Abstract
Clinically, autologous gastrointestinal segments are traditionally used for urinary diversion. However, this procedure often causes many serious complications. Tissue engineering may provide an alternative treatment method in urinary diversion. This research aims to produce tissue-engineered tubular substitutions by using homologous adipose-derived stem cells, smooth muscle cells, and bladder acellular matrix in developing urinary diversion in a rabbit model. Adipose-derived stem cells and smooth muscle cells of rabbit were obtained and cultured in vitro. These cultured adipose-derived stem cells and smooth muscle cells were seeded onto the two sides of the bladder acellular matrix and then incubated for seven days. The cell-seeded matrix was used to build tissue-engineered tubular substitutions, which were then implanted and wrapped into the omentum in vivo for two weeks to promote angiogenesis. In the experimental group, the bladder of 20 rabbits was totally resected, and the above tissue-engineered tubular substitutions were used for urinary diversion. In the control group, bladder acellular matrix tubular substitutions with unseeded cells were implanted into the omentum and were used as urinary diversion on another five rabbits with the same process. The implants were harvested, and histological examination was conducted at 2, 4, 8, and 16 weeks after operation. Intravenous urography assessment was performed at 16 weeks postoperatively. All the rabbits were alive in the experimental group until they were sacrificed. Histological analysis of the construct displayed the presence of multilayer urothelial cells on the luminal side and organized smooth muscle tissue on the other side, and different diameters of neovascularization were clearly identified in the substitutions obtained. No leakage, stricture, or obstructions were noted with intravenous urography assessment. All the animals in the control group died within two weeks, and urine leakage, scar formation, and inflammation were detected through autopsy. This study demonstrates the feasibility of tissue-engineered tubular substitutions constructed using homologous adipose-derived stem cells, smooth muscle cells, and bladder acellular matrix for urinary diversion in a rabbit model.
Keywords: Tissue engineering, adipose-derived stem cells, smooth muscle cells, bladder acellular matrix, epithelium, omentum, urinary diversion
Introduction
Congenital disorders or acquired abnormalities may result in bladder tissue damage or loss, often requiring significant surgical repair and reconstruction. Gastrointestinal segments are traditionally used as urinary reservoir or conduit for urine collection and excretion, particularly for patients suffering from bladder cancer requiring radical cystectomy.1 Although using autologous bowels is currently the gold standard of therapy for these patients, this strategy incurs significant complications, such as twice trauma, chronic urinary tract infections, electrolyte imbalance, and mucus secretion, which severely influence the quality of life in patients.2,3 Thus, finding other artificial alternatives to divert urine and eliminate complications related to urinary diversion is desirable. Tissue engineering technology, as an emerging and developing research field, may offer an alternative to address numerous current therapeutic shortcomings in urinary diversion for patients who develop bladder diseases requiring radical cystectomy. Tissue engineering technology aims to utilize autologous sources of patient cells and biocompatible materials to encourage regenerative processes of new tissues and organs.
In the urinary system, the epithelium possesses specialized and unique features that act as blood–urine barrier to protect the underlying muscle tissues and extracellular space.4 Cell seeding highlights the importance of the epithelium for successful bladder restoration and reconstruction in animals or humans.5 However, sometimes patients with malignant bladder disease cannot acquire sufficient and safe urothelial cells. Hence, stem cells derived from autologous patients for tissue repair and reconstruction are promising as novel sources of seeding cells. Adipose-derived stem cells (ADSCs), which extensively exist in adipose tissues and are harvested with no trauma risk, possess self-renewal and multidirectional differentiation abilities. Therefore, ADSCs are considered to have the potential for bladder tissue regeneration. Recent studies have shown that ADSCs can differentiate into epithelial cells, and after co-culture with urothelial cells, ADSCs can detect the expression of urothelial-specific protein-uroplakin Ib and uroplakin II.6,7 In addition, ADSCs contain secretory protein groups and can release various growth factors, which may accelerate vascularization of ischemic tissues.8
Bladder acellular matrix (BAM) is a kind of natural tissue engineering scaffold material obtained through mechanical and chemical methods to remove the cell ingredients of the bladder tissue, leaving bioactive components of extracellular matrix. BAM has excellent biocompatibility and physical performance. After decellularization, BAM can save numerous endogenous growth factors on the surface, such as the basic fibroblast growth factor, vascular endothelial growth factor, and transforming growth factor beta. These growth factors are conducive to cell adhesion, migration, and proliferation.9
Lack of sufficient blood supply caused by inflammatory response triggered by transplanted graft often leads to ischemia and extensive fibrosis.10 Therefore, adequate blood supplement is one of the necessary conditions for grafting. The omentum is considered as an excellent biological incubator, promoting neovascularization in vivo.11
In this study, we isolated and cultivated ADSCs and smooth muscle cells (SMCs) of the bladder of rabbits in vitro. Next, ADSCs and SMCs were seeded onto the two sides of the BAM to build tissue-engineered tubular substitutions (TETSs). The TETSs were then transferred and incubated into the omentum of the rabbits for two weeks in vivo. We also attempted to research the feasibility of TETSs as transplant used in urinary diversion after radical cystectomy of a rabbit model.
