Abstract
Renal injury has a strong relationship to the subsequent development of renal fibrosis. In developing renal fibrosis, tubular epithelial cells in the kidney underwent epithelial–mesenchymal transition (EMT). Matrix metalloproteinase 7 (MMP7) was reported to reduce E-cadherin and induce EMT by up-regulation of β-catenin/lymphoid enhancer-binding factor 1 (LEF1) signaling. In this research, we tried to evaluate the role of resveratrol (RSV) on EMT process in renal injury and fibrosis. Human tubular epithelial cell HK-2 cells were treated with aristolochic acid (AAs) and transforming growth factor-β(TGF-β) to induce EMT with or without the administration of RSV. The inhibitory role of RSV on EMT in renal injury and fibrosis was determined by Western blotting, real-time PCR, and immunofluorescence staining. The EMT repressing role of RSV was also evaluated in vivo by renal ischemia-reperfusion (I/R) injury and unilateral ureteral obstruction (UUO) models. The underlying mechanism was investigated by shRNA interfering MMP7 and sirtuin 1 (SIRT1) expression. The results indicated that RSV reversed human kidney 2 (HK-2) cell EMT, renal I/R injury, and renal fibrosis. MMP7 inhibition was responsible for RSV-induced EMT repression. SIRT1 was up-regulated by RSV inhibited TGF-β pathway on MMP7 via deacetylating Smad4. In conclusion, RSV attenuated renal injury and fibrosis by inhibiting EMT process which was attributed to the fact that the up-regulated SIRT1 by RSV deacetylated Smad4 and inhibited MMP7 expression.
Keywords: Resveratrol, epithelial–mesenchymal, transition, renal fibrosis, matrix metalloproteinase 7
Introduction
Renal injury is a complex disorder that occurs in a variety of settings with clinical manifestations. It has a strong relationship to the subsequent development of chronic kidney diseases, like renal fibrosis. In developing renal fibrosis, tubular epithelial cells in the kidney underwent epithelial–mesenchymal transition (EMT). The blockage of EMT reduced fibrosis after renal obstructive injury.1 In injury and fibrosis, matrix metalloproteinases (MMPs) participated in regulating abnormal epithelial response to injury, fibroblast proliferation, extracellular matrix accumulation, and aberrant tissue remodeling.2 It has been reported that MMP7 expression dramatically increased during several renal disease states and acute lung injury.3–5 Chen et al.6 found that MMP7 was over-expressed in the aging kidney. The role of MMP7 on the induction EMT of human squamous carcinoma cell line was reported by Shibata et al.7 They found that MMP7 could reduce E-cadherin and induce EMT by up-regulation of β-catenin/LEF1 signaling.
Resveratrol (RSV; trans-3,5,4'-trihydroxystilbene), a stilbene polyphenol from grapes, wine, mulberries, and peanuts, has been reported to have various pharmacological effects, including protection against coronary heart disease, anti-inflammatory properties, and chemo-preventive of cancer, etc.8–10 In basic research, RSV is also a star natural compound in attenuating renal injury and fibrosis, including glycerol-induced renal injury,11 sepsis-induced renal injury,12 cisplatin-induced renal injury,13 ischemia-reperfusion (I/R) injury,14 and ureteral obstruction (UUO)-induced renal fibrosis,15 etc. Most of the researches focused on the anti-inflammatory and anti-oxidation effects of RSV. RSV ameliorated renal injury and renal fibrosis by suppressing the inflammatory process and by inhibiting lipid peroxidation.11 The inhibitory effect of RSV on EMT has been revealed in prostate cancer,16 ovarian cancer,17 breast cancer,18 and pancreatic cancer.19 In a recent research by Bai et al.,20 RSV inhibited EMT in renal tubular cells by antagonizing the hedgehog signaling pathway. Based on these understandings, we speculated that as the activator of sirtuin 1 (SIRT1),12 whose loss in tubular epithelial cells exacerbated injury induced kidney fibrosis,21 RSV could inhibit EMT in renal injury and renal fibrosis by activating SIRT1.
In this study, we investigated the role of RSV in EMT process which is a key step in renal injury and fibrosis and try to uncover the underlying mechanism beneath the protective role of RSV from a novel aspect.
