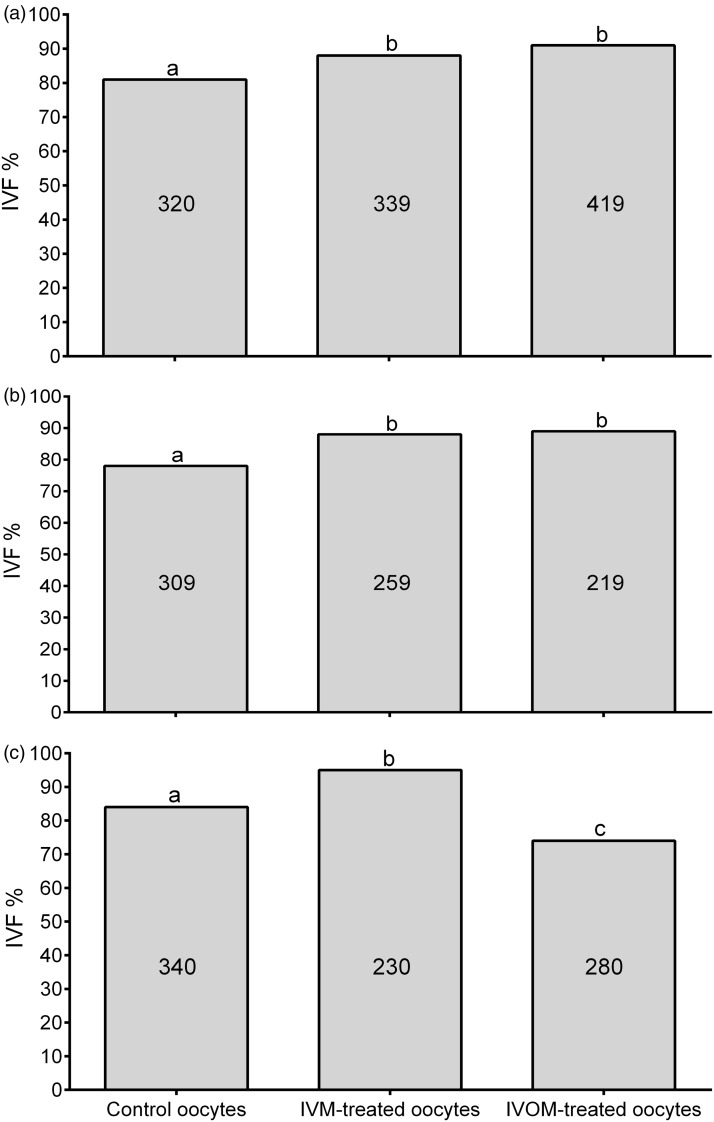
Administration of 7.5 mg CLZ in superovulated mice 7 h pre-hCG injection (Group I) resulted in mice ovulating 97.3% MI oocytes when compared to 3.9% MI oocytes resulting from their corresponding control mice (P < 0.0001, Table 1). Those MI oocytes were synchronized to undergo maturation in vitro or in vivo in similar periods of time. In this regard, MI oocytes resulting from Group I mice had in vitro or in vivo maturation periods of 3 h with MII oocyte yields of 96.3 or 98.8%, respectively, which were not significantly different from the 96.1% MII oocytes resulting from the control group. However, both in vitro and in vivo MI oocytes matured into MII oocytes resulting from Group I had higher IVF rates of 88.2 and 90.6%, respectively, than that of 81.6% observed in their corresponding control group (P < 0.02, Figure 1(a)).
Table 1.
Maturation rates of ovulated MI oocytes resulting from mice treated with 7.5 mg CLZ 7 h pre-hCG (Group I)
| Group | GV |
MI |
MII |
|||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Maturation stages at ovulation | 0.0 (153) | 0.0 (147) | 3.9a (153) | 97.3b (147) | 96.1a (153) | 2.7b (147) |
| IVM for 3 h | – | 0.0 (133) | – | 3.7a (133) | – | 96.3a (133) |
| IVOM for 3 h | – | 0.0 (170) | – | 1.2a (170) | – | 98.8a (170) |
Data are presented as percent (total number of oocytes). Values with different superscripts are significantly different (P < 0.0001) within each oocyte maturation stage of germinal vesicle (GV), metaphase I (MI), or metaphase II (MII). Groups of mice of 7–8 were used for each experiment.
