Abstract
Long-term insulin delivery can reduce blood glucose variability in diabetic patients. In this study, its impact on oxidative stress status, inflammation, and liver injury was investigated. Diabetes was induced in Wistar rats with a single dose of streptozotocin (100 mg/kg). Untreated rats and rats administered Insuplant® (2 UI/200 g/day) through a subcutaneous osmotic pump for one or four weeks were compared with non-diabetic controls. Body weight, fructosamine level, total cholesterol, Insulin Growth Factor-1 (IGF-1) level, lipid peroxidation, and total antioxidant capacity were measured. Hepatic injury was determined through the measurement of glycogen content, reactive oxygen species (ROS) production, and macrophage infiltration. Liver oxidative stress status was evaluated through the measurement of superoxide dismutase (SOD), catalase (CAT), and nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) expression, and p38 mitogen-activated protein kinase (p38MAPK) activation. Induction of diabetes led to increased plasma oxidative stress and inflammation. Moreover, ROS production and macrophage infiltration increased in addition to SOD, CAT, and NADPH oxidase expression. Intensive insulin therapy improved metabolic control in diabetic animals as seen by a restoration of hepatic glycogen, plasma IGF-1 levels, and a decrease in plasma oxidative stress. However, insulin treatment did not result in a decrease in acute inflammation in diabetic rats as seen by continued ROS production and macrophage infiltration in the liver, and a decrease of p38MAPK activation. These results suggest that the onset of diabetes induces liver oxidative stress and inflammation, and that subcutaneous insulin administration cannot completely reverse these changes. Targeting oxidative stress and/or inflammation in diabetic patients could be an interesting strategy to improve therapeutic options.
Keywords: Insulin therapy, oxidative stress, diabetes, liver
Introduction
Diabetes is one of the most common chronic diseases worldwide affecting more than 340 million people. It is characterized by prolonged hyperglycemia in the postprandial and/or fasting state1,2 as a result of impaired insulin-mediated glucose metabolism. Uncontrolled hyperglycemia leads to the progressive development of microvascular and macrovascular complications, causing morbidity and mortality in diabetic patients.3–6 Indeed, hyperglycemia is the pivotal factor leading to increased oxidative stress and reactive oxygen species (ROS) production which can activate pathways including glucose autoxidation, protein glycation, and the subsequent oxidative degradation of glycated proteins.7–9 Moreover, fluctuations in acute glucose concentration are related to oxidative stress.10 While a certain level of ROS is necessary to maintain cell survival, high levels of ROS lead to activation of cell defenses and, if cells cannot re-establish equilibrium, to cell death.11,12 One of the major sources of ROS in the tissues of diabetic patients, in response to high glucose, is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase,13,14 which catalyzes the generation of superoxide anions. Superoxide is well known as a mediator of inflammation and an inducer of apoptosis. When overproduced, ROS can lead to intracellular protein, lipid (lipid peroxidation), membrane, and DNA damage, and activate inflammation via several stress pathways such as nuclear factor-kappa B, stress-activated protein kinase/c-Jun NH(2)-terminal kinase, or p38 mitogen-activated protein kinase.15
ROS levels are regulated through a variety of cellular defense mechanisms consisting of enzymatic antioxidants.16,17 Maintenance of acceptable and necessary levels of ROS, through their sequestration and transformation, is carried out by a pool of dedicated enzymes including superoxide dismutase (SOD) and catalase (CAT).18,19 Reports in the literature regarding the effects of diabetes-induced hyperglycemia on antioxidant enzymes are contradictory. Hyperglycemia has been shown to enhance, inhibit, or maintain the level of antioxidant enzyme expression in diabetic animals, with wide variations depending on animal age and duration of diabetes,20–22 or tissues examined.23,24 These discrepancies may arise due to variations in enzyme activity over time; for example, compensatory increases in enzyme activity in response to increased oxidative stress or direct inhibitory effects of ROS depending on the type of tissue under examination. With diabetes some tissues, such as heart, kidney, and retina, are more susceptible to damage, while others are more resistant.25
The liver plays a pivotal role in glucose homeostasis through glycogen storage in the fed state and glucose production through glycogenolysis and gluconeogenesis in the postabsorptive period; under physiological conditions, hepatocytes are the main site of glucose metabolism. Insulin enhances glycogen synthesis within the liver and prevents glucose production.
Numerous studies have demonstrated the existence of hepatic and systemic oxidative stress in diabetic animal models and tested antioxidant molecules;26–29 however, few of these were carried out on insulin-treated diabetic rats mimicking the human therapeutic approach. A reduction in blood glucose variability in response to long-term insulin delivery has been reported previously;30,31 however, its impact on oxidative stress and liver metabolism has not yet been described.
In the present study, we developed an experimental diabetic animal model in which type 1 diabetes was induced by a single high dose of streptozotocin (STZ). We then administered insulin subcutaneously through an osmotic pump for either one or four weeks and assessed the levels of plasma and hepatic oxidative stress, and liver tissue defenses during insulin treatment.
Materials and methods
Chemicals
Amyloglucosidase (AMGD) was purchased from Roche Diagnostics (Meylan, France); glucose and phosphate-buffered saline from Fisher Scientific (Illkirch, France); and eosin, Harris hematoxylin, ethanol, and toluene from Labonord (Templemars, France). All other products were purchased from Sigma-Aldrich (St Quentin Fallavier, France).
Animals and ethics statement
This study was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH, publication no. 85-23, revised 1996). The laboratory in which the work was conducted was licensed by the Department of Veterinary Service (license N° B67-482-28), and the protocol of the study was approved by the Departmental Direction of Populations Protection (license N° 67-318). All efforts to minimize animal suffering and reduce the numbers of animal used were made. Male Wistar rats (180–200 g) (Depré, Saint Doulchard, France) were housed in a thermoneutral environment (23 ± 1℃) with a 12:12 h photoperiod and provided food (Safe-A04, Villemoisson-sur-Orge, France) and water ad libitum. All rats were weighed once a week and on the day of death.
