Abstract
Our previous study found that CLOCK knockdown in the testes of male mice led to a reduced fertility, which might be associated with the lower acrosin activity. In this present study, we examined the differential expression in proteins of CLOCK knockdown sperm. Clock gene expression was knocked down in cells to confirm those differentially expressions and serine protease inhibitor SERPINA3K was identified as a potential target. The up-regulated SERPINA3K revealed an inverse relationship with Clock knockdown. Direct treatment of normal sperm with recombinant SERPINA3K protein inhibited the acrosin activity and reduced in vitro fertilization rate. The luciferase reporter gene assay showed that the down-regulated of Clock gene could activate the Serpina3k promoter, but this activation was not affected by the mutation of E-box core sequence. Co-IP demonstrated a natural interaction between SERPIAN3K and RORs (α and β). Taken together, these results demonstrated that SERPINA3K is involved in the Clock gene-mediated male fertility by regulating acrosin activity and provide the first evidence that SERPINA3K could be regulated by Clock gene via retinoic acid-related orphan receptor response elements.
Keywords: Clock gene, male reproduction, acrosin activity, serine protease inhibitor, SERPINA3K
Introduction
Circadian system is widely existed in all living organisms, synchronizing their metabolism and behavior to environmental conditions.1 In mammals, the circadian network is generated and maintained via an approximately 24 h cycle of transcriptional–translational feedback loops of clock and clock-controlled genes2,3 through clock elements such as E-box, D-box, and retinoic acid-related orphan receptor response elements (RORE).4–6 Both E-box and RORE together contribute toward defining the Cry1 circadian expression pattern.7
The core circadian gene Clock regulates its targets by forming heterodimers with BMAL1 and binding to E-box elements in the promoters of target genes,8,9 activating their transcription.8–12 The nuclear receptors Rev-erbα and retinoid-related orphan receptor (ROR) are crucial components of the molecular circadian clock,13,14 which function oppositely by competitively binds to RORE,15 controlling circadian oscillation of clock genes.4 Rev-erbα, a negative component of the circadian network, not only has the capability to reset the peripheral tissues molecular oscillators16 but also participates in the entrainment of central clocks, functioning as a synchronizing hinge of the clock gene machinery.17 Retinoid-related orphan receptor α (RORα) was revealed to play an important role in the maintaining circadian clock function.18
In mammals, circadian clock genes are expressed in many organs19 and perform other functions in additional of circadian function. Circadian clock genes are expressed during spermatogenesis in a stage-specific and circadian-independent manner, suggesting that these clock proteins have non-circadian functions in the spermatogenesis.20 A recent genomic DNA SNP selection and genotyping provides evidence that genetic variability in the CLOCK gene might be associated with male infertility.21 Our previous study also found that in mice, the male fertility was reduced when the CLOCK expression was knocked down in their testes, revealed by the smaller litter size, lower in vitro fertility rate, lower blastula formation rate, and lower acrosin activity of the knockdown male mice, and down-regulated acrosin activity in the sperm might be responsible.22 However, the regulatory pathway of circadian clock genes in male fertility is still unknown.
In this study, we screened the differential expression proteins of the sperm in testis CLOCK knockdowned mice and determined the effect of CLOCK down-regulation on them (these proteins) in MS1 cells with the small interfering RNA (siRNA) strategy, as well as investigated the underlying molecular mechanisms for Clock gene to regulate acrosin activity.
Materials and methods
Plasmids and siRNA
Clock small hairpin RNA expression plasmid (pClock.shRNA) and its control (pCtrl.shRNA) were constructed by Genesil Biotechnology Co., Ltd. (Wuhan, China). The entire DNA sequence inserted in a hU6 promoter-driven pGensil-1 plasmid to construct pClock.shRNA was 5′-GGA TCC GCT TCA GAC TCA TTA TTA TTT CAA GAC GAT AAT AAT GAG TCT GAA GCT TTT TTG TCG ACA AGC TT-3′ including the restriction enzyme cutting sites of BamHI and HindIII, and its expression was driven by hU6 promoter. The luciferase reporter plasmid, pGL3-Basic, was purchased from Promega Co. (Madison, USA). The promoter region, a 2000-bp fragment upstream of the transcription start site, of Mus musculus Serpina3k (Gene ID: 20714) was amplified by PCR, digested with KpnI and XhoI, and inserted into the KpnI and XhoI sites of pGL3-basic, generating pGL3-S3k-pro. The primers were 5′-GCG GTA CCT GAA CAA GGT GGT TAT AGT GA-3′ and 5′-ATC TCG AGT CCC CAG TGA ACA GTG ATG CC-3′. The pET41 vector for recombinant SERPINA3K protein expression and purification was purchased from Novagen Co. (Madison, USA). A reverse transcription reaction was done with total RNA extracted from male mouse liver. The entire coding sequence of Mus musculus Serpina3k (Gene ID: 20714) was amplified by PCR, which was directly digested and inserted between the restriction enzyme cutting sites of BamHI and SalI, named Serpina3k/pET41. The primers were 5′-GCG GAT CCA TGG CCT TCA TTG TAG CTA TG-3′ and 5′-GAG TCG ACC TTG GGG TTA TTG ACT TTG GC-3′. All these inserted sequences were verified by sequencing.