IVM: in vitro maturation; IVOM: in vivo oocyte maturation.
Figure 1.
IVF rates (2–4 cell embryo stage) of ovulated immature oocytes resulting from superovulated mice treated with 7.5 mg CLZ. (a) IVF rates of ovulated MI oocytes resulting from superovulated mice treated with 7.5 mg CLZ 7 h pre-hCG (Group I). (b) IVF rates of ovulated MI oocytes resulting from superovulated mice treated with 7.5 mg CLZ 4 h pre-hCG (Group II). (c) IVF rates of ovulated GV oocytes resulting from superovulated mice treated with 7.5 mg CLZ 4 h pre-hCG and 2 h post-hCG (Group III). Columns with different superscripts are significantly different (P < 0.02 for Figure 1(a), P < 0.002 for Figure 1(b), and P < 0.0001 for Figure 1(c)). Numbers inside columns represent the total number of used oocytes in each subgroup, and 12–16 mice were used in each subgroup
Administration of 7.5 mg CLZ in superovulated mice 4 h pre-hCG injection (Group II) also resulted in mice ovulating 95.6% MI oocytes in comparison to 4.2% MI oocytes resulting from their control group (P < 0.0001, Table 2). Both in vitro and in vivo maturation periods of these MI oocytes were synchronized to 6 h with MII oocyte yields of 98.3 and 95.1%, respectively. These MII oocyte yields were not significantly different from those of controls (95.8%). Both in vitro and in vivo MI oocytes matured into MII oocytes in Group II showed IVF rates higher than those observed in their corresponding control groups (P < 0.002, Figure 1(b)).
Table 2.
Maturation rates of ovulated MI oocytes resulting from mice treated with 7.5 mg CLZ 4 h pre-hCG (Group II)
| Group | GV |
MI |
MII |
|||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Maturation stages at ovulation | 0.0 (140) | 4.4 (136) | 4.2a (140) | 95.6b (136) | 95.8 (140) | 0.0 (136) |
| IVM for 6 h | – | 0.0 (120) | – | 1.7a (120) | – | 98.3 (120) |
| IVOM for 6 h | – | 0.0 (163) | – | 4.9a (163) | – | 95.1 (163) |
Data are presented as percent (total number of oocytes). Values with different superscripts are significantly different (P < 0.0001) within each oocyte maturation stage of germinal vesicle (GV), metaphase I (MI), or metaphase II (MII). Groups of mice of 7–8 were used for each experiment.
IVM: in vitro maturation; IVOM: in vivo oocyte maturation.
GV oocytes
Multiple administrations of CLZ in superovulated mice (Group III) resulted in ovulation of 91.6% GV oocytes in comparison to their corresponding controls that did not ovulate GV oocytes (Table 3). The ovulated GV oocytes were synchronized to undergo maturation in vitro or in vivo in similar periods of time of 10 h to result in 98.4 or 95.0% MII oocytes, respectively. These MII oocyte yields were not significantly different from their corresponding control group (99.2%). IVM of ovulated GV oocytes showed high IVF rates when compared to that of control groups (95% versus 84%, respectively, P < 0.0001, Figure 1(c)). Conversely, IVOM of ovulated GV oocytes showed lower rate of IVF than that of the control group (73.6% versus 84%, respectively, P < 0.0001).
Table 3.
Maturation rates of ovulated GV oocytes resulting from mice treated with 7.5 mg CLZ 4 h pre-hCG and 2 h post-hCG (Group III)
| Group | GV |
MI |
MII |
|||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Maturation stages at ovulation | 0.0 (133) | 91.6 (120) | 0.8a (133) | 8.4b (120) | 99.2a (133) | 0.0 (120) |
| IVM for 10 h | – | 0.0 (126) | – | 1.6a (126) | – | 98.4a (126) |
| IVOM for 106 h | – | 0.0 (160) | – | 5.0b (160) | – | 95.0a (160) |
Data are presented as percent (total number of oocytes). Values with different superscripts are significantly different (P < 0.03) within each oocyte maturation stage of germinal vesicle (GV), metaphase I (MI), or metaphase II (MII). Groups of mice of 7–8 were used for each experiment.