Materials and methods
Cell and reagents
Human tubular epithelial cell human kidney 2 (HK-2) cells were from ATCC. HK-2 cells were supported in RPMI-1640 medium (Gibco, Gaithersburg, MD) containing 10% fetal bovine serum (FBS) (Gibco) at 37 ℃ in an air supplemented with 5% CO2 under humidified condition. RSV and aristolochic acids (AAs) were purchased from Sigma Aldrich (St. Louis, MO). Recombinant human transforming growth factor-β (TGF-β) was purchased from PeproTech (Rocky Hill City, NJ). Primary antibodies against E-cadherin, α-smooth muscle actin (α-SMA), type I collagen (COL1A1), β-catenin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MMP7, and SIRT1 were obtained from Cell Signaling Technology (Beverly, MA). The antibody against acetyl-lysine was purchased from Santa Cruz Biotechnology.
Renal I/R injury and UUO models
Renal I/R injury and UUO models were generated in eight-week-old male C57BL/6 mice (n = 8 per group). I/R injury was performed by clamping both renal pedicles for 30 min with vascular clamps. Blood sample from I/R injury model was collected every day after surgery. Mice were sacrificed four days following the experiment and kidneys were collected for further analysis. For UUO model, 4-0 silk was used to ligate the left ureter of the anesthetized mice.15 The mice in the RSV treatment group received a daily dose of 20 mg/kg (body weight) of RSV by gavage. Mice in control group, I/R injury, and UUO group were treated with vehicle. The mice in UUO model were sacrificed six weeks after left ureter ligation and kidneys were collected.
Western blotting
Protein levels were detected by Western blotting following published protocols.21 The protein levels were determined by incubating with primary antibodies followed by horseradish peroxidase (HRP)-conjugated second antibody. The signal was developed with enhanced chemiluminescence (ECL) (Millipore, Switzerland).
qRT-PCR
Total RNA was extracted by TRIzol reagent (TaKaRa, Japan) and reverse transcribed to cDNA using Primescript RT master mix (TaKaRa). The relative mRNA levels were determined by quantitative real-time PCR using the SYBR Green PCR Kit (TaKaRa) on ABI PRISM 7300Sequence Detector. GAPDH was set as internal control. The mRNA relative expression level was calculated by 2−ΔΔCt method. The primer sequences were as the following: for hE-cadherin gene, forward primer 5'-CGAGAGCTACACGTTCACGG-3', reverse primer 5'-GGGTGTCGAGGGAAAAATAGG-3'; for hα-SMA gene, forward primer 5'-TCTG GAGGCACAACTGGCATCGT-3', reverse primer 5'-TACATATGTTGTCCCCC TGATAG-3'; for hCOL1A1 gene, forward primer 5'-GAGGGCCAAGACGAAG ACATC-3', reverse primer 5'-CAGATCACGTCATCGCACAAC-3'; for hMMP7 gene, forward primer 5'-GAGTGAGCTACAGTGGGAACA-3', reverse primer 5'-CTATGACGCGGGAGTTTAACAT-3'; for hSIRT1 gene, forward primer 5'-TAGCCTT GTCAGATAAGGAAGGA-3', reverse primer 5'-ACAGCTTCACAGTCAACTTT GT-3'; for mE-cadherin gene, forward primer 5'-CAGGTCTCCTCATGGCTTT GC-3', reverse primer 5'-CTTCCGAAAAGAAGGCTGTCC-3'; for mα-SMA gene, forward primer 5'-GTCCCAGACATCAGGGAGTAA-3', reverse primer 5'-GTC CCAGACATCAGGGAGTAA-3', reverse primer 5'-TCGGATACTTCAGCGTC AGGA-3'; for mCOL1A1 gene, forward primer 5'-GCTCCTCTTAGGGGCCACT-3', reverse primer 5'-CCACGTCTCACCATTGGGG-3'; for hGAPDH gene, forward primer 5'-GGAGCGAGATCCCTCCAAAAT-3', reverse primer 5'-GGCTGTTGTC ATACTTCTCATGG-3'; for mGAPDH gene, forward primer 5'-AGGTCGGTGTG AACGGATTTG-3', reverse primer 5'-TGTAGACCATGTAGTTGAGGTCA-3'.