Clock small interfering RNA (Clock.siRNA) and its control (Ctrl.siRNA) were synthesized from Integrated DNA Technologies, Inc (Iowa, USA). The duplex sequences of Clock.siRNA were 5′-GCU ACA CCU CAG UUC AUC AUU-3′ and 5′-UGA UGA ACU GAG GUG UAG CUA-3′, the Ctrl.siRNA sequences were 5′-UAG CGA CUA AAC ACA UCA A-3′ and 5′-UUG AUG UGU UUA GUC GCU A-3′.
Site-directed mutagenesis of putative E-Box sites
Mutants of putative E-Boxes in the pGL3-s3k-pro reporter plasmid were generated using Site-directed Gene Mutagenesis Kit (Beyotime Institute of Biotechnology, China) and Dpn I restriction enzyme (Fermentas, Canada). Briefly, 30 ng of pGL3-s3k-pro plasmid DNA was used as template for mutant strand synthesis in a total volume of 100 μl for each mutagenesis. The primer sequences for the mutagenesis were as follows: MuE1 (5′-CAT TGT CTT TGA AAT GTT CAA CAA ACA AAA TAC-3′ and 5′-GTA TTT TGT TTG TTG AAC ATT TCA AAG ACA ATG-3′), MuE2 (5′-CTC TGT GAT AAC TCT GGC CAT GTC AAG TTG ACA C-3′ and 5′-GTG TCA ACT TGA CAT GGC CAG AGT TAT CAC AGA G-3′), MuE3 (5′-TTG AGG CAT TTC CTT GAC CAA AGC TCC TTT CTC-3′ and 5′-GAG AAA GGA GCT TTG GTC AAG GAA ATG CCT CAA-3′), MuE4 (5′-GGT GTT TTG TTT TTC TGT ACA AAG TTG AGA AAT GC-3′ and 5′-GCA TTT CTC AAC TTT GTA CAG AAA AAC AAA ACA CC-3′), MuE5 (5′-GCT CTA ACA CTG TAT TGG ACA GAG GCT GAT CCA G-3′ and 5′-CTG GAT CAG CCT CTG TCC AAT ACA GTG TTA GAG C-3′). The italicized region in each primer represents the sequence to which the E box was mutated. When the PCR reaction was finished and cooled to room temperature, 1 μL of DpnI was added and incubated at 37℃ for 3 h to cut the parental plasmid DNA to pieces. Then, 10 μL of this reaction product was transformed into competent Escherichia coli DH5α. These mutations were confirmed by sequencing.
Animal husbandry
All animals used in this study were sexually matured mice (8–10 weeks in male mice, 6–8 weeks in females) of the ICR strain. All animal procedures were approved by the Ethical Committee of Investigation of Laboratory Animals of Sichuan University. Mice were kept in cages under a 12 h light:12 h dark lighting schedule, with food and water available ad libitum.