IVM: in vitro maturation; IVOM: in vivo oocyte maturation.
Timing of oocyte meiotic maturation events in vitro and in vivo
GV breakdown
Ovulated GV oocytes (Group III) were synchronized to have GVBD at similar times and yields whether matured in vitro or in vivo when compared to control ovarian GV oocytes. For example, most of ovulated GV oocytes resulting from Group III mice were capable of undergoing GVBD in vitro or in vivo within 1.5 h and with similar yields of MI oocytes of 94.1 and 86.3%, respectively (P > 0.05, Table 4). Conversely, control ovarian GV oocytes showed 20 or 0% of oocytes at the MI stage after 1.5 h of IVM or IVOM, respectively (Table 5). In addition, while most of control ovarian GV oocytes finished GVBD by 3 h when matured in vitro with MI oocyte yield of 81.5%, such control oocytes started GVBD only after 3 h when matured in vivo with MI oocyte yield of 2% (P < 0.0001). This yield of MI oocytes increased to 29% when oocytes were collected 6 h post-hCG injection, which was still not synchronized to the high yield (83.8%) of MI oocytes resulting from control ovarian oocytes matured in vitro for 6 h (P < 0.0001). At 10 h post-hCG, few GV oocytes were collected from large preovulatory follicles when compared to those collected 6 h post-hCG (25% versus 69%, P < 0.0001). At ovulation, only MI and MII oocytes were observed, indicating that GVBD was continuous up to the onset of ovulation.
Table 4.
Timing of meiotic events in ovulated GV oocytes (Group III) matured in vitro or in vivo
| Incubation period (h) | GV |
MI |
MII |
|||
|---|---|---|---|---|---|---|
| IVM | IVOM | IVM | IVOM | IVM | IVOM | |
| 1.5 | 5.9 (118) | 13.7 (95) | 94.1a (118) | 86.3a (95) | 0.0 (118) | 0.0 (95) |
| 10 | 0.0 (126) | 0.0 (160) | 1.6b (126) | 5.0b (160) | 98.4 (126) | 95.0 (160) |
The meiotic stages of oocytes in Group III mice at ovulation were 91.6% GV, 8.4% MI, and 0% MII (n = 120 oocytes). Data are presented as percent (total number of oocytes). Values with different superscripts are significantly different (P < 0.0001) within each oocyte maturation stage of germinal vesicle (GV), metaphase I (MI), or metaphase II (MII). Groups of mice of 8–10 were used for each experiment.
IVM: in vitro maturation; IVOM: in vivo oocyte maturation.
Table 5.
Timing of meiotic events of control ovarian GV oocytes underwent IVM or IVOM
| Incubation period (h) | GV |
MI |
MII |
|||
|---|---|---|---|---|---|---|
| IVM | IVOM | IVM | IVOM | IVM | IVOM | |
| 1.5 | 80.0a (85) | 100.0b (102) | 20.0a (85) | 0.0 (102) | 0.0 (85) | 0.0 (102) |
| 3 | 18.5c (92) | 98.0b (198) | 81.5b (92) | 2.0c (198) | 0.0 (92) | 0.0 (198) |
| 6 | 16.2c (88) | 69.0d (283) | 83.8b (88) | 29.0ad (283) | 0.0 (88) | 2.0a (283) |
| 10 | 14.0c (79) | 25.0c (184) | 35.4ad (79) | 38.4d (184) | 50.6b (79) | 36.6c (184) |
| 14 | 14.3c (99) | 0.0 (157) | 4.0c (99) | 5.0c (157) | 81.7d (99) | 95e (157) |
Data are presented as percent (total number of oocytes). Values with different superscripts are significantly different (P < 0.03) within each oocyte maturation stage of germinal vesicle (GV), metaphase I (MI), or metaphase II (MII). Groups of mice of 8–10 were used for each time point.
IVM: in vitro maturation of control ovarian GV oocytes; IVOM: in vivo oocyte maturation of control ovarian GV oocytes.