Induction of diabetes
Diabetes mellitus was induced by a single intraperitoneal injection of STZ (100 mg/kg, n = 30). After three days, glycemia was measured in tail-vein blood samples using a glucometer (Accu-Chek Performa, Roche Diagnostic, France). Rats with a blood glucose level >250 mg/dL and c-peptide level <100 ng/mL were considered. Only 20 diabetic rats were then selected and randomized into two diabetic groups: untreated (DIAB) (n = 10) or treated with Insuplant® (Sanofi-Aventis Deutschland GmbH, Germany) (INS) (n = 10) which was administered through a subcutaneous osmotic pump. The control group (C) was composed of non-diabetic rats of the same age (n = 10).
Surgery and samples
Twenty-four hours after diagnosis of diabetes, the INS group were anesthetized (ketamine/xylazine (100 µL/100 g)) and an osmotic minipump (Alzet, model 2006, Charles River Laboratories Inc., Wilmington, MA, USA) was inserted subcutaneously. The infusion rate was set to 2 UI/200g rat/day for either one or four weeks.
One week and four weeks after starting insulin treatment, five rats per group were killed. After general anesthesia induced by a mixture of 2.7 mL of xylazine (Rompun® 2%, Bayer, Puteaux, France) and 10 mL of ketamine (Imalgène® 1000, Merial, Lyon, France) at a dose of 100 µL/100 g; the stages of sleep of animals (vibrissae inert, loss of palpebral reflex, non-responsiveness to external stimuli) were checked. Blood was sampled from the abdominal aorta and rats were killed by exsanguination. Plasma samples were taken for measurement of plasma, metabolic, and oxidative parameters. Liver tissue and vessels were embedded in Tissue-Tek® O.C.T. (Leica Microsystem SAS, Nanterre, France) and snap frozen in liquid nitrogen.
Biochemical analysis of plasma
Metabolic parameters
Tail vein blood glucose at death was measured before anesthesia using a glucometer. To determine the efficiency of subcutaneous insulin therapy, human insulin was measured by ELISA (Mercodia Insulin ELISA, Uppsala, Sweden). Plasma fructosamine was measured using a colorimetric method from the Cerba Laboratory (Cergy Pontoise, France), and Insulin Growth Factor-1 (IGF-1) was measured with a Quantikine® mouse/rat IGF-1 immunoassay (R&D systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Plasma cholesterol was measured using the Cholesterol RTU™ (Biomérieux, Lyon, France) colorimetric method and a cholesterol calibrator.
Inflammatory parameters
α2-macroglobulin was measured according to the manufacturer’s instructions (Rat α2-macroglobulin ELISA, GenWay Biotech, Inc. San Diego, USA).
Oxidative parameters
Lipid peroxidation was evaluated by measuring thiobarbituric acid reactive substances (TBARS) using the OxiSelect™ TBARS Assay Kit-MDA (Malondialdehyde) Quantitation (Cell Biolabs Inc., San Diego, USA) according to the manufacturer’s instructions.
Total antioxidant capacity (TAOC) was measured using a Trolox ((+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalent antioxidant capacity method described by Re et al.32 Briefly, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (7 mmol/L) was added to potassium persulfate (2.5 mmol/L) to generate ABTSċ+ radical cations. The ABTS•+ (pH 7.4) was used at an absorbance of 0.7 ± 0.02 at 734 nm as measured using a microplate reader (iMarkTM, Biorad, Marnes-la-Coquette, France). Exactly 4 min after the addition of 200 µL diluted ABTS•+ solution to 2 µL plasma or Trolox (0.4 µmol/L) standards, absorbance was read at 734 nm. Results are expressed as µM equivalent of Trolox.
Histological studies
Hepatic glycogen quantification
A piece of fresh liver (100 mg) was placed in a tube with 450 µL sodium acetate buffer (0.2 mol/L, pH 4.5) and mixed briefly with an ULTRA-TURRAX (Polytron PT-MR2100, Kinematica AG, Luzern, Switzerland). An aliquot of homogenate was mixed with AMGD and incubated at 55℃ for 90 min to degrade glycogen into glucose residuals. Samples (10 µL) or reference standards of glucose (0–1 g/L) were incubated with 250 µL of extemporary glucose reagent (250 µL of ortho-dianisidine 10 g/L in distilled water + 50 mL glucose oxidase 0.25 g/L/peroxidase 0.04 g/L in phosphate buffer) at room temperature for 10 min, and absorbance was read at 450 nm. Samples were analyzed in duplicate, and the results expressed as microgram glycogen per microgram liver (adapted from Gustavsson et al.33).
Characterization of hepatic oxidative stress
The oxidative fluorescent dye dihydroethidine (2.5 µmol/L, 30 min, 37℃) was used to evaluate in situ formation of ROS on unfixed 10 µm-thick sections of liver using a method described by Dal-Ros et al.34 The level of ROS was determined using a microscope and the whole fluorescence of tissue samples was quantified with the imaging software (NIS-Elements BR, Nikon, France) at a 20 ×magnification. Levels of ROS were expressed as the percentage of red pixels per five random fields per animal in comparison with control values (100%).
Hepatic inflammation
In situ macrophages were measured on frozen 10 µm sections of liver. After fixation with 4% paraformaldehyde, sections were incubated with rabbit anti-ionized calcium binding adapter molecule 1 (Iba 1) (1:1000, rabbit, Wako Chemicals GmbH, Germany), then incubated with an anti-rabbit IgG biotinylated secondary antibody (1:200, goat, Vector Laboratories Inc., Burlingame, USA) followed by conjugated horseradish peroxidase (HRP) streptavidin (Vector Laboratories), and finally by incubation with 3,3′-diaminobenzidine (Vector Laboratories) and counterstaining with diluted Harris hematoxylin. Macrophage density was expressed as the percentage of brown pixels per five random fields per animal in comparison with control values (100%).