Materials and methods
Animals
All animal handling and procedures were operated according to the guidelines of the Institute of Laboratory Animal Resources in Wuhan University. This project was examined and approved by the Laboratory Animal Ethics Committee of the Medical Department of Wuhan University. New Zealand white rabbits (male, 2.0–2.5 kg) obtained from the Experimental Animal Center of Wuhan University were housed in the Medical Department of Wuhan University Animal Care Facility under conventional conditions, with free access to drinks and food.
Isolation and culture of ADSCs and SMCs
ADSCs were isolated from subcutaneous adipose tissues of the New Zealand white rabbits. Approximately 10 g of adipose tissues was obtained from the inguinal subcutaneous site under anesthesia and collected in phosphate-buffered saline (PBS) with antibiotics. ADSCs were harvested through the collagenase digestion method. The adipose tissues were washed three times in PBS, thoroughly minced, and then transferred into 0.1% type I collagenase at 37℃ for digestion for 1 h. The enzyme digestion process was terminated using Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 150 U/mL penicillin, and 150 U/mL streptomycin. The solution was centrifuged at 805 g for 10 min. The cell pellets at the bottom of the centrifuge tube were resuspended using the above configured DMEM and then inoculated in a 10-cm diameter Petri dish at 37℃ with 5% CO2. After 24 h, the DMEM was renewed to remove the non-adherent cells and residual red blood cells. In the following incubation, the DMEM was changed every three days, and passage 3–5 cells were used for the next planting experiments.
The isolation and culture of the bladder SMCs were referenced to the previous method called tissue-explant technique.12 Ten New Zealand white rabbits received partial cystectomy, and muscle biopsies were harvested from the bladder wall. Primary cells were obtained by explanting muscle tissues in 10 cm diameter Petri dish using DMEM plus 10% FBS at 5% CO2 and 37℃ condition. The muscle cells expanded for seven days, and sufficient cells were digested, washed, and inoculated into a new Petri dish. In addition, passage 3–5 SMCs were used for the following plantation.
Identification of ADSCs and SMCs
Flow cytometry analysis was performed to determine the phenotypic characteristic of stem cell molecular markers on the ADSCs. The passage 3 ADSCs expanded approximately 2 × 106 cells, digested, and then incubated in 100 μl of PBS containing 10 μl of monoclonal mouse anti-rabbit primary antibodies integrated with fluorescein isothiocyanate for 45 min under 4℃ and dark condition. After washing in PBS twice, the cells were resuspended in 500 μl of PBS and analyzed using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Primary antibodies for CD90, CD44, CD34, and CD45 were purchased from Abcam, and the data were analyzed using CellQuest software (BD Biosciences). The SMC phenotype characteristic was evaluated and identified via cell immunofluorescence staining for smooth muscle alpha actin (SMA).