First polar body emission
Ovulated GV oocytes (Group III) were synchronized to mature into MII oocytes at similar times and yields whether matured in vitro or in vivo when compared to control ovarian GV oocytes. For example, ovulated GV oocytes matured in vitro or in vivo for 10 h resulted in similar yields of MII oocytes of 98.4 or 95%, respectively (P > 0.05, Table 4). Conversely, scoring of control ovarian GV oocytes matured in vitro or in vivo for 10 h showed MII oocyte rates of 50.6 or 36.6%, respectively (P < 0.03, Table 5). The latter yields were also lower than those observed in ovulated GV oocytes matured in vitro or in vivo (P < 0.0001). Scoring of control ovarian GV oocytes matured in vitro or in vivo for 14 h showed MII oocyte rates of 81.7 or 95%, respectively (P < 0.001). Extending the IVM period to 18 h in control ovarian GV oocytes did not significantly improve the yields of MII oocytes (data not shown). Moreover, the first appearance of 1st polar body 6 h post-hCG in control ovarian GV oocytes suggests that the emission of 1st polar bodies started first 6 h post-hCG and continued until the onset of ovulation.
Discussion
Administration of CLZ in superovulated mice results in ovulation of GV or MI oocytes, which can mature in vitro or in vivo into MII oocytes with yields similar to those observed in control superovulated mice. The resultant MII oocytes from CLZ treated mice were observed to have high fertilization rates when compared to those of superovulated mature oocytes. CLZ also synchronizes meiotic events, such as GVBD and 1st polar body emission, and time periods required for oocyte maturation in vitro or in vivo. For instance, ovulated GV oocytes matured in vitro or in vivo required 1.5 h for most of oocytes to undergo GVBD. Conversely, only small numbers of ovarian GV oocytes underwent GVBD when matured in vitro for 1.5 h whereas scoring of oocytes from large preovulatory follicles at different timings after the hCG injection revealed that GVBD occurred first 3 h post-hCG injection and continued until the onset of ovulation. As for 1st polar body emission, most of ovulated GV oocytes required 10 h of IVM or IVOM to emit 1st polar bodies whereas only smaller numbers of ovarian GV oocytes matured in vitro or in vivo for 10 h emitted 1st polar bodies. It is also interesting that some MII oocytes were collected as early as 6 h post-hCG administration in control mice. This directs attention to the occurrence of premature polar body emission in some oocytes in superovulation, and consequently the ovulation of aged MII oocytes with compromised viability and fertility. This emphasizes the usefulness of synchronization of oocyte maturation in superovulation programs.
Superovulation in rodents has been found to adversely affect embryonic development, cause abnormal microfilament distribution,19 and increase retardation of fetal growth.23 A dose–response relationship between PMSG dose and the incidence of polyploidy has also been noted.20 In IVF clinics, serial measurements of serum estradiol and applications of transvaginal ultrasonography are used to monitor follicular development and to adjust hormonal dosage administrations in superovulation programs.24 Nevertheless, a delay of embryonic development and low birth weights have also been observed after IVF treatment in humans.25,26 In addition, fully grown GV oocytes with active transcriptional activities can respond to administered gonadotropins and terminate the transcriptional activity at earlier times.7,8 It is thus possible that superovulation results in oocytes that lack some essential transcripts and proteins that are required for oocyte maturation and embryonic development. Administration of CLZ may reduce some of these adverse effects resulting from superovulatory hormones because temporal arrest of oocyte maturation in superovulation allows for more GV oocytes to finish synthesis and accumulation of transcripts and proteins that aid in sustaining oocyte maturation and embryonic development. Moreover, temporal arrest of ovarian GV oocytes matured in vitro has been shown to positively affect GV oocytes. Such oocytes were observed to have high rates of nucleoli surrounded with chromatin, chromosomes aligned at equators of normal spindles, maturation, and fertilization in humans,11,12 rodents,9,10,13 and bovine.9 Therefore, the results of high levels of synchronization and fertilization rates observed in this in vivo study using CLZ may also be due to the same positive effects observed in ovarian GV oocytes matured in vitro upon temporal arrest using other PDE3A inhibitors.