Western blotting assays
Preparation of liver tissue homogenates
Liver tissue (5 mg) from experimental rats was homogenized using NP-40 buffer (NaCl 150 mmol/L, 1.0% Triton X-100, Tris 50 mmol/L, pH 8) with a protease/phosphatase inhibitor cocktail (Roche Diagnostics, Meylan, France) using an ULTRA-TURRAX. Supernatants were collected and protein contents measured by the Bradford method.35
Western blotting
Total protein (20–40 µg) was separated on a 4–12% Bis-Tris Criterion™ XT Precast Gel (Bio-Rad, Marne-La-Coquette, France) and transferred to an Immobilon PVDF membrane (Millipore, Molsheim, France). Anti-GAPDH (1:500, rabbit, Cell Signaling, Danvers, MA, USA), -CAT (1:1000, mouse, Sigma-Aldrich), -MnSOD (manganese superoxide dismutase, 1:500, mouse, Sigma-Aldrich), -phosphorylated-p38 (1:500, rabbit, Cell Signaling, Danvers, MA, USA), and -p67 phox subunits of NADPH oxidase (1:100, mouse, Santa Cruz, CA, USA) antibodies were incubated with membranes overnight at 4℃. Membranes were incubated for 1 h at room temperature with a HRP-conjugated secondary antibody (1:2000–1:4000) and developed using the Luminata™ Forte Western HRP substrate (Millipore, Molsheim, France) with Chemidoc XRS (Bio-Rad, Marne-La-Coquette, France). The relative quantity of the protein of interest compared with the reference protein GAPDH was measured with Image J software (NIH, USA).
Statistical analysis
All data followed a normal distribution. Values are expressed as mean ± SEM, with n indicating the number of rats. Statistical analysis was performed using the Student’s t-test for unpaired data, or two-way ANOVA followed by Fischer’s protected least-significant difference test where appropriate (Sigmastat 3.10, Systat Software, Point Richmond, United States). P value of < 0.05 was considered statistically significant.
Results
Subcutaneous insulin administration improves metabolic control in diabetic rats
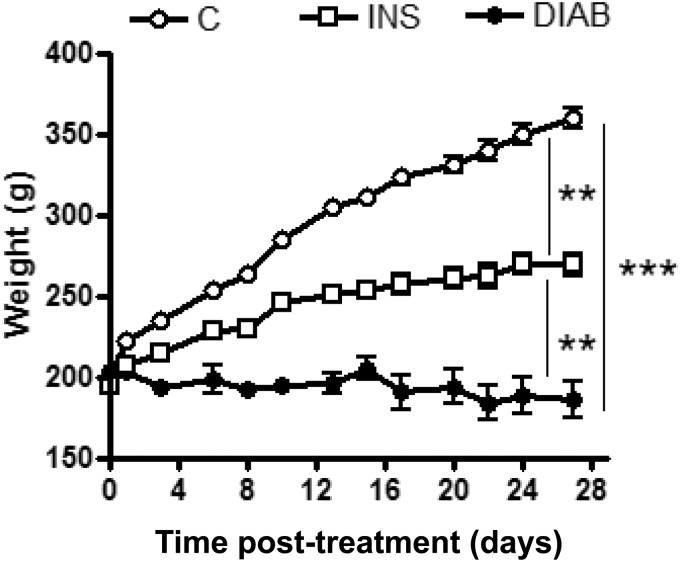
After four weeks, insulin-treated diabetic rats were heavier than non-treated diabetic rats (p < 0.01) but lighter than the control group (Figure 1). Moreover, frequent hypoglycemia was observed in insulin-treated rats when fasting, preventing measurement of fasting glycemia. Indeed, plasma levels of human insulin in treated rats were maintained for the duration of treatment (83.75 ± 4.48 mU/L at one week, and 93.34 ± 36.67 mU/L at four weeks). Glycemia in untreated diabetic rats remained very high throughout the study (>550 g/L) (Table 1). In accordance with this data, plasma fructosamine, an indicator of glycemic control in rats, was significantly higher in untreated diabetic rats after four weeks than that in insulin-treated diabetic rats (p < 0.001), even if these levels remained higher than those of control rats (Table 1). In the same way, the level of plasma cholesterol was significantly higher in diabetic rats after four weeks (p < 0.001), although it was comparable to the control group after four weeks for insulin-treated diabetic rats (Table 1).
Figure 1.
Weight of rats throughout the study. Results are given as mean ± SEM for control rats (C), non-treated diabetic rats (DIAB), and insulin-treated rats (INS) throughout the four weeks of study, n = 5; mean ± SEM. **p < 0.01, ***p < 0.001
Table 1.
General metabolic characteristics of rats at sacrifices.
| Body weight (g) | Tail vein glycemia (mg/dL) | Plasmatic fructosamine (µmol/L) | Total cholesterol (µmol/L) | |
|---|---|---|---|---|
| W1 C | 266.9 ± 6.56 | 126 ± 5 | 135.33 ± 12.44 | 0.795 ± 0.01 |
| W1 DIAB | 185.6 ± 8.99*** | 587 ± 13 | 245 ± 4.29*** | 1.095 ± 0.26 |
| W1 INS | 218.8 ± 11.93 | 548 ± 33*** | 222 ± 12.17*** | 0.825 ± 0.025 |
| W4 C | 361.9 ± 10.93 | 115 ± 3 | 130.25 ± 4.96 | 0.763 ± 0.04 |
| W4DIAB | 171.3 ± 25.61*** | 584 ± 16*** | 312 ± 6.56*** | 1.547 ± 0.28*** |
| W4 INS | 263.8 ± 8.05***### | 477 ± 51***### | 249.25 ± 12.23***### | 0.709 ± 0.04## |
Note: Effects of diabetes on body weight, tail vein glycemia, plasmatic fructosamine, and total cholesterol.