Preparation of homologous BAM
Bladder specimens were obtained from 30 male New Zealand white rabbits, and the entire decellularization process was under aseptic conditions. The bladder specimens were rinsed in PBS plus 0.1% w/v EDTA and 10 KIU/ml Trasylol to help inhibit the proteinase activity. The bladder tissues were transferred into hypotonic Tris buffer at 4℃ for 24 h and then incubated in 0.1% w/v sodium dodecyl sulfate on an orbital shaker at ambient temperature for another 24 h. The bladder tissues were washed in PBS, immersed into 10 mM Tris-HCl added with 2000 Kunitz units of deoxyribonuclease I, and agitated at 37℃ for 24 h. The bladder tissues were then transferred into the mixed solution with 4% sodium deoxycholate and 0.1% sodium azide and stirred at normal temperature for 24 h. This procedure was repeated twice. Finally, the bladder materials were washed three times with aseptic PBS and then sterilized with 0.1% v/v peracetic acid. The resultant material was stored in a penicillin/streptomycin solution (PH 7.2) at 4℃. Hematoxylin and eosin staining (H&E staining), Sirius Red staining, Masson’s trichrome staining, and scanning electron microscope (SEM) were used to evaluate the histological structure and residual of the cellular content of the rabbit bladders, which can reveal the acellularity degree.
Seeding cells onto the BAM and producing TETSs
Passage 3–5 ADSCs were digested using trypsin into cell suspensions, which were seeded onto the BAM with a density of 2 × 107/cm2, and were cultivated for three days. Passage 3–5 SMCs were digested and planted onto the other side of BAM. The compound matrix was cultured in DMEM for one week, and the medium was refreshed daily. The cellular proliferation status on the compound matrix was then evaluated via H&E staining and SEM. With ADSCs made on the lumen surface, the compound matrix surrounded an 8 F guiding catheter and was tailored into 4 cm in length to produce TETSs.
Transferring TETSs into the omentum of rabbits
Under general anesthesia, 20 TETSs were wrapped into the omentum of rabbits for two weeks before being used in urinary diversion. H&E staining and immunohistochemistry staining were performed on the TETSs to detect the regeneration of epithelial cells and neovascularization of tissues. Meanwhile, five BAM tubular substitutions with unseeded cells were also incubated in the omentum of the rabbits for two weeks as additional control group.
Implanting TETSs into the rabbits for urinary diversion
In the experimental group, the rabbits received 3% sodium pentobarbital for anesthesia, and a small abdominal longitudinal incision along the midline was made above the symphysis pubis. After revealing the bladder and identifying the bilateral ureter, the bladder was totally resected. The urethra was then seamed, and the bilateral ureters were anastomosed onto the TETSs in a simple continuous pattern. The TETSs passed through the peritoneum and were embedded into the peritoneum, ending in the abdominal wall stoma. Finally, the distal TETSs were anastomosed in the site of the abdominal wall stoma using a catheter placed in the stoma for one week to drain the urine flowing out of the body. In the control group, BAM tubular substitutions with unseeded cells were implanted into another five rabbits via the same process.
Histologic analysis and intravenous urography assessment
Five rabbits from each experimental group were euthanized 2, 4, 8, and 16 weeks after transplantation, and the embedded TETSs were harvested for histologic analysis. Another five rabbits that served as the control group were also analyzed for the histologic appearance. After 10% formalin fixing and paraffin embedding, a 5 -µm-thick tissue section was made for H&E and immunohistochemical staining methods. Epidermal cell layers were assessed via immunohistochemistry using antibody to cytokeratin AE1/AE3, zonula occludens-1 (ZO-1), and uroplakin IIIa. Smooth muscle and neovascularization were identified through immunohistochemistry for the α-SMA and CD31 monoclonal antibodies. Intravenous urography assessment was performed to survey the imaging of the kidney, ureter, and TETSs 16 weeks postoperation.
Results
Morphological characterization and identification of cells
ADSCs were successfully isolated from rabbit adipose tissue, and the primary cells showed typical spindle fibroblast-like characteristics (Figure 1(a) and (b)). To characterize the ADSC population, we examined the phenotypic characteristics of mesenchymal stem cells via flow cytometry analysis. Expanded cells were detected with high-level expression of CD90 (99.98%) and CD44 (99.94%), but without significant expression of CD34 (2.73%) and CD45 (2.55%). This result is in accordance with previous literature about the characteristics of ADSC phenotypic markers (Figure 1(c)–(f)).
Figure 1.
Morphological characterization and identification of ADSCs. (a) Primary culture after three days (× 100). (b) After incubation for 10 days, the cells proliferated rapidly and displayed a spindle fibroblastic appearance (×100). Flow cytometry analysis demonstrated expression of (c) CD90 (99.98%) and (d) CD44 (99.94%), and there was no significant expression of (e) CD34 (2.73%), and (f) CD45 (2.55%). (A color version of this figure is available in the online journal.)