Although temporal arrest of maturation of ovarian GV oocytes in vitro was reported to be associated with high oocyte quality and embryonic rates,9–13 other investigators have reported the lack of such beneficial effects.27,28 It is possible that concentrations and types of PDE3A inhibitors used and/or durations of arresting of oocytes at the GV stage are factors that may explain such discrepancies. Similarly, we observed that although arresting of oocytes at the GV stage in vivo followed by IVM for 10 h improved the IVF rate more than that of control MII oocytes, oviductal maturation of ovulated GV oocytes for 10 h reduced IVF rates. Interestingly, oviductal maturation of MI oocytes for 3 or 6 h into MII oocytes showed significantly higher IVF rates in the present study. In humans, delaying IVF after oocyte collection showed high levels of fertilization, fetal development, and pregnancy.29–31 Moreover, delaying oocyte retrieval after the hCG injection showed similar results.32 These results indicate that prolonged in vitro or in vivo maturation of oocytes is beneficial and increases fertilization rates. In the present study, the maturation period of ovulated GV oocytes in vivo or in vitro was similar (10 h), excluding the possibility of oocytes or factor(s) within oocytes undergoing aging. Therefore, it is suggested that other in vivo factor(s) such as CLZ concentrations upon multiple administrations may affect viability and normality of oocytes when at the GV stage.
Genetic polymorphisms in hepatic CYP 450, CYP3A (CYP3A5*3) and CYP2C19 (CYP2C19*2 and CYP2C19*3), were found to cause substantial interindividual variability in CLZ clearance when administered orally in humans.33 It is possible that such genetic polymorphisms in hepatic enzymes are also present in mice that result in CLZ inhibitory concentrations being maintained longer in some of the treated mice. As a result, meiotic maturation kinetics might have been disrupted. It is therefore suggested that some of the MII oocytes, obtained from IVOM of ovulated GV oocytes, may have disrupted meiotic maturation and consequently reduced fertilization capability.
Until the time of preparation of this report, synchronization of oocyte maturation was conducted in vitro but not in vivo and was found to improve oocyte maturation and fertilization rates in humans.9–13 The main shortcoming for not extrapolating these promising in vitro observations into in vivo clinical application is due to the lack of a safe PDE3A inhibitor that can be administered in vivo without inducing cardiovascular adverse effects. In the present study, we have selected CLZ, which is an FDA approved therapeutic (pletal®) with both PDE3A and cellular adenosine uptake inhibitory effects. The latter effect is believed to counterbalance the adverse effects associated with inhibition of PDE3A in the cardiac tissue. Inhibition of adenosine uptake by cells increases adenosine circulatory levels. Adenosine affects adenosine 1 receptor on myocytes leading to activation of Gi protein and deactivation of protein kinase A and therefore inhibition of the positive inotropic like effects resultant from the inhibition of PDE3A in the heart. This explains the wide margin of safety and the safe long-term use of this medication in patients over other PDE3A inhibitors.34–36
Many studies have shown that PDE3A inhibitors are capable of inhibiting oocyte maturation in vitro and in vivo in different animal species.37–40 Other studies have shown that PDE3A inhibitors are also capable of inhibiting human oocyte maturation in vitro.11,12 Inhibition of human oocyte maturation in vivo has not been studied. It is expected that administration of a safe PDE3A inhibitor such as CLZ in women is capable of inhibiting oocyte maturation at the GV or MI stage depending on time and frequency of administration. If this would be the case, we would suggest the potential scenario of inclusion of CLZ in superovulation programs in order to synchronize oocyte maturation and to improve IVF success in infertile patients. Ovarian stimulated patients could be treated with an appropriate CLZ dose(s). Oocytes at the GV or MI stage could be collected before ovulation as currently conducted in IVF clinics and matured in vitro for an appropriate time period before fertilization. Such a modified superovulation program might improve IVF success. Moreover, we have recently produced normal live births from ovulated GV and MI oocytes in mice treated with CLZ (unpublished data). It is also noteworthy to mention that the evaluated dose of CLZ used in this research was extrapolated from the therapeutic doses of CLZ prescribed in humans.21 Therefore, CLZ is not only able to block oocyte meiotic maturation as a potential nonsteroidal contraceptive agent21,41,42 but also can be utilized to produce oocytes of high fertilization capacity. From the later point of view, administration of CLZ in superovulation protocols may improve IVF outcomes in humans.