Immunofluorescence staining
HK-2 cells were labeled with antibodies against β-catenin and MMP7. The slides were exposed to AlexFluor594 anti-rabbit secondary antibodies (Invitrogen, CA). The nucleus was stained with DAPI (Invitrogen). The staining was visualized by fluorescence microscope.
Renal function
The serum creatinine was detected by S-Cr ELISA kit (ExCell Bio, China). The blood urea nitrogen (BUN) level was determined by a commercial ELISA kit (Neogen, Shanghai) following the manufacturer's instructions.
Immunoprecipitation
The immunoprecipitations were carried out by using Pierce Direct IP Kit (Thermo Scientific). HK-2 cells were incubated with agarose beads coupled with Smad4 antibody. The Smad4 acetylation was determined by incubation with acetyl-lysine antibody.
Statistical analysis
All the data were expressed as mean ± SD from three individual experiments and were analyzed by the Student's t-test and two-way ANOVA with Bonferroni correction. P < 0.05 was considered significant.
Results
RSV reversed HK-2 cell EMT induced by AAs and TGF-β
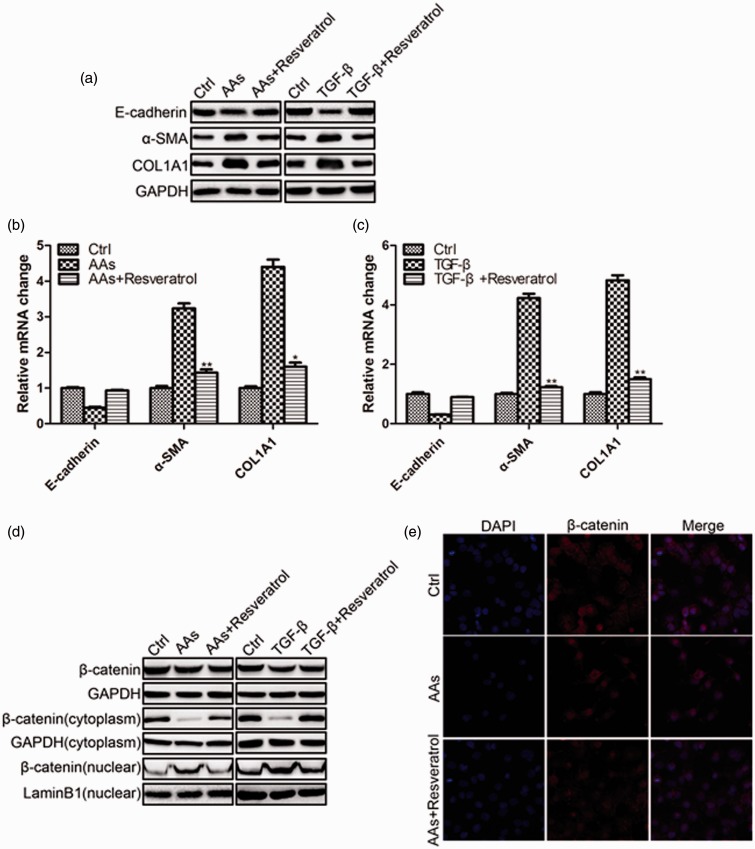
To induce EMT in HK-2 cells, we used AAs and TGF-β in the experiments. HK-2 cells were incubated with AAs or TGF-β for 48 h, and were harvested for protein and mRNA analysis. As shown in Figure 1(a) and (b), AAs inhibited E-cadherin and increased α-SMA and COL1A1 protein and mRNA levels. A similar result was observed in TGF-β treated HK-2 cells (Figure 1(a) and (c)). This confirmed that HK-2 cell underwent EMT. However, the administration of RSV inhibited E-cadherin decrease and α-SMA and COL1A1 increase. RSV blocked the EMT process induced by AAs and TGF-β. Because nuclear re-localization of β-catenin/LEF was one of the major criteria for EMT, we further detected the location of β-catenin in the cells by separating the cytoplasm and nuclear protein. We found a translocation of β-catenin from the cytoplasm to the nuclear under AAs and TGF-β treatment which could be inhibited by RSV administration (Figure 1(d)). The immunofluorescence staining of β-catenin was in consistence with the Western blotting results (Figure 1(e)).