Values are mean ± SEM of five different rats.
* Significant results versus age-matched control rats (C) and # versus age-matched diabetic- rats (DIAB) or insulin treated rats (INS). *p < 0.05, ***p < 0.001 ; ##p < 0.01, ###pp < 0.001
Subcutaneous insulin therapy restores hepatic metabolism
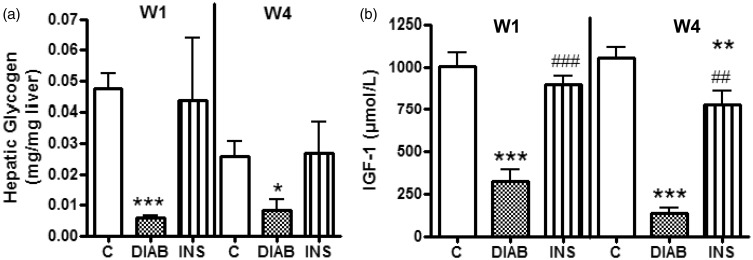
Glycogen content of rat livers was comparable between insulin-treated diabetic rats and the control group throughout the study. In contrast, the level of hepatic glycogen was significantly lower in untreated diabetic rats after one (p < 0.001) and four weeks (p < 0.05) (Figure 2(a)).
Figure 2.
Measurement of hepatic glycogen content (a) and plasma IGF-1 levels (b). Results are given as mean ± SEM for control rats (C), non-treated diabetic rats (DIAB), and insulin-treated rats (INS) after one week (W1) and four weeks (W4), n = 5; mean ± SEM *p > 0.05, **p < 0.01, ***p < 0.001 compared with C-rats, ##p < 0.01, ###p < 0.001 compared with DIAB rats
As IGF-1 production is mediated by insulin, we compared the level of plasma IGF-1 between insulin-treated and untreated diabetic rats, and observed that IGF-1 production was higher in insulin-treated diabetic rats after one (p < 0.001) and four weeks of treatment (p < 0.01) (Figure 2(a)).
Subcutaneous insulin therapy does not reduce liver oxidative stress
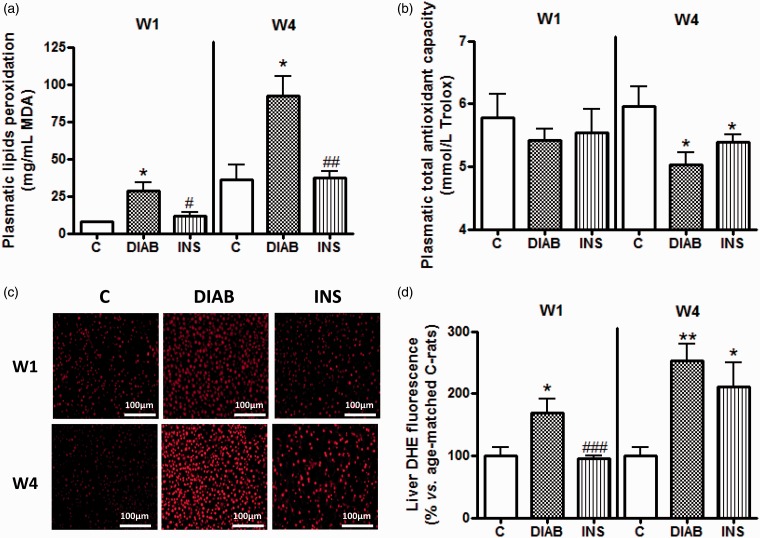
Insulin therapy also led to a reduction in the level of plasma lipid peroxidation from one week of treatment in comparison with untreated diabetic rats (p < 0.05). Moreover, this reduction was maintained after four weeks attesting to the effectiveness of subcutaneous insulin administration (p < 0.01, Figure 3(a)). However, the TAOC of insulin-treated diabetic rats was significantly lower than that of the control rats (p < 0.05), and comparable to that of untreated diabetic rats, after four weeks of treatment (Figure 3(b)).
Figure 3.
Measurement of plasma oxidative stress as defined by (a) lipid peroxidation, (b) total antioxidant capacity and tissue oxidative stress with the hepatic ROS production (c, d). (a and b) Results are given as mean ± SEM. (c) Similar results have been observed for four additional rats in each group. (d) Corresponding cumulative data. For all experiments: n = 5 for control rats (C), non-treated diabetic rats (DIAB), and insulin-treated rats (INS) after one week (W1) and four weeks (W4) of treatment. *p > 0.05, **p < 0.01, ***p < 0.001 compared with C-rats, #p < 0.05, ##p < 0.01, ###p < 0.001 compared with DIAB rats
Excess ROS production in the liver of diabetic rats was only reduced after one week of insulin treatment (p < 0.001 compared with diabetic rats). After four weeks, the level of ROS was similar between treated and untreated diabetic rats, and was significantly higher than that of the control group (p < 0.05, Figure 3(c) and (d)).