For the SMCs, after three days of primary culture, the cells proliferated on the surfaces of Petri dish and reached about 30% confluence (Figure 2(a)). The cells reached 80%–90% confluence in seven days and showed a classic spindle-shaped morphology under an optical microscope (Figure 2(b)). In addition, immunofluorescence staining for cells displayed α-SMA-positive in the cytoplast (Figure 2(c)).
Figure 2.
Morphological characterization and immunofluorescence staining of SMCs. (a) After three days of primary culture, the cells proliferated on the surfaces of Petri dish and reached about 30% confluence (×100). (b) After seven days of culture, the cells reached 80%–90% confluence and displayed a classic spindle-shaped morphology (×100). (c) α-SMA immunofluorescence staining (in green) of SMCs in combination with DAPI cell nuclei stain (in blue) (× 200). (A color version of this figure is available in the online journal.)
Characteristics of BAM and matrix with seeded cells
After the cells were removed, BAM presented a translucent film appearance (Figure 3(a)). Compared with native bladder, H&E staining, Masson’s trichrome staining, Sirius Red staining, and SEM showed that no cells adhered on the matrices (Figure 3(b)–(e)). After seeding and incubating the ADSCs and SMCs onto the BAM for seven days in DMEM, H&E staining and SEM of the composites revealed that the ADSCs and SMCs fused well on the two aspects of BAM (Figure 4(a)–(d)).
Figure 3.
Characteristics of BAM. (a) After decellularization procedure, the BAM appeared as a translucent film. (b) H&E staining of BAM (×400). (c) Masson’s trichrome staining of BAM (×400). (d) Sirius Red staining of BAM (×400). (e) Scanning electron microscope of BAM (×400). (A color version of this figure is available in the online journal.)
Figure 4.
The feature of BAM seeding. After seeding cells onto the BAM, the cells fused well on the three-dimensional BAM scaffold. (a) H&E staining of luminal side (×400). (b) H&E staining of outside side (×400). (c) Scanning electron microscope of luminal surface (×2000). (d) Scanning electron microscope of outside surface (×2000). (A color version of this figure is available in the online journal.)
Regeneration of epithelium and neovascularization after omental incubation
After being wrapped in the omentum for two weeks, the TETSs can be detected with a one-layer epithelium structure via H&E staining and anti-AE1/AE3 immunohistochemistry staining, and neovascularization via anti-CD31 and anti-α-SMA immunohistochemical evaluation (Figure 5(a), (c), (e), and (f)). However, no obvious epithelium structures were found in the unseeded BAM substitutions (Figure 5(b) and (d)).
Figure 5.
Regeneration of epithelium and neovascularization after omental incubation. (a) H&E staining of TETSs (× 400). (b) H&E staining of the unseeded BAM (× 400). (c) Anti-AE1/AE3 immunohistochemistry staining displayed a one-layer epithelium structure (× 200). (d) Anti-AE1/AE3 immunohistochemistry staining showed no obvious epithelium in the unseeded BAM (× 200). (e) Anti-CD31 immunohistochemistry staining revealed neovascularization of TETSs (× 200). (f) Anti-α-SMA immunohistochemistry staining revealed vascular walls in TETSs (× 200). (A color version of this figure is available in the online journal.)