Figure 1.
RSV reversed HK-2 cell EMT induced by AAs and TGF-β. a. Western blotting results of E-cadherin, α-SMA, and COL1A1 in HK-2 cells 48 h following the treatment of AAs (10 μmol/L)/TGF-β (10 ng/mL) with or without the presence of RSV (20 μmol/L). b. Real-time PCR results of E-cadherin, α-SMA, and COL1A1 in HK-2 cells 48 h following the treatment of AAs (10 μmol/L) with or without the presence of RSV (20 μmol/L). c. Real-time PCR results of E-cadherin, α-SMA, and COL1A1 in HK-2 cells 48 h following the treatment of TGF-β (10 ng/mL) with or without the presence of RSV (20 μmol/L). d. RSV reversed HK-2 cell EMT by inhibiting the translocation of β-catenin into the nuclear. e. Immunofluorescence staining of β-catenin in HK-2 cells 48 h following the treatment of AAs with or without the presence of RSV (*P < 0.05 AAs vs. AAs + RSV, **P < 0.01 AAs vs. AAs + RSV). (A color version of this figure is available in the online journal.)
RSV reversed renal I/R injury and renal fibrosis induced by UUO
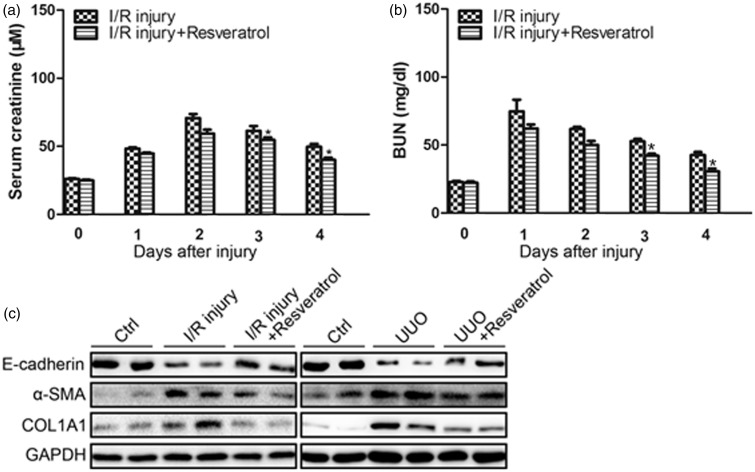
To verify the inhibitory effect on EMT by RSV in vivo, we adopted acute renal injury and renal fibrosis model in mice. After I/R, the serum creatinine and BUN were detected. In I/R injury group, the creatinine elevated sharply and peaked at the second day, and then slowly declined. The creatinine in RSV-treated group was lower than AAs group (Figure 2(a)). The BUN changes were similar to creatinine changes. RSV decreased the BUN elevation after I/R injury (Figure 2(b)). The EMT markers E-cadherin, α-SMA and COL1A1 after renal injury and fibrosis were detected by Western blotting. After I/R injury and renal fibrosis, E-cadherin decreased in the kidney, while α-SMA and COL1A1 increased, which proved that EMT existed in renal injury and fibrosis. A daily dose of 20 mg/(kg body weight) of RSV partially inhibited the changes of the EMT markers (Figure 2(c)).
Figure 2.
RSV reversed renal I/R injury and renal fibrosis induced by UUO. a. Serum creatinine changes over time following I/R injury (n = 6 in each group). b. BUN levels over time following I/R injury (n = 6 in each group). c. Western blotting results of E-cadherin, α-SMA, and COL1A1 in the kidney of mice after I/R injury for 48 h with or without the presence of RSV (left panel). Western blotting results of E-cadherin, α-SMA, and COL1A1 in the kidney of mice after UUO for six weeks with or without the presence of RSV (right panel). (*P < 0.05 I/R injury vs. I/R injury + RSV)
The above results proved that RSV attenuated renal injury and fibrosis by inhibiting EMT process in vitro and in vivo.