Plasmid transfection and sperm preparation
All sperm were prepared from 20 sexually matured male mice. The testes of half number of these mice were injected with the pClock.shRNA plasmid using an in vivo-jetPET™ (Polyplus, France) according to the manufacturer’s instructions, and the other half with the pCtrl.shRNA control plasmid. Briefly, mice were anesthetized with diethyl ether and both testes were injected with 20 μL of the plasmid-liposome complex using a microinjector (32 Ga). Eighteen days after siRNA plasmids injection, the male mice were anesthetized with an intraperitoneal injection of 40 mg/kg pentobarbital. Both caudae epididymides were surgically removed and punctured with a sharply pointed forceps. Dense masses of spermatozoa squeezed from the epididymis were placed in the bottom of a 1.5-mL Eppendorf tube containing 0.5 mL protection solution (10 mmol/L Tri-HCl, 50 mmol/L EDTA, 50 mmol/L NaCl) which was preheated at 37℃ before use. The tube was incubated for 10 min at 37℃ to allow spermatozoa to disperse. The upper 300–400 μL of the sperm suspension in the tube was collected and transferred to a 1.5-mL Eppendorf tube for freeze-drying. The Eppendorf tubes with sperm suspension were plunged into liquid nitrogen for 20 s and then connected to Edwards Super Modulyo Freeze Dryer (Edwards High Vacuum, Part of BOC Ltd, UK). When lyophilization completed, each Eppendorf tube was sealed with parafilm file.
Sperm proteomics
The lyophilized sperm samples were immediately shipped to Applied Biomics, Inc. (Hayward, CA) for 2D-DIGE and MALDI-MS/MS analysis. The 2D/MASS results were analyzed using the DAVID Functional Annotation Bioinformatics Microarray Analysis method (http://david.abcc.ncifcrf.gov/home.jsp).23,24
Cell culture and transfection
Mile sven 1 (MS1) cells (Mus musculus endothelial cell line) were obtained from ATCC (Manassas, VA) and cultured at 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, USA) supplemented with 10% fetal calf serum (Hyclone) and 100 IU/mL penicillin/streptomycin (BioWhittaker, USA) at 37℃. These cells were plated in six-well plates and were transfected with the siRNA, pGL3-s3k-pro plasmid and its mutants. Lipofectamine™ 2000 transfection reagent (Invitrogen, CA) was used for all in vitro cell transfection according to the manufacturer’s instructions.
RNA extraction and qRT-PCR
Total RNA of MS1 cells was extracted with Trizol reagent (Invitrogen, USA) 48 h after siRNA transfection, followed by treatment of DNAse I (Sigma, USA). cDNA was synthesized by oligo (dT) priming with RevertAid™ First Strand cDNA Synthesis Kit according to the manufacturer’s instruction (Fermentas, Canada). Semi-quantitative reverse-transcription real-time PCR (qRT-PCR) was carried out using SYBR-Green Realtime PCR Master Mix with SYBR-Green I (BIO-RAD, USA) in a total volume of 20 μl. PCR amplifications were performed in a MiniOpticon Real-time PCR System (BIO-RAD, USA) in triplicate. The relative quantification of gene expression was analyzed from the measured threshold cycles (CT) by using the 2-ΔΔ(Ct) method. The data were normalized by determination of the amount of glyceraldehyde-3-phosphate dehydrogenase (gapdh) mRNA in each sample. Primers were designed by Primer Premier and all of them spanned an intron. The sequences were as follow: Clock (Gene ID: 12753): 5′-GAT GAC AAG GAC AAA GCA A-3′ and 5′-CGT TAC CAG GAA GCA TAG AC-3′; Serpina3k (Gene ID: 20714): 5′-GAC CCT GAG GAA ATG GAG-3′ and 5′-TAG CGA TGG AGA ACT TGG-3′; Serpina1c (Gene ID: 20702): 5′-TCC ATC TTG GCT CCC ACT AC-3′ and 5′-CAT TTT CAG CTT GCT GTC CC-3′; A2m (Gene ID: 232345): 5′-TCA GCA CCA CAG AAA CCA ATC T-3′ and 5′-CTT TGG TTC CCA CGG ACA GA-3′; Pebp1 (Gene ID: 42644): 5′-ATC CCA AAT TCA GGG AGT GGC-3′ and 5′-CCA GAC ATA GCG GTG GAG AC-3′; Pgk2 (Gene ID: 18663): 5′-GGA GGA AGA AGG TAA GGG TAA AG-3′ and 5′-AGA ACT GTG AGC CCG ATG TG-3′; Pkm (Gene ID: 18746): 5′-TTA CCA GCG ACC CCA CA-3′ and 5′-AGT CAC GGC AAT GAT AGG AG-3′; Pdhb (Gene ID: 68263): 5′-GCC ACA AGT TGG AGT AGG AGC-3’ and 5′-TGC GTA AGG CAT AGG GAC AT-3′; and gapdh (Gene ID: 14433): 5′-GGT GCT GAG TAT GTC GTG GAG T-3′ and 5′-GCG GAG ATG ATG ACC CTT TT-3′.