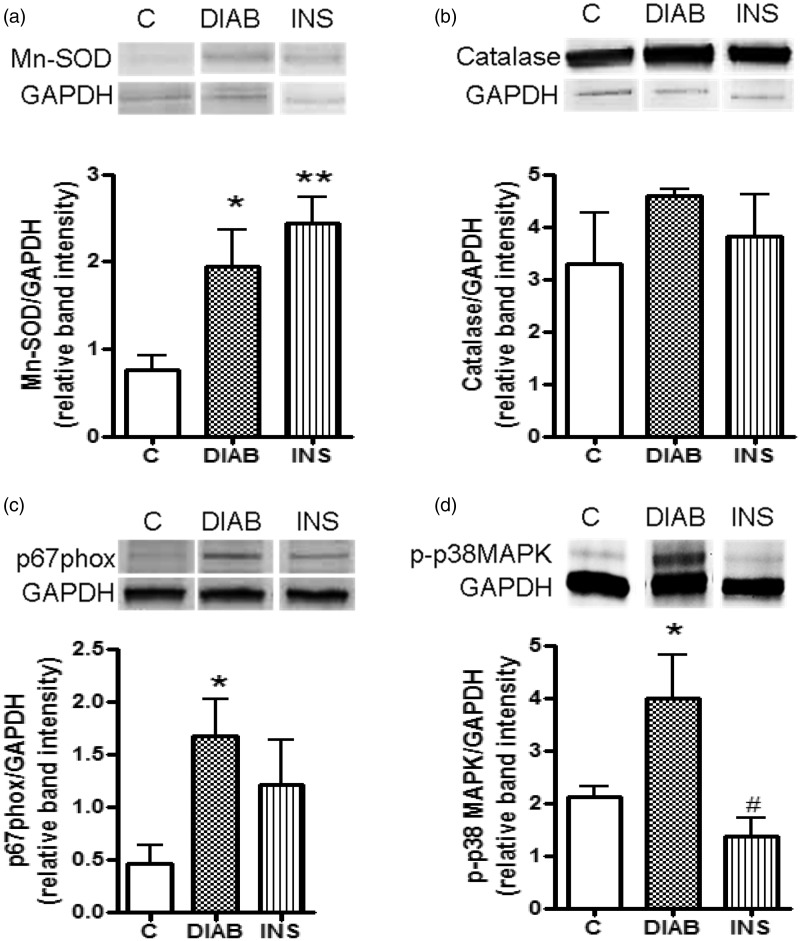
Finally, a study of oxidative stress signaling pathways in the liver was performed after four weeks of treatment. Mn-SOD was overexpressed in diabetic rats (p < 0.05) and insulin-treated diabetic rats (p < 0.01) in comparison with the control group (Figure 4(a)). In contrast, CAT expression was comparable in all three groups (Figure 4(b)). Expression of p67phox, a subunit of the pro-oxidant enzyme NADPH oxidase, was significantly higher in non-treated diabetic rats than that in control group (p < 0.05) (Figure 4(c)). Moreover, p38-MAPKinase activation was observed in untreated diabetic rats only (p < 0.05) (Figure 4(d)).
Figure 4.
Hepatic oxidative stress signaling pathways after four weeks of treatment. Based on expression of manganese superoxide dismutase (Mn-SOD) (a), catalase (CAT) (b), p67phox (a subunit of NADPH oxidase) (c), and p38-MAPKinase (d). The relative intensities of the bands were determined using Image J software n = 5; mean ± SEM for control rats (C), non-treated diabetic rats (DIAB), and insulin-treated rats (INS). *p > 0.05, **p < 0.01 compared with C-rats, #p < 0.05 compared with DIAB rats
Subcutaneous insulin treatment did not lead to improvements in liver inflammatory status
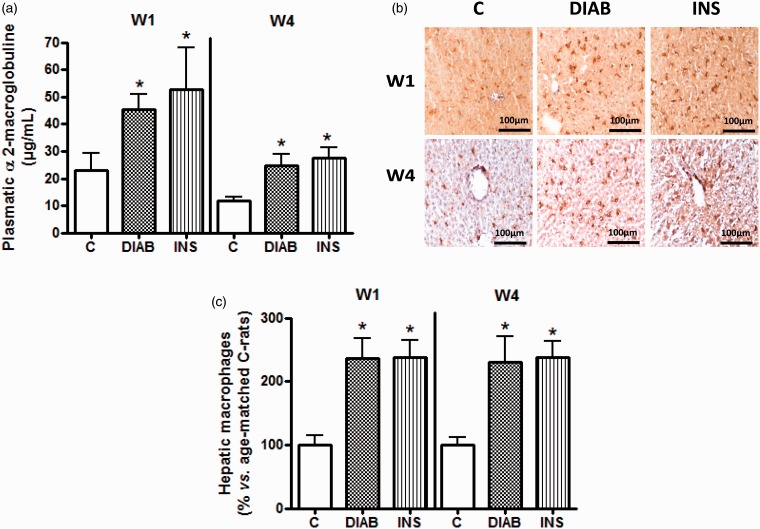
α2-macroglobulin, a protein characteristic of acute inflammation in rats, was measured (Figure 5(a)). We observed that after one and four weeks of insulin treatment, the level of α2-macroglobulin was comparable to that of untreated diabetic rats, and was higher than that of the control group (Figure 5(a)). At four weeks, α2-macroglobulin concentration in control group was 11.87 ± 1.2 µg/mL, compared to 24.65 ± 4.11 µg/mL in diabetic rats, and 27.64 ± 3.73 µg/mL in insulin-treated diabetic rats (p < 0.05).
Figure 5.
Measurement of plasma inflammation as defined by α2-macroglobulin level (a), and tissue inflammation as measured by the visualization and quantification of macrophage infiltration in the liver (b and c). (a) Results are given as mean ± SEM. (b) Similar results have been observed for four additional rats in each group. (c) Corresponding cumulative data. In all experiments: n = 5 for control rats (C), non-treated diabetic rats (DIAB), and insulin-treated rats (INS) after one week (W1) and four weeks (W4) of treatment. *p > 0.05 compared with C-rats
Analysis of liver inflammation was carried out through the measurement of macrophage infiltration. This infiltration was higher after one week in diabetic rats with or without insulin treatment (p < 0.05) and was maintained after four weeks (Figure 5(b) and (c)).