MMP7 inhibition was responsible for RSV-induced EMT repression
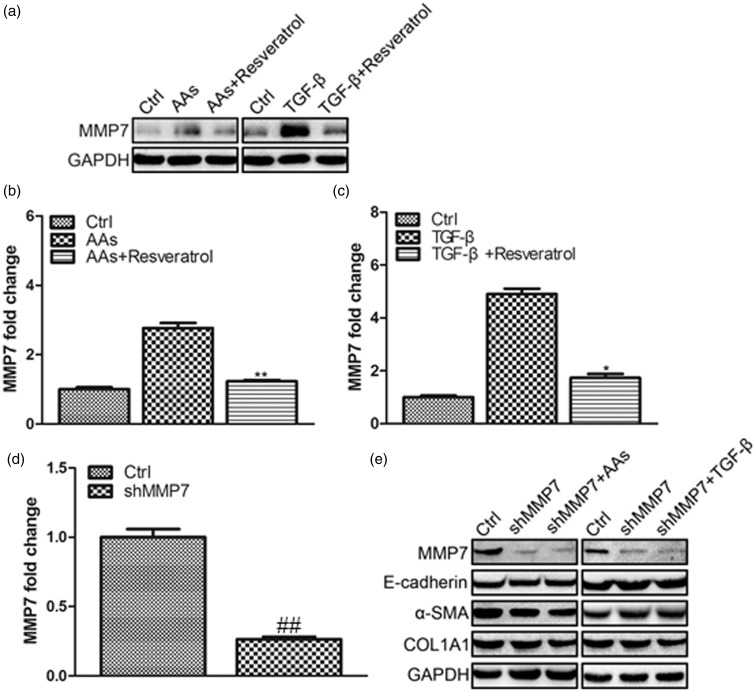
Because MMP7 was reported to increase in renal injury and it could reduce E-cadherin and induce EMT,6,7 we further detected the level of MMP7 in HK-2 cells. We found the increase of MMP7 in HK-2 cells after AAS and TGF-β treatment and RSV inhibited MMP7 elevation (Figure 3(a) to (c)). As MMP7 could induce EMT through nuclear β-catenin translocation, we speculated that MMP7 might be a key protein in AAs and TGF-β induced EMT. Therefore, we further studied MMP7 expression in HK-2 by transfecting MMP7 shRNA. The interfering efficiency was determined by real-time PCR method which showed an interfering efficiency of about 70% (Figure 3(d)). After MMP7 shRNA transfection for 48 h, HK-2 cells were incubated with AAs/TGF-β for another 48 h. The Western blotting results showed that MMP7 shRNA transfection inhibited AAs/TGF-β induced E-cadherin decrease and α-SMA and COL1A1 increase.
Figure 3.
MMP7 inhibition was responsible for RSV-induced EMT repression. a. Western blotting results of MMP7 in HK-2 cells 48 h following the treatment of AAs (10 μmol/L)/TGF-β (10 ng/mL) with or without the presence of RSV (20 μmol/L). b. Real-time PCR results of MMP7 in HK-2 cells 48 h following the treatment of AAs (10 μmol/L) with or without the presence of RSV (20 μmol/L). c. Real-time PCR results of MMP7 in HK-2 cells 48 h following the treatment of TGF-β (10 ng/mL) with or without the presence of RSV (20 μmol/L). d. Interfering efficiency of MMP7 shRNA verified by real-time PCR. e. Inhibition of MMP7 reversed AAs (10 μmol/L)/TGF-β (10 ng/mL) induced EMT. (*P < 0.01 AAs vs. AAs + RSV, *P < 0.05 TGF-β vs. TGF-β + RSV, ##P < 0.01 Ctrl vs. shMM7)
SIRT1 up-regulation by RSV inhibited TGF-β pathway on MMP7 by deacetylating Smad4
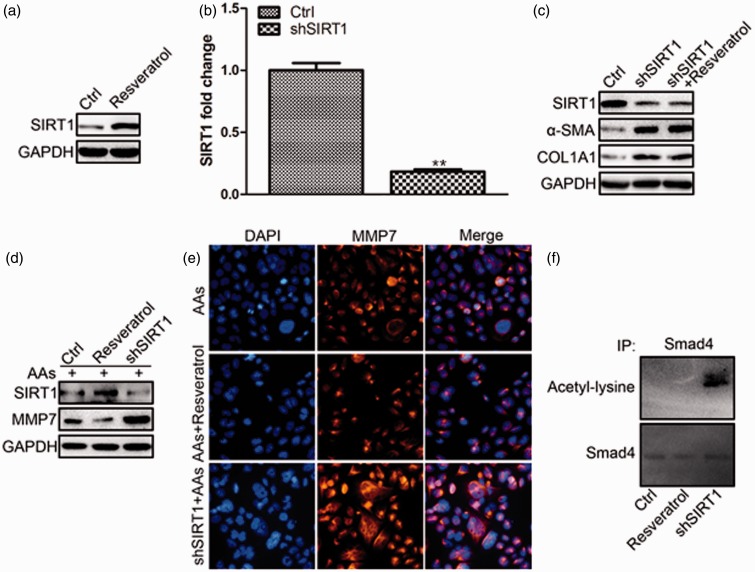
Because SIRT1 could be activated by RSV22 and the loss of SIRT1 in tubular epithelial cells exacerbated injury induced kidney fibrosis,21 we further detected the SIRT1 level after RSV treatment. RSV up-regulated the protein level of SIRT1 dramatically (Figure 4(a)). To confirm the necessary role of SIRT1 in RSV-induced EMT repression, SIRT1 