Preparation of protein lysates and Western blotting
MS1 cells were harvested 72 h after transfection with siRNA. Sperm samples were obtained 18 days after transfection with plasmid. Proteins were extracted with RIPA Lysis Buffer (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. Protein concentration was determined by Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instruction. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, followed by blotting onto membranes. Blots were probed with an anti-SERPINA3K polyclonal antibody (sc-271603; Santa Cruz, USA) and an HRP-labeled rabbit anti-goat IgG antibody (ZB-2306; ZSGB-BIO, China) for detecting SERPINA3K, the dilution of anti-SERPINA3K is 1:200. β-actin was used as a loading control; it was detected using an anti-β-actin monoclonal antibody (TA-09; ZSGB-BIO, China) and an HRP-labeled goat anti-mouse IgG antibody (ZB-5305; ZSGB-BIO, China). Proteins were visualized using an Enhanced Chemiluminescence detection system (BIO-RAD, USA). Protein expression on the Western blot was quantitated using Quantity One, a software package from BIO-RAD.
Recombinant SERPINA3K purification
The serpina3k/pET41 plasmid expressing SERPINA3K was introduced into E. coli strain ROSETTA-gami2. The expression and purification followed the protocol recommended by CWBIOTECH Company (Beijing, China). The recombinant SERPINA3K protein was confirmed by Western blot and dialyzed to remove imidazole, and its purity was estimated to be more than 90% by SDS-PAGE followed by Coomassie brilliant blue staining. The cytotoxicity of the purified protein (at 1, 2.5, and 5 mg/mL) was determined to be minimal using an MTT assay25 on SERPINA3K-treated MS1 Cells.
In vitro fertilization and sperm acrosin activity
Caudae epididymal sperm of untreated sexually mature mice were collected for in vitro fertilization (IVF) and acrosin activity test. Those sperm were first centrifuged in the TYH at 1000 rpm for 10 min for precipitation and incubated at 37℃ for 30 min. Then the sperm of good motility rose to the supernatant were removed for the following tests. Spermatozoa capacitation and IVF were performed in modified Krebs-Ringer bicarbonate solution (TYH medium) following methods described elsewhere.26,27 After capacitation, sperm were washed with TYH to remove the excessive SERPINAK, then grouped and respectively incubated with the recombinant SERPINA3K at a final concentration of 2.5 mg/mL for 30 min, phosphate-buffered Saline (PBS), bovine serum albumin (BSA; Sigma, USA), and heat-inactivated recombinant SERPINA3K protein (SERPINA3K*) were used as controls.
For IVF, female mice were given one dose of pregnant mare serum gonadotrophin (7.5 IU, PMSG; Ningbo Hormone Product Co., Ltd., Ningbo, China), followed 48 h later by human chorionic gonadotropin (hCG; 7.5 IU, Ningbo Hormone Product Co., Ltd., Ningbo, China) via intraperitoneal injection to induce superovulation. Oocytes were collected approximately 15 h after hCG injection. Only oocytes in metaphase II (M II) were selected for IVF culturing. Metaphase II oocytes are recognized by the presence of the extruded first polar body.28,29 When co-incubated capacitated sperm were added into the drops of M II oocyte, the time to disperse the cumulus around the oocytes and to form the male pronucleus in each oocyte was recorded. The number of oocytes and two-cell embryos was also recorded. The ratio of two-cell embryos to the total of two-cell embryos plus metaphase oocytes was recorded as the in vitro fertility rate. Ten IVF experiments were conducted, with one male of each treatment group included in each experiment.