Histological and intravenous urography evaluation
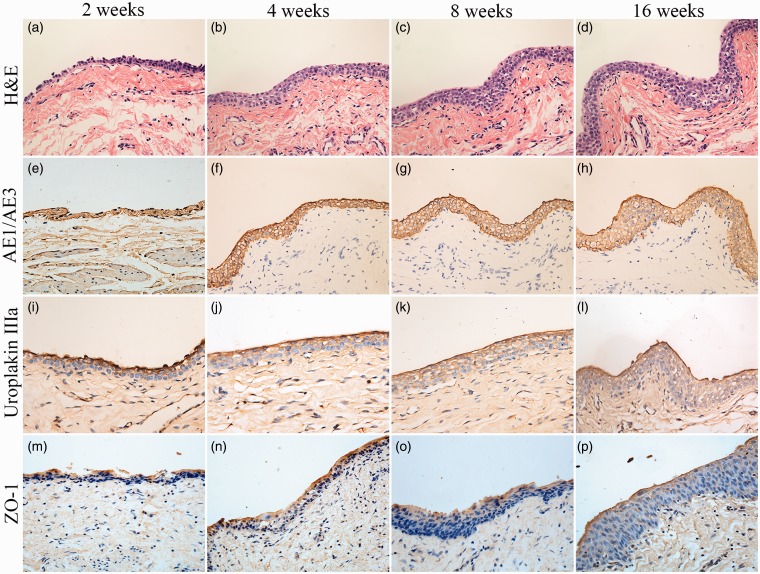
In the experimental group, all the animals survived until they were sacrificed. The histological features of the implanted composites were as follows. At two weeks after transplantation, H&E staining revealed a thin polarized structure with epithelium covering the luminal aspect of the tubular transplants (Figure 6(a)), and at four weeks, the transitional epithelial cell layer thickened (Figure 6(b)). Eventually, at 8 and 16 weeks of implantation, the number of layers of the TETSs in both experimental groups continuously increased and a multilayer uroepithelium-like lining covered the lumen of the tubular grafts (Figure 6(c) and (d)). Immunohistochemical studies confirmed that the urothelial cells of the construct luminal sides were positively obtained with anti-AE1/AE3 (Figure 6(e)–(h)), anti-uroplakin IIIa (Figure 6(i)–(l)), and anti-ZO-1 antibodies (Figure 6(m)–(p)). At 16 weeks, all the TETSs showed that the SMCs were positively stained with the anti-α-SMA antibody (Figure 7(a)) and displayed well-developed revascularization, which was stained positively with anti-CD31 and anti-α-SMA antibodies (Figure 7(b) and (c)). In addition, intravenous urography revealed that the urinary tract was unblocked and that no leakage, stricture, or obstruction occurred in the TETSs and ureter (Figure 8). By contrast, in the control group, the five animals with unseeded BAM substitutions died within two weeks after urinary diversion. The autopsy of these rabbits showed urine leakage out of tubular transplants accompanied with evident inflammatory reaction, gross damage, and scar formation.
Figure 6.
Histologic characteristics of TETSs in the experimental group after urinary diversion at 2, 4, 8, and 16 weeks. (a, b, c, and d) H&E staining displayed the regeneration of epithelium layers of TETSs (× 400). (e–h, i–l, and m–p) Immunohistochemical staining of AE1/AE3, uroplakin IIIa, and ZO-1 revealed the regeneration of epithelium of TETSs, respectively (× 400). (A color version of this figure is available in the online journal.)
Figure 7.
Immunohistochemistry analysis for regeneration of smooth muscle and neovascularization at 16 weeks (× 200). (a) Anti-α-SMA antibody positive displayed organized smooth muscle tissues. (b)Anti-CD31 antibody positive showed well-developed angiogenesis. (c) Anti-α-SMA antibody positive revealed vessel wall. (A color version of this figure is available in the online journal.)
Figure 8.
Intravenous urography observation. Intravenous urography at 16 weeks postoperatively showed urinary tract was unblocked and that no leakage, stricture, or obstruction occurred in the TETSs and bilateral ureters. (A color version of this figure is available in the online journal.)
Discussion
Congenital and acquired bladder pathologies, such as ectopocystis, neurogenic bladder, and muscle-invasive bladder cancer, routinely require cystoplasty. Clinically, ileal conduit, ureteral skin stoma, and orthotopic neobladder are the three main surgical diversion procedures currently used. Employing autologous intestine segments is the gold standard of bladder reconstruction treatment. However, this strategy is associated with early and long-term complications, including bowel resection and anastomosis, urolithiasis, excessive mucous production, bowel obstruction, metabolic abnormalities, and secondary malignancies.13,14 Tissue engineering may provide a novel method for urinary diversion without intestine substitution.