shRNA was transfected into HK-2 cells. Figure 4(b) showed the interfering efficiency of SIRT1 shRNA to be about 80%. SIRT1 interfering induced EMT in HK-2 cells as manifested by the increase of α-SMA and COL1A1 protein level which could not be repressed by RSV (Figure 4(c)). The increased α-SMA and COL1A1 level might be the result of increased MMP7, because SIRT1 interfering also promoted the increase of MMP7 protein level (Figure 4(d) and (e)). Because SIRT1 deacetylated the positive regulator of TGF-β signalingSmad4 to inhibit the expression of MMP7,21 we immunoprecipitated Smad4 from HK-2 cells after RSV incubation/ SIRT1 shRNA transfection and probed Western blotting with antibody against acetyl-lysine. We found SIRT1 interfering increased the acetylation of Smad4 (Figure 4(f)).
Figure 4.
SIRT1 up-regulation by RSV inhibited TGF-β pathway on MMP7 by deacetylating Smad4. a. RSV up-regulated SIRT1 protein level. b. Interfering efficiency of SIRT1 shRNA verified by real-time PCR. c. Inhibition of SIRT1 induced HK-2 cell EMT. d. SIRT1 up-regulation inhibited MMP7 protein level. e. SIRT1 up-regulation by RSV inhibited MMP7 protein level visualized by immunofluorescence staining of MMP7 in HK-2 cells. f. SIRT1 up-regulation by RSV deacetylated Smad4 which inhibited MMP7 activity (**P < 0.01 Ctrl vs. shSIRT1). (A color version of this figure is available in the online journal.)
The above results proved that RSV up-regulated SIRT1 which deacetylated Smad4 and inhibited MMP7 expression, thus leading to EMT repression.
Discussion
In the present study, we evaluated the inhibitory role of RSV on EMT process in renal injury and fibrosis. RSV reversed the reduction of E-cadherin and the increase of α-SMA and COL1A1 in both protein and mRNA levels. RSV inhibited the profibrotic protein MMP7 level, therefore, attenuated UUO-induced renal fibrosis. The underlying mechanism was also investigated. We found that SIRT1 up-regulation by RSV was responsible for the downstream blockage of EMT process. SIRT1 inhibited MMP7 protein level by deacetylating Smad4 which was a transcription factor for MMP7 expression, and the interfering of SIRT1 induced the up-regulation of MMP7 and EMT.