The sperm acrosin activity was determined by a procedure described elsewhere,12 with modifications. Briefly for acrosin activity, prepared solutions for this assay: Solution A (11% Ficoll in 0.12 M NaC1, 0.025 M Hepes buffer at pH 7.4), Solution B (0.01% Triton X-100 in 55 mM Hepes, 55 mM NaCl at pH 8.0), Solution C (500 mM Benzamidine in water), Solution D (23 mM BAPNA in DMSO), and Solution E (one part Solution B and three parts Solution D). Sperm of both groups were adjusted to a concentration of 8 × 106 sperm/mL with PBS and 0.25 mL of each sperm suspension was placed into a 1.5-mL Eppendorf tube to which 0.5 mL of Solution A was added. After centrifugation at 1500 × g for 10 min, the supernatant was removed except for 100 μl remaining for the subsequent steps. To the control tube, 100 μl of Solution C was added. Then, 1.0 mL of Solution E was added to all tubes, mixed thoroughly by vortexing and incubated at 23℃ for 3 h; mixing the contents of the tubes once every hour. After incubation, 100 μl of Solution C was added to end the reaction. Samples were shaken and then centrifuged at 1500 × g for 10 min. Supernatant (0.2 mL) from both the experimental and control tubes was added to different wells of a 96-well plate and a similar amount of Solution E was added to another well, which served as the zero control. Absorbance was determined using a Microplate Reader at a wavelength of 410 nm. One international unit (IU) of acrosin activity was defined as the amount of enzyme that hydrolysed 1 mmol BAPNA/min at 23℃. Formula: Acrosin activity (μIU)/2 × 106 sperm = (ODtest − ODcontrol) × 106/1485 × 2.
Sequence analysis
The upstream sequence of Mus musculus serpina3k (Gene ID: 20714) was downloaded from the NCBI Gene database. This gene sequence spanning from 2 kb upstream to the transcription start site was examined in the NCBI BLAST database for the follow consensus sequences of the binding elements allowing for a 1-base mismatch (N stands for any nucleatide):
E-Box: CAN NTG
RORE: [A/T]A[A/T] NT[A/G] GGT CA
Luciferase reporter gene assay
The firefly luciferase activity was assessed using a luciferase assay system (Promega, Madison. WI). Briefly, 48 h after transfection with pGL3-s3k-pro plasmid (or its mutant) and siRNA, cells were washed twice with Mg2+- and Ca2+-free PBS buffer (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4 in distilled water at pH 7.4). Four volumes of Mg2+- and Ca2+-free PBS buffer was added to 1 volume of Reporter Lysis 5 × Buffer before use. One hundred microliters of this diluted Reporter Lysis Buffer were added to each well of the six-well plate and incubated for 15 min. The cell lysate was transferred to a microcentrifuge tube, and a single freeze-thaw cycle was performed to ensure complete lysis. Then, lysates were centrifuged at 13,800 × g for 2 min at 4℃. The supernatant was carefully transferred to a fresh tube. Protein concentration was determined by Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instruction, and protein concentrations of the samples were adjusted to a similar level with 1x Reporter Lysis Buffer. Twenty microliters of the supernatant of each sample were dispensed into a 96-well plate, and 100 μL of Luciferase Assay Reagent was added to each well. A Fluoroskan Ascent FL Microplate Fluorometer and Luminometer (Thermo Scientific, USA) was used to determine the luciferase activity. A 10-s shaking step was programmed to thoroughly mix the samples before initiation of reading. To normalize the transfection efficiency variations, pSV-β-Galactosidase Control Vector was included in all transfections and β-galactosidase activity was measured by β-galactosidase Assay Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instruction. Luciferase activity was expressed as the relative light unit (rlu), which was normalized by dividing by β-galactosidase activity.
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) was applied to quantify the protein–protein interaction between SERPINA3K and RORs (RORα, RORβ, and RORγ) in normally cultured MS1 cells. Western blot analysis used the whole cellular protein was conducted as control. Briefly, 700 μg of cellular proteins were labeled using anti-SERPINA3K polyclonal antibody (sc-271603; Santa Cruz, USA) following overnight incubation with gently rotation at 4℃. The protein-antibody immunoprecipitates were collected by protein A/G PLUS-Agarose, (SC-2003, Santa Cruz, USA). After five times of washing with the RIPA Lysis Buffer (Beyotime Institute of Biotechnology, China), the samples were boiled and centrifuged to pellet the agarose beads. Western blot analysis of the RORs protein in the supernatant was then conducted, using anti-RORα polyclonal antibody (sc-6062; Santa Cruz, USA), anti-RORβ polyclonal antibody (sc-21354; Santa Cruz, USA), and anti-RORγ polyclonal antibody (sc-28559; Santa Cruz, USA). Proteins were visualized using an Enhanced Chemiluminescence detection system (BIO-RAD, USA). Protein expression on the Western blot was quantitated using Quantity One, a software package from BIO-RAD.
Statistical analysis
IVF rates of two groups were compared by Chi-square test. Other experimental data are presented as mean ± SEM of at least three independent experiments. The statistical significance of differences between experimental groups was determined by Student’s t-test (SPSS 11.0, SPSS Inc). Probability values (P) of less than 0.05 were considered statistically significant.