Acknowledgments
This study was supported by the Iraqi Ministry of Health and Texas A&M University.
Author Contributions
All authors participated in the design of this study, analysis of the data, interpretation of the results, and critically reviewed the paper. AMT wrote the paper.
References
- 1.Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: Cytoplasmic components and their effects on early development. Theriogenology 2001; 55: 1255–76. [DOI] [PubMed] [Google Scholar]
- 2.Krisher RL. The effect of oocyte quality on development. J Anim Sci 2004; 82: 14–23. [DOI] [PubMed] [Google Scholar]
- 3.Watson AJ. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J Anim Sci 2007; 85: 1–3. [DOI] [PubMed] [Google Scholar]
- 4.Raghu HM, Nandi S, Reddy SM. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod Fertil Dev 2002; 14: 55–61. [DOI] [PubMed] [Google Scholar]
- 5.Otoi T, Fujii M, Tanaka M, Ooka A, Suzuki T. Oocyte diameter in relation to meiotic competence and sperm penetration. Theriogenology 2000; 54: 535–42. [DOI] [PubMed] [Google Scholar]
- 6.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum Reprod 2011; 26: 2165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouniol Baly C, Hamraoui L, Guibert J, Beaujean N, Szöllösi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod 1999; 60: 580–7. [DOI] [PubMed] [Google Scholar]
- 8.De La Fuente R, Eppig J. Transcriptional activity of the mouse oocyte genome: Companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol 2001; 229: 224–36. [DOI] [PubMed] [Google Scholar]
- 9.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod 2010; 12: 2999–3011. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod 2003; 69: 2045–52. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, Smitz J. Meiotic arrest in vitro by phosphodiesterase 3-inhbitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod 2006; 74: 177–84. [DOI] [PubMed] [Google Scholar]
- 12.Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Van der Elst J. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Hum Reprod 2007; 22: 1239–46. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte L, Nogueira D, Gerris J, Dhont M, De Sutter P. Effect of temporary nuclear arrest by phosphodiesterase 3-inhibitor on morphological and functional aspects of in vitro matured mouse Oocytes. Mol Reprod Dev 2008; 75: 1021–30. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Mal S, Miao D, Liu D, Bao S, Tan J. Cortical granules behave differently in mouse oocytes matured under different conditions. Hum Reprod 2005; 20: 3402–13. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006; 131: 193–205. [DOI] [PubMed] [Google Scholar]
- 16.Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 1996; 122: 815–22. [DOI] [PubMed] [Google Scholar]
- 17.Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction 2004; 128: 153–62. [DOI] [PubMed] [Google Scholar]
- 18.Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development 1991; 113: 789–95. [DOI] [PubMed] [Google Scholar]
- 19.Lee ST, Kim TM, Cho MY, Moon SY, Han JY, Lim JM. Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development. Fertil Steril 2005; 83: 1264–74. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Kalousek DK, Yuen BH, Moon YS. Investigation of effects of pregnant mare serum gonadotropin (PMSG) on the chromosomal complement of CD-1 mouse embryos. J Assist Reprod Genet 1997; 14: 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taiyeb AM, Ridha MT, Sayes CM, Dees WL, Kraemer DC. Cilostazol administered to female mice induces ovulation of immature oocytes: A contraceptive animal model. Life Sci 2014; 96: 46–52. [DOI] [PubMed] [Google Scholar]
- 22.Gill J. Design and analysis of experiments in the animal and medical sciences, Iowa: The Iowa State University Press, 1987. [Google Scholar]
- 23.Van der Auwera I, D’Hooghe T. Superovulation of female mice delays embryonic and fetal development. Hum Reprod 2001; 16: 1237–43. [DOI] [PubMed] [Google Scholar]