RSV is a polyphenol found in red wine that is associated with a number of health benefits.22–24 The renal protective effects of RSV have been reported previously. RSV can ameliorate several types of renal injury through its antioxidant effect and SIRT1 activation.23 SIRT1 is an NAD+-dependent deacetylase and functions in multiple cellular processes like apoptosis, mitochondrial biogenesis, inflammation, etc. through the deacetylation of target proteins.25 He et al.26 found that SIRT1 is an important protective factor for mouse renal medullary interstitial cells following oxidative stress. Jung et al.13 found that SIRT1 up-regulation by RSV decreased acetylation of NF-κB p65 subunit and attenuate cisplatin-induced renal cell damage.
In renal injury and fibrosis, renal tubular epithelial cells undergo EMT in which they lose the epithelial phenotype and acquire the new features of mesenchyme.27 When renal fibrogenesis sets in, more than one-third of all disease-related fibroblasts originate from tubular epithelial cells at the site of injury.28 This reinforces the notion that EMT exists in renal injury and fibrosis. In the EMT process of renal tubular cells, loss of epithelial adhesion properties, de novo α-SMA expression and actin reorganization, disruption of tubular basement membrane, and enhanced cell migration happen. In this research, human tubular cell HK-2 cells were treated with AAs and TGF-β to induce EMT in vitro, while renal I/R injury and UUO models in mice were used to induce EMT in vivo. The renal I/R injury and longtime UUO models can mimic renal epithelial injury and renal fibrosis, respectively. We found that AAs and TGF-β could induce EMT in HK-2 cells. Renal I/R injury and longtime UUO also induced EMT in the kidney. The induced EMT could be reversed by RSV administration. RSV attenuated renal injury and fibrosis by inhibiting EMT process.
The growth factor TGF-β can initiate and maintain EMT in a variety of biological system and pathophysiological context by activating major signaling pathways and transcriptional regulators integrated in extensive signaling networks.29 Boutet et al.30 found that aberrant Snail1 activation in the adult kidney is sufficient to induce renal fibrosis in transgenic mice. TGF-β signaling regulates Snail activation, which triggers EMT and the conversion of epithelial cells into myofiroblasts.31 Thiery et al.,31 found that in renal proximal tubular cells, TGF-β-induced β-catenin was required for synthesis of α-SMA as a marker of EMT. In this research, we found the translocation of β-catenin into the nucleus which could be inhibited by RSV.
It has been reported that MMP7 expression increases during several renal diseases and in aging kidneys.3,6 MMP7 can reduce E-cadherin and induce EMT by up-regulation of β-catenin/LEF1 signaling.7 In TGF-β signaling, there is a strong relationship between MMP7 and Smad4. Kitamura et al. found that MMP7 was not required for the invasion or fibrosis of the colon cancer whose Smad4-dependent TGF-β family signaling is blocked.32 Simic et al.21 found that TGF-β regulated MMP7 expression, while SIRT1 deacetylated Smad4 which re-pressed the activity of MMP7. Some scientists33,34 also found that by associating and deacetylating Smad4, SIRT1 enzyme could influence MMP7 expression and enzyme activity. In this research, we found that the up-regulated SIRT1 by RSV deacetylated Smad4 and inhibited MMP7 expression.
In summary, we found that RSV attenuated renal injury and fibrosis by inhibiting EMT process. The up-regulated SIRT1 by RSV inhibited MMP7 expression by deacetylating Smad4.
ACKNOWLEDGMENT
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Natural Science Fund of China (No. 81070552).