Finally, no macroscopic or physiopathological modifications, in particular signs of steatosis of the liver, were observed for animals undergoing Insuplant® treatment, as determined by hematoxylin/eosin coloration (data not shown).
Discussion
In this work, we have confirmed that hyperglycemia induced by diabetes is the pivotal mechanism leading to plasma and liver oxidative stress, and an increase in the production of ROS in diabetes. The control of blood glucose levels through intensive subcutaneous insulin administration led to a decrease in plasma oxidative stress but not to a reduction in the oxidative or inflammatory status of the liver.
The administration of STZ is a well-established experimental tool in the induction of type 1 diabetes. Its selective toxicity toward insulin-secreting β-cells leads to hyperglycemia and hypoinsulinemia.36,37 As expected in our study, diabetic animals exhibited higher blood glucose levels and lower body weight at the end of the experiment in comparison to the onset. Good control of diabetes, which often requires intensive insulin therapy, is desirable in type 1 diabetes.38 The intensive subcutaneous insulin administration performed using a mini pump in our study led to an improvement in the metabolic control of diabetic rats, as confirmed by a decrease in fructosamine levels and an increase in body weight after four weeks of treatment. Moreover, blood insulin concentration was maintained at the same level throughout the study attesting to the efficiency of this therapy. The improvement in the metabolic status of diabetic rats is comparable to results from studies in humans.39 Moreover, plasma cholesterol increased dramatically in diabetic rats, and similar elevated cholesterol levels have been reported for diabetic human patients.40 It is well known that insulin deficiency leads to inactivation of the lipoprotein lipase promoting hepatic conversion of free fatty acids into phospholipids and cholesterol which are then discharged into the blood resulting in elevated serum phospholipid levels.41 In insulin-treated rats, cholesterol concentration was maintained at a level comparable to that of control rats. In fact, insulin can activate the action of lipolytic hormones on peripheral fat which hydrolyses triglycerides and prevents the mobilization of free fatty acids.42
In addition to metabolic parameters, the evaluation of plasma oxidative status in diabetic rats showed an increase in lipid peroxidation; again, the administration of insulin restored lipid peroxidation levels to those of control rats. These data confirm that hyperglycemia causes oxidative stress,43 and the regulation of glycemic control during insulin therapy can reduce this stress.
TAOC, which reflects the collective reducing contribution of individual non-protein antioxidants or electron donating components,44 was evaluated. In our study, the level of TAOC decreased in insulin-treated rats after four weeks of treatment and was comparable to that of untreated diabetic rats, suggesting that even if lipid peroxidation is reduced after insulin therapy, oxidative stress was not reduced sufficiently to improve TAOC status. In accordance with these results, ROS production in the liver was significantly higher in untreated diabetic rats after one week and four weeks but insulin therapy did not result in a decrease in ROS production after four weeks. ROS production was associated with elevated SOD levels, and MAPKinase and NADPH oxidase activation in STZ-induced diabetic rats was comparable to control rats. The administration of insulin led to a decrease in MAPKinase activation in diabetic rats. In fact, tissue antioxidant status was altered with diabetes as previously described in the literature;22 however, insulin treatment partially reversed these changes. These effects were not due to the experimental model of diabetes used as STZ is rapidly metabolized.45 Moreover, no alterations in the oxidative status of the livers of STZ-injected animals that failed to develop diabetes were detected (data not shown). In agreement with our results, Palma et al.30 recently demonstrated that insulin therapy can prevent an increase in TBARS levels in serum but does not lead to an improvement in the antioxidant defenses of the liver.
It is well known that type 1 diabetes is considered an inflammatory process, and the effects of hyperglycemia are mediated by elevations in the levels of pro-inflammatory proteins.46 Indeed, the levels of α2-macroglobulin, a typical acute-phase protein in rats47–49 that is comparable to C-reactive protein in humans,50 suggest an increase in inflammation after one week, which is maintained after four weeks in diabetic rats. Moreover, insulin therapy did not lead to a reduction in this acute inflammation.
Oxidative stress and inflammation have been reported to cause direct organ damage in diabetic rats51 and humans.52 In this study, macrophage infiltration in the liver was observed in diabetic rats and, once again, insulin therapy did not lead to a decrease in this infiltration. Infiltrating macrophages are a source of pro-inflammatory cytokines in the liver.53 Our study showed that intensive insulin therapy cannot reduce the systemic and hepatic inflammation induced by diabetes.
The poor regulation of glycemia in diabetic rats with insulin therapy was not the cause of these results as insulin therapy restored glycogen storage in the liver and IGF-1 levels in the serum. In fact, some previous studies show that glycogen levels in the liver are lower in diabetic animals; however, accumulation of glycogen in the livers of insulin-treated animals is similar to that of control rats54–56 and is often accompanied by an increase in serum IGF-1 levels toward normal values.31,57–59
In summary, we have explored the effects of intensive subcutaneous insulin administration using a subcutaneous pump in a type 1 diabetic rat model. We found that insulin treatment could restore metabolic control in diabetic rats, and this was associated with a reduction in plasma oxidative stress. However, the important outcome of this study is that insulin treatment did not lead to a decrease in the acute inflammation associated with oxidative stress and macrophage infiltration in the livers of these rats. As liver inflammation affects insulin sensitivity and can induce systemic insulin resistance,60,61 targeting oxidative stress and/or inflammation in diabetic patients could improve their therapeutic options.
ACKNOWLEDGEMENTS
We thank the “Vaincre le Diabète” foundation and the ASDIA (Assistance Service Diabète) company for funding this project, Sanofi-Aventis for providing hepatic histological data, Dr F. Foufelle and her technician (UMR872 INSERM, Centre de recherche des Cordeliers, Paris, France) for help with hepatic glycogen extraction, and N. Dali-Youcef (IGBMC, UMR7104-U964 Equipe de Biologie Moléculaire et Cellulaire des Cancers du Sein, Illkirch, France, and Laboratoire de Biochimie et de Biologie Moléculaire Plateau technique de biologie NHC-CHU de Strasbourg, France).