Two main tissue engineering techniques serve as viable options to reconstruct urinary organs, including acellular matrix alone and acellular matrix seeded with cells. Both techniques are potential alternatives for tissue-engineered tissues or organs in various animal models. However, the primary drawbacks of unseeded acellular matrix method include inflammation and failure of the implanted matrix precipitated by direct contact to the cytotoxic urine environment after transplantation. By contrast, cell-seeded matrix displays important advantages and avoids the above shortcomings in urological regeneration. Yuan et al.15 seeded human umbilical mesenchymal stem cells (HUMSCs) onto the BAM and demonstrated the feasibility of bladder reconstruction in a canine model. By comparing HUMSC-BAM in the experimental group and unseeded-BAMG in the control group, the authors found through immunohistochemistry staining that the seeded matrix better supports the formation of a multilayered urothelium and a well-developed smooth muscle at 12 weeks. Orabi et al.16 seeded autologous bladder epithelial and SMCs onto preconfigured tubular acellular matrices to repair a long segment defect of the urethra in a canine model, and tubularized constructs without seeded cells served as the control group. After CT urethrograms and histology assessment at predetermined time points, the authors observed that cell-seeded tubularized matrices can reestablish long urethral defects, whereas acellular collagen matrices alone cause poor tissue development and formation of strictures.
In the urinary system, urothelium is important and serves many functions within the urinary tract, which can shield the acellular matrix and newly formed tissues from the cytotoxin effects of urine, and serve as a crucial barrier to prevent urinary tract infection.17 Urothelial cells perform many critical functions that require specialized features of components called apical plasma membrane and tight junctions.18 Proper barrier function of urothelial cells depends on the precise formation of apical plasma membrane, which contains lipid composition and epithelium-specific glycoproteins called uroplakins.19 The uroplakins of all mammalian urothelium comprise four major subunits, namely, uroplakins Ia and Ib, uroplakin II, and uroplakin IIIa, which synthesize a unique crystalline 2D tetramer of 16-nm particles.19 Uroplakins, which are integral membrane proteins and integrated into plaques that possess a special shield to water and toxic molecules in urine, are synthesized in large numbers only by urothelial cells of terminally differentiated stage. Hence, uroplakins can be perceived as major differentiation markers of urothelial cells.20 Tight junction protein ZO-1 serves as another important constituent to maintain the high-resistance urothelial barrier.21 ZO-1 is regarded as multidomain scaffolding proteins that interlink the tight junction, a membrane-associated complex that controls the transport of ions, macromolecules, and immune cells.22
However, for many patients with urological malignancy or extensive tissue and organ damage, organ biopsy cannot yield enough safe or sufficient cells for expansion and transplantation. Under such conditions, scholars should seek other cell sources for urinary tissue engineering. In recent years, homologous stem cells have attracted investigators’ attention and have gradually become an ideal origin of transplantation cells for urinary system tissue engineering. Among numerous stem cells, ADSCs, which are harvested abundantly and leave less trauma to the donor site, attract more attention and serve as potential alternative seeding cell resources for tissue-engineered urinary diversion.23 Several researchers found that ADSCs can potentially differentiate into epithelial lineage through induction of contributing factors.6,24 Some scholars have reported that rabbit ADSCs can be induced toward epithelial differentiation with a 3D culture system in vitro. The induced ADSCs present a stratified epithelial-like morphology and express epithelial specific proteins.25 Through follow-up study, scholars confirmed that epithelial-differentiated rabbit ADSCs can be used as potential substitute of urothelial cells for urethral tissue engineering.26 In addition, Zhang et al.27 incubated ADSCs in a conditioned medium and in a Transwell co-culture system with an immortalized urothelial cell line in vitro; they confirmed that ADSCs can potentially be differentiated toward urothelium-like cell lines. Given the aforementioned striking features, in the present study, we selected ADSCs as the seeded cell source to accelerate the epithelialization of conduits. ADSCs could differentiate into urothelial cell lineages, with the expression of urothelial-specific proteins confirmed via immunohistochemical assessment.