Author contributions
ZX and QZ conceived and designed the study; ZX, CC, and WZ performed laboratory experiments; ZX, CC, and TM analyzed data and interpreted the results; ZX, CC, and QZ created the figures and drafted the manuscript; ZX, CC, WZ, and QZ edited and revised manuscript; all authors approved the final version of the manuscript.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Wu ST, Sun GH, Hsu CY, Huang CS, Wu YH, Wang HH, Sun KH. Tumor necrosis factor-alpha induces epithelial-mesenchymal transition of renal cell carcinoma cells via a nuclear factor kappa B-independent mechanism. Exp Biol Med 2011; 236: 1022–9. [DOI] [PubMed] [Google Scholar]
- 2.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008; 5: 0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002; 11: 635–46. [DOI] [PubMed] [Google Scholar]
- 4.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003; 162: 1831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Feng P, Li X, Song J, Chen W. Expression of MMP-2, MT1-MMP, and TMP-2 by cultured rabbit corneal fibroblasts under mechanical stretch. Exp Biol Med 2014; 239: 907–12. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Bridenbaugh EA, Akintola AD, Catania JM, Vaidya VS, Bonventre JV, Dearman AC, Sampson HW, Zawieja DC, Burghardt RC. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol 2007; 293: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata SI, Marushima H, Asakura T, Matsuura T, Eda H, Aoki K, Matsudaira H, Ueda K, Ohkawa K. Three-dimensional culture using a radial flow bioireactor induces matrix metalloprotease 7-mediated EMT-like process in tumor cells via TGF beta 1/Smad pathway. Int J Oncol 2009; 34: 1433–48. [PubMed] [Google Scholar]
- 8.Fremont L. Biological effects of resveratrol. Life Sci 2000; 66: 663–73. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 2004; 24: 2783–840. [PubMed] [Google Scholar]
- 10.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002; 106: 1652–8. [DOI] [PubMed] [Google Scholar]
- 11.de Jesus Soares T, Volpini RA, Francescato HD, Costa RS, da Silva CG, Coimbra TM. Effects of resveratrol on glycerol-induced renal injury. Life Sci 2007; 81: 647–56. [DOI] [PubMed] [Google Scholar]
- 12.Kolgazi M, Sener G, Cetinel S, Gedik N, Alican I. Resveratrol reduces renal and lung injury caused by sepsis in rats. J Surg Res 2006; 134: 315–21. [DOI] [PubMed] [Google Scholar]
- 13.Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK, Lee SY, Han MK, Kim DH, Kim W. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-kappaB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun 2012; 419: 206–10. [DOI] [PubMed] [Google Scholar]
- 14.Chander V, Chopra K. Role of nitric oxide in resveratrol-induced renal protective effects of ischemic preconditioning. J Vasc Surg 2005; 42: 1198–205. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Tian S, Han J, Xiong P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren Fail 2014; 36: 285–91. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Chong T, Wang Z, Chen H, Li H, Cao J, Zhang P, Li H. A novel anti-cancer effect of resveratrol: reversal of epithelial-mesenchymal transition in prostate cancer cells. Mol Med Rep 2014; 10: 1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baribeau S, Chaudhry P, Parent S, Asselin E. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. Plos One 2014; 9: e86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way T-D, Chen WJ. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol 2013; 272: 746–56. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu Q, Duan W, Yu S, Wang F. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappa B pathway. Curr Med Chem 2013; 20: 4185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai YH, Lu H, Wu CZ, Liang Y, Wang SL, Lin CC, Chen BC, Xia P. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem Pharmacol 2014; 92: 484–93. [DOI] [PubMed] [Google Scholar]
- 21.Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep 2013; 3: 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–6. [DOI] [PubMed] [Google Scholar]
- 23.Kitada M, Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev 2013; 2013: 568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, Yang Y, Chen X, Liu G. Resveratrol inhibits TGF-beta 1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013; 303: 139–46. [DOI] [PubMed] [Google Scholar]
- 25.Guarente L. Sirtuins, aging, and medicine. New Eng J Med 2011; 364: 2235–44. [DOI] [PubMed] [Google Scholar]
- 26.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 2010; 120: 1056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004; 15: 1–12. [DOI] [PubMed] [Google Scholar]
- 28.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002; 110: 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005; 24: 5764–74. [DOI] [PubMed] [Google Scholar]
- 30.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. Embo J 2006; 25: 5603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiery JP, Acloque H, Huang RYJ, Angela Nieto M. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–90. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura T, Biyajima K, Aoki M, Oshima M, Taketo MM. Matrix metalloproteinase 7 is required for tumor formation, but dispensable for invasion and fibrosis in SMAD4-deficient intestinal adenocarcinomas. Lab Invest 2009; 89: 98–105. [DOI] [PubMed] [Google Scholar]
- 33.Chen IC, Chiang WF, Huang HH, Chen PF, Shen YY, Chiang HC. Role of SIRT1 in regulation of epithelial-to-mesenchymal transition in oral squamous cell carcinoma metastasis. Mol Cancer 2014; 13: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep 2013; 3: 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]