Authors’ contribution
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; SD, ES, WB, CP, and SS conducted the experiments, SD, NJD, and SS wrote the manuscript.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hanefeld M, Temelkova-Kurktschiev T. Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis 2002; 12: 98–107. [PubMed] [Google Scholar]
- 2.Yki-Jarvinen H. Acute and chronic effects of hyperglycaemia on glucose metabolism: implications for the development of new therapies. Diabetes Med 1997; 14: S32–7. [DOI] [PubMed] [Google Scholar]
- 3.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108: 1527–32. [DOI] [PubMed] [Google Scholar]
- 4.Johnson EL. Glycemic variability in type 2 diabetes mellitus: oxidative stress and macrovascular complications. Adv Exp Med Biol 2012; 771: 139–54. [DOI] [PubMed] [Google Scholar]
- 5.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep 2013; 13: 435–44. [DOI] [PubMed] [Google Scholar]
- 6.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003; 108: 1655–61. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabetes Med 1997; 14: S45–9. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A. Hyperglycaemia: the bridge between non-enzymatic glycation and oxidative stress in the pathogenesis of diabetic complications. Diabetes Nutr Metab 1999; 12: 42–6. [PubMed] [Google Scholar]
- 9.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann NY Acad Sci 2013; 1281: 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care 2011; 34: S120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabater B, Martin M. Hypothesis: increase of the ratio singlet oxygen plus superoxide radical to hydrogen peroxide changes stress defense response to programmed leaf death. Front Plant Sci 2013; 4: 479.–479.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Jin X, Kei Lam CW, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med 2011; 49: 1773–82. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 2009; 82: 9–20. [DOI] [PubMed] [Google Scholar]
- 14.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol 2010; 88: 241–8. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 16.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Org J 2012; 5: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 2005; 38: 995–1014. [DOI] [PubMed] [Google Scholar]
- 18.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on beta cells and diabetes. Antioxid Redox Signal 2011; 14: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17:24–38. [DOI] [PubMed]
- 20.Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci 1998; 94: 623–32. [DOI] [PubMed] [Google Scholar]
- 21.McDermott BM, Flatt PR, Strain JJ. Effects of copper deficiency and experimental diabetes on tissue antioxidant enzyme levels in rats. Ann Nutr Metab 1994; 38: 263–9. [DOI] [PubMed] [Google Scholar]
- 22.Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat. Effects of insulin treatment. Diabetes 1987; 36: 1014–8. [DOI] [PubMed] [Google Scholar]
- 23.Godin DV, Wohaieb SA, Garnett ME, Goumeniouk AD. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol Cell Biochem 1988; 84: 223–31. [DOI] [PubMed] [Google Scholar]
- 24.Mak DH, Ip SP, Li PC, Poon MK, Ko KM. Alterations in tissue glutathione antioxidant system in streptozotocin-induced diabetic rats. Mol Cell Biochem 1996; 162: 153–8. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan RP, Fu B, Oliver RL, Siddals KW, Donn R, Hudson JE, White A, Laing I, Ollier WE, Heald AH, Gibson J. Insulin-like growth factor-II and insulin-like growth factor binding protein-2 prospectively predict longitudinal elevation of HDL-cholesterol in type 2 diabetes. Ann Clin Biochem 2014;51:468–75. [DOI] [PubMed]
- 26.Belviranli M, Gokbel H, Okudan N, Buyukbas S. Oxidative stress and anti-oxidant status in diabetic rat liver: effect of plant polyphenols. Arch Physiol Biochem 2012; 118: 237–43. [DOI] [PubMed] [Google Scholar]
- 27.Ozcelik D, Tuncdemir M, Ozturk M, Uzun H. Evaluation of trace elements and oxidative stress levels in the liver and kidney of streptozotocin-induced experimental diabetic rat model. Gen Physiol Biophys 2011; 30: 356–63. [DOI] [PubMed] [Google Scholar]
- 28.Sugiura M, Ohshima M, Ogawa K, Yano M. Chronic administration of Satsuma mandarin fruit (Citrus unshiu Marc.) improves oxidative stress in streptozotocin-induced diabetic rat liver. Biol Pharmaceut Bull 2006; 29: 588–91. [DOI] [PubMed] [Google Scholar]
- 29.Szaleczky E, Prechl J, Feher J, Somogyi A. Alterations in enzymatic antioxidant defence in diabetes mellitus—a rational approach. Postgrad Med J 1999; 75: 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma HE, Wolkmer P, Gallio M, Correa MM, Schmatz R, Thome GR, Pereira LB, Castro VS, Pereira AB, Bueno A, de Oliveira LS, Rosolen D, Mann TR, de Cecco BS, Graca DL, Lopes ST, Mazzanti CM. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol Cell Biochem 2014;368:199–210. [DOI] [PubMed]
- 31.Russell-Jones DL, Rattray M, Wilson VJ, Jones RH, Sonksen PH, Thomas CR. Intraperitoneal insulin is more potent than subcutaneous insulin at restoring hepatic insulin-like growth factor-I mRNA levels in the diabetic rat: a functional role for the portal vascular link. J Mol Endocrinol 1992; 9: 257–63. [DOI] [PubMed] [Google Scholar]
- 32.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–7. [DOI] [PubMed] [Google Scholar]