Optimal scaffolds for urinary diversion require biocompatibility to support cell adhesion, proliferation, and differentiation, as well as possess a certain intensity to sustain the shape of tissue-engineered grafts, but they do not induce a detrimental host toxic and inflammatory response after transplantation. Naturally derived biomaterials and synthetic materials are explored as urinary diversion substitutes. Synthetic materials possess excellent mechanical strength and can be tailored and fabricated with specific mechanical properties, including elasticity, porosity, and degradation rate. However, the lack of cytokines and growth factors that support and induce the growth and differentiation of cells restricts these synthetic polymers.28 By contrast, at least 10 growth factors, including VEGF, BMP4, PDGF-BB, KGF, TGFbeta1, IGF, bFGF, EGF, and TGFalpha were found to be preserved in the BAM.9 These active proteins can provide an excellent microenvironment to orchestrate growth, migration, proliferation, and differentiation of cells.9 Meanwhile, BAM preserves a porous 3D structure that can support a maximal regeneration property.29 In our study, BAM served as a scaffold to construct TETSs. Expectedly, BAM displayed excellent cell biocompatibility. Davis et al.30 seeded human urothelial cells (HUCs) on porcine small intestinal submucosa (SIS) and BAM. They found that BAM demonstrates significantly greater HUC viability and proliferative ability compared with SIS. These findings all demonstrate that BAM has high regenerative potential for cells and tissues. Moreover, the findings suggest that BAM can be applied as a type of “off the shelf” scaffold material to load seeding cells for graft production, eventually hoping to promote host tissue regeneration.
A significant limitation that hampers the development of tissue engineering is fibrosis of the construct, which may be mainly caused by microvascular ischemia. Therefore, developing alternative methods to quickly revascularize TETSs is essential. Many researchers have previously developed a method to effectively improve the vascularization for tissue engineering grafts, which is a strategy known as wrapping transplants with the omentum.11,31,32 This strategy is also verified in this study. In this research, we used omental prewrapping as an in vivo bioreactor to promote neovascularization of TETSs. After two weeks, the formation degree of epithelial structure and neovascularization in the experimental group was evidently higher than that in the control group.
In addition, in this study, intravenous urography demonstrated no hydroureteronephrosis, urine leakage, stricture, or obstruction in the urinary tract at 16 weeks. This result indicates that TETSs applied to urinary diversion can be responsible for the functional urine drainage effect.
In summary, the results presented in this study showed the feasibility of constructing TETSs using ADSCs, SMCs, and BAM for urinary diversion in a rabbit model. Histological evaluations demonstrated multilayered urothelial cells lining the luminal aspect of the graft. These cells are important in restoring the blood–urine barrier creating a urine-free environment for the subsequent remainder of tissue regeneration. SMCs proliferated and formed a corresponding muscular layer externally. Furthermore, the omentum could serve as an excellent bioreactor to promote neovascularization and regeneration of tissues in vivo. TETSs may become an emerging tissue-engineered apparatus for urinary diversion.
ACKNOWLEDGEMENTS
This work was supported by the Fundamental Research Funds for the Central Universities of China (Grant No. 2042014KF0106), the National Natural Science Funds of China (Grant No. 31400835), and the Nature Science Foundation of Hubei Province (Grant No. 2013CFB265).
Author contributions
All authors participated in the design, interpretation of the experiments, analysis of the results, and review of the manuscript; LM, WL, and YX conducted the experiments; LM wrote the manuscript; and LM and WL contributed equally to this work.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Biers SM, Venn SN, Greenwell TJ. The past, present and future of augmentation cystoplasty. BJU Int 2012; 109: 1280–93. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Wiener JS. Evaluating outcomes of enterocystoplasty in patients with spina bifida: a review of the literature. J Urol 2008; 180: 2323–9. [DOI] [PubMed] [Google Scholar]
- 3.Husmann DA, Rathbun SR. Long-term follow up of enteric bladder augmentations: the risk for malignancy. J Pediatr Urol 2008; 4: 381–5; discussion 6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Atala A. Urothelial cell culture: stratified urothelial sheet and three-dimensional growth of urothelial structure. Methods Mol Biol 2013; 945: 383–99. [DOI] [PubMed] [Google Scholar]