- 24.Tur R, Barri PN, Coroleu B, Buxaderas R, Martínez F, Balasch J. Risk factors for high-order multiple implantation after ovarian stimulation with gonadotrophins: Evidence from a large series of 1878 consecutive pregnancies in a single centre. Hum Reprod 2001; 16: 2124–9. [DOI] [PubMed] [Google Scholar]
- 25.FIVNAT (French In vitro National). Pregnancies and births resulting from in vitro fertilization: French national registry, analysis of data 1986 to 1990. Fertil Steril 1995; 64: 746–56. [DOI] [PubMed] [Google Scholar]
- 26.Sundstrom I, Ildgruben A, Hogberg U. Treatment-related and treatment-independent deliveries among infertile couples, a long-term follow-up. Acta Obstet Gynecol Scand 1997; 76: 238–43. [PubMed] [Google Scholar]
- 27.Curnow EC, Ryan JP, Saunders DM, Hayes ES. Oocyte prematuration in the presence of milrinone improves nuclear but not cytoplasmic maturation of macaque oocytes. Reprod Fertil Dev 2011; 23: 224–5. [Google Scholar]
- 28.Jee BC, Chen HY, Chian RC. Effect of a phosphodiesterase type 3 inhibitor in oocyte maturation medium on subsequent mouse embryo development. Fertil Steril 2009; 91: 2037–42. [DOI] [PubMed] [Google Scholar]
- 29.Patrat C, Kaffel A, Delaroche L, Guibert J, Jouannet P, Epelboin S, De Ziegler D, Wolf JP, Fauque P. Optimal timing for oocyte denudation and intracytoplasmic sperm injection. Obstet Gynecol Int 2012; 2012: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundstrom P, Nilsson BO. Meiotic and cytoplasmic maturation of oocytes collected in stimulated cycles is asynchronous. Hum Reprod 1988; 3: 613–9. [DOI] [PubMed] [Google Scholar]
- 31.Trounson AO, Mohr LR, Wood C, Leeton JF. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil 1982; 64: 285–94. [DOI] [PubMed] [Google Scholar]
- 32.Son W, Chung J, Chian R, Herrero B, Demirtas E, Elizur S, Gidoni Y, Sylvestre C, Dean N, Tan SL. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod 2008; 23: 2010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo H, Cho H, Lee Y. Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol 2010; 69: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev 2001; 19: 369–86. [DOI] [PubMed] [Google Scholar]
- 35.Sun B, Le SN, Lin S, Fong M, Guertin M, Liu Y, Tandon NN, Yoshitake M, Kambayashi J. New mechanism of action for cilostazol: Interplay between adenosine and cilostazol in inhibiting platelet activation. J Cardiovasc Pharmacol 2002; 40: 577–85. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Cone J, Fong M, Yoshitake M, Kambayashi J, Liu Y. Interplay between inhibition of adenosine uptake and phosphodiesterase type 3 on cardiac function by cilostazol, an agent to treat intermittent claudication. J Cardiovasc Pharmacol 2001; 38: 775–83. [DOI] [PubMed] [Google Scholar]
- 37.Wiersma A, Hirsch B, Tsafriri A, Hanssen RGJM, van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest 1998; 102: 532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod 2004; 71: 1142–9. [DOI] [PubMed] [Google Scholar]
- 39.Jensen J, Schwinof K, Zelinski Wooten M, Conti M, DePaolo L, Stouffer R. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod 2002; 17: 2079–84. [DOI] [PubMed] [Google Scholar]
- 40.Grupen C, Fung M, Armstrong D. Effects of milrinone and butyrolactone-I on porcine oocyte meiotic progression and developmental competence. Reprod Fertil Dev 2006; 18: 309–17. [DOI] [PubMed] [Google Scholar]
- 41.Taiyeb AM, Sayes CM, Ridha Albarzanchi MT, Fajt V, Dees WL, Kraemer DC. Cilostazol blocks pregnancy in naturally cycling mice. Contraception 2013; 87: 443–8. [DOI] [PubMed] [Google Scholar]
- 42.Taiyeb AM, Dees WL, Ridha-Albarzanchi MT, Sayes CM, Kraemer DC. In vitro effects of cilostazol, a phosphodiesterase IIIA inhibitor, on mouse oocyte maturation and morphology. Clin Exp Pharm Physiol 2014; 41: 147–53. [DOI] [PubMed] [Google Scholar]