- 33.Gustavsson C, Yassin K, Wahlstrom E, Cheung L, Lindberg J, Brismar K, Ostenson CG, Norstedt G, Tollet-Egnell P. Sex-different hepaticglycogen content and glucose output in rats. BMC Biochem 2010; 11: 38.–38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dal-Ros S, Oswald-Mammosser M, Pestrikova T, Schott C, Boehm N, Bronner C, Chataigneau T, Geny B, Schini-Kerth VB. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology 2010; 138: 1574–84. [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–54. [DOI] [PubMed] [Google Scholar]
- 36.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976; 193: 415–7. [DOI] [PubMed] [Google Scholar]
- 37.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001; 50: 537–46. [PubMed] [Google Scholar]
- 38.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003; 289: 2254–64. [DOI] [PubMed] [Google Scholar]
- 39.Harrington J, Pena AS, Wilson L, Gent R, Dowling K, Baghurst P, Couper J. Vascular function and glucose variability improve transiently following initiation of continuous subcutaneous insulin infusion in children with type 1 diabetes. Pediatr Diabetes 2013; 14: 504–11. [DOI] [PubMed] [Google Scholar]
- 40.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, Cowan LD, Gray RS, Welty TK, Go OT, Howard WJ. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscl Thromb Vasc Biol 2000; 20: 830–5. [DOI] [PubMed] [Google Scholar]
- 41.Pushparaj PN, Low HK, Manikandan J, Tan BK, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol 2007; 111: 430–4. [DOI] [PubMed] [Google Scholar]
- 42.Briones ER, Mao SJ, Palumbo PJ, O’Fallon WM, Chenoweth W, Kottke BA. Analysis of plasma lipids and apolipoproteins in insulin-dependent and noninsulin-dependent diabetics. Metabol Clin Exp 1984; 33: 42–9. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhry J, Ghosh NN, Roy K, Chandra R. Antihyperglycemic effect of a new thiazolidinedione analogue and its role in ameliorating oxidative stress in alloxan-induced diabetic rats. Life Sci 2007; 80: 1135–42. [DOI] [PubMed] [Google Scholar]
- 44.Fayh AP, Krause M, Rodrigues-Krause J, Ribeiro JL, Ribeiro JP, Friedman R, Moreira JC, Reischak-Oliveira A. Effects of L-arginine supplementation on blood flow, oxidative stress status and exercise responses in young adults with uncomplicated type I diabetes. Eur J Nutr 2013; 52: 975–83. [DOI] [PubMed] [Google Scholar]
- 45.Bhuyan BK, Kuentzel SL, Gray LG, Fraser TJ, Wallach D, Neil GL. Tissue distribution of streptozotocin (NSC-85998). Cancer Chemother Rep Part 1 1974; 58: 157–65. [PubMed] [Google Scholar]
- 46.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi S, Jinbo T, Iguchi K, Shimizu M, Shimada T, Nomura M, Ishida Y, Yamamoto S. A comparison of the concentrations of C-reactive protein and alpha1-acid glycoprotein in the serum of young and adult dogs with acute inflammation. Veterinary Res Commun 2001; 25: 117–26. [DOI] [PubMed] [Google Scholar]
- 48.Jinbo T, Sakamoto T, Yamamoto S. Serum alpha2-macroglobulin and cytokine measurements in an acute inflammation model in rats. Lab Anim 2002; 36: 153–7. [DOI] [PubMed] [Google Scholar]
- 49.van Westrhenen R, Westra WM, van den Born J, Krediet RT, Keuning ED, Hiralall J, Dragt C, Hekking LH. Alpha-2-macroglobulin and albumin are useful serum proteins to detect subclinical peritonitis in the rat. Perit Dial Int 2006; 26: 101–7. [PubMed] [Google Scholar]
- 50.Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 2008; 19: 104–8. [DOI] [PubMed] [Google Scholar]
- 51.Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan A, Suzuki K, Kawachi H, Watanabe K. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab 2011; 8: 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu CC, Chen JS, Lu KC, Chen CC, Lin SH, Chu P, Sytwu HK, Lin YF. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta 2010; 411: 700–4. [DOI] [PubMed] [Google Scholar]
- 53.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000; 343: 1467–76. [DOI] [PubMed] [Google Scholar]
- 54.Osborn MJ, Felts JM, Chaikoff IL. Transitory increase in fat content and size of liver induced by insulin in alloxandiabetic rats. J Biol Chem 1953; 203: 173–81. [PubMed] [Google Scholar]
- 55.Spiro RG, Hastings AB. Studies on carbohydrate metabolism in rat liver slices. XI. Effect of prolonged insulin administration to the alloxan-diabetic animal. J Biol Chem 1958; 230: 751–9. [PubMed] [Google Scholar]
- 56.Steiner DF, King J. Induced synthesis of hepatic uridine diphosphate glucose-glycogen glucosyltransferase after administration of insulin to alloxan-diabetic rats. J Biol Chem 1964; 239: 1292–8. [PubMed] [Google Scholar]
- 57.Scheiwiller E, Guler HP, Merryweather J, Scandella C, Maerki W, Zapf J, Froesch ER. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature 1986; 323: 169–71. [DOI] [PubMed] [Google Scholar]
- 58.Scott CD, Baxter RC. Production of insulin-like growth factor I and its binding protein in rat hepatocytes cultured from diabetic and insulin-treated diabetic rats. Endocrinology 1986; 119: 2346–52. [DOI] [PubMed] [Google Scholar]
- 59.Rudolf MC, Coustan DR, Sherwin RS, Bates SE, Felig P, Genel M, Tamborlane WV. Efficacy of the insulin pump in the home treatment of pregnant diabetics. Diabetes 1981; 30: 891–5. [DOI] [PubMed] [Google Scholar]
- 60.Adiels M, Taskinen MR, Boren J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diabetes Rep 2008; 8: 60–4. [DOI] [PubMed] [Google Scholar]
- 61.Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol 2006; 40: 68–76. [DOI] [PubMed] [Google Scholar]