- 5.Horst M, Madduri S, Gobet R, Sulser T, Milleret V, Hall H, Atala A, Eberli D. Engineering functional bladder tissues. J Tissue Eng Regener Med 2013; 7: 515–22. [DOI] [PubMed] [Google Scholar]
- 6.Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 2005; 330: 142–50. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Huang J, Lin T, Zhang C, Yin X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem Biophys Res Commun 2009; 390: 931–6. [DOI] [PubMed] [Google Scholar]
- 8.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109: 1292–8. [DOI] [PubMed] [Google Scholar]
- 9.Chun SY, Lim GJ, Kwon TG, Kwak EK, Kim BW, Atala A, Yoo JJ. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials 2007; 28: 4251–6. [DOI] [PubMed] [Google Scholar]
- 10.Schultheiss D, Gabouev AI, Cebotari S, Tudorache I, Walles T, Schlote N, Wefer J, Kaufmann PM, Haverich A, Jonas U, Stief CG, Mertsching H. Biological vascularized matrix for bladder tissue engineering: matrix preparation, reseeding technique and short-term implantation in a porcine model. J Urol 2005; 173: 276–80. [DOI] [PubMed] [Google Scholar]
- 11.Baumert H, Simon P, Hekmati M, Fromont G, Levy M, Balaton A, Molinié V, Malavaud B. Development of a seeded scaffold in the great omentum: feasibility of an in vivo bioreactor for bladder tissue engineering. Eur Urol 2007; 52: 884–90. [DOI] [PubMed] [Google Scholar]
- 12.Freeman MR, Yoo JJ, Raab G, Soker S, Adam RM, Schneck FX, Renshaw AA, Klagsbrun M, Atala A. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Invest 1997; 99: 1028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert SM, Hensle TW. Metabolic consequences and long-term complications of enterocystoplasty in children: a review. J Urol 2005; 173: 1080–6. [DOI] [PubMed] [Google Scholar]
- 14.Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol 2004; 172: 1649–51; discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, Zhuang Y, Xiong J, Zhi W, Liu L, Wei Q, Han P. Human umbilical mesenchymal stem cells-seeded bladder acellular matrix grafts for reconstruction of bladder defects in a canine model. PLoS One 2013; 8: e80959–e80959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orabi H, AbouShwareb T, Zhang Y, Yoo JJ, Atala A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol 2013; 63: 531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamany T, Van Batavia J, Mendelsohn C. Formation and regeneration of the urothelium. Curr Opin Organ Transplant 2014; 19: 323–30. [DOI] [PubMed] [Google Scholar]
- 18.Ardelt PU, Woodhouse CR, Riedmiller H, Gerharz EW. The efferent segment in continent cutaneous urinary diversion: a comprehensive review of the literature. BJU Int 2012; 109: 288–97. [DOI] [PubMed] [Google Scholar]
- 19.Katnik-Prastowska I, Lis J, Matejuk A. Glycosylation of uroplakins. Implications for bladder physiopathology. Glycoconjugate J 2014; 31: 623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee G. Uroplakins in the lower urinary tract. Int Neurourol J 2011; 15: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu HT, Shie JH, Chen SH, Wang YS, Kuo HC. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology 2012; 80: 225.e13–8. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci 2013; 126: 1565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells 2014; 6: 312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope 2010; 120: 125–31. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Xu Y, Fu Q, Li C. Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture. Tissue Eng Part A 2012; 18: 1760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Xu Y, Xie H, Li C, Song L, Feng C, Zhang Q, Xie M, Wang Y, Lv X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A 2014; 20: 774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Xu MX, Zhou Z, Zhang K, Zhou J, Zhao Y, Wang Z, Lu MJ. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS One 2014; 9: e95583–e95583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin HK, Madihally SV, Palmer B, Frimberger D, Fung KM, Kropp BP. Biomatrices for bladder reconstruction. Adv Drug Deliv Rev 2015;82–83:47–63. [DOI] [PubMed]
- 29.Liu Y, Bharadwaj S, Lee SJ, Atala A, Zhang Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials 2009; 30: 3865–73. [DOI] [PubMed] [Google Scholar]
- 30.Davis NF, Callanan A, McGuire BB, Flood HD, McGloughlin TM. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology 2011; 77: 1007.e1–7. [DOI] [PubMed] [Google Scholar]
- 31.Liao W, Yang S, Song C, Li X, Li Y, Xiong Y. Construction of ureteral grafts by seeding bone marrow mesenchymal stem cells and smooth muscle cells into bladder acellular matrix. Transplant Proc 2013; 45: 730–4. [DOI] [PubMed] [Google Scholar]
- 32.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006; 367: 1241–6. [DOI] [PubMed] [Google Scholar]