Results
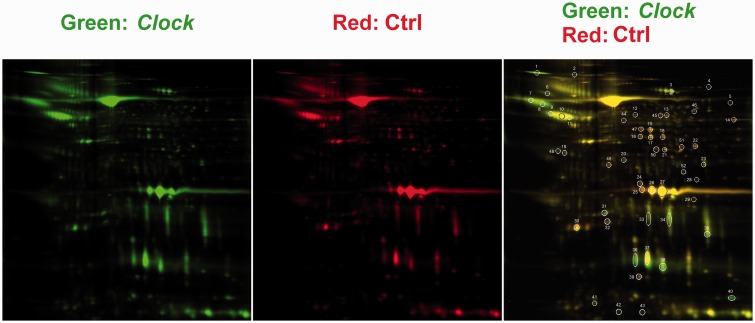
To understand how Clock gene in round spermatids regulates the acrosin activity of mature sperm in male mice, a proteomics analysis of sperm was performed to search for the differentially expressed proteins when Clock expression was knocked down during round spermatid period. First, the pClock.shRNA expression plasmid was introduced into the testes of sexually mature mice as we previously described,22 and pCtrl.shRNA expression plasmid was injected as a control. Then, profiled protein expression patterns in these two groups of sperm were screened by 2D/MASS proteomics approach. DIA software was used to detect and quantified protein spots. There were a total of 52 protein spots with a threshold value of 1.4-fold between these two types of sperm (Figure 1). These protein spots were selected and subjected to MALDI-MS/MS for identification. Fifty-one of 52 spots showed high confidence in the protein identification, which are listed in Table 1.
Figure 1.
Proteomic analysis of sperm by 2D-DIGE and MALDI-MS/MS. Total sperm extracts were assayed. pClockshRNA sperm samples (10 mice per group, sperm of each group were mixed together) were labeled with Cy3 (green), and the pCtrl sperm samples were labeled with Cy5 (red). The spots circled and numbered represent 52 differentially expressed protein spots, 51 of which were identified by MALDI-MS/MS. (A color version of this figure is available in the online journal)
Table 1.
Fifty-one proteins showed high confidence in the protein identification
| Spot number | Accession no. | Protein name | Ctrl/Clock* |
|---|---|---|---|
| 1 | gi|18044689 | Serine (or cysteine) peptidase inhibitor, clade A, member 3K [Mus musculus] | -2.46 |
| 2 | gi|81886949 | RecName: Full = CUB and zona pellucida-like domain-containing protein 1; Short = CUB and ZP domain-cont | -1.79 |
| 3 | gi|20330802 | serotransferrin precursor [Mus musculus] | -1.48 |
| 4 | gi|18079339 | aconitate hydratase, mitochondrial precursor [Mus musculus] | 1.58 |
| 5 | gi|148706296 | complement component 3, isoform CRA_c [Mus musculus] | -2.34 |
| 6 | gi|134034200 | RecName: Full = Liver carboxylesterase N; AltName: Full = Lung surfactant convertase; AltName: Full = PES | -1.47 |
| 7 | gi|16741103 | Serine (or cysteine) peptidase inhibitor, clade A, member 3K [Mus musculus] | -1.78 |
| 8 | gi|16741103 | Serine (or cysteine) peptidase inhibitor, clade A, member 3K [Mus musculus] | -2.26 |
| 9 | gi|14602605 | Serine (or cysteine) peptidase inhibitor, clade A, member 1C [Mus musculus] | -1.62 |
| 10 | gi|14602605 | Serine (or cysteine) peptidase inhibitor, clade A, member 1C [Mus musculus] | -1.68 |
| 11 | gi|6678083 | alpha-1-antitrypsin 1-3 [Mus musculus] | -1.95 |
| 12 | gi|33859809 | fibrinogen beta chain precursor [Mus musculus] | -1.47 |
| 13 | gi|148704970 | dihydrolipoamide dehydrogenase [Mus musculus] | 1.65 |
| 14 | gi|148677501 | ATP synthase, H + transporting, mitochondrial F1 complex, alpha subunit, isoform 1, isoform CRA_e [Mus musculus] | 1.61 |
| 15 | gi|309265176 | PREDICTED: alpha-enolase-like isoform 7 [Mus musculus] | 1.67 |
| 16 | gi|226246531 | phosphoglycerate kinase 2 [Mus musculus] | 1.60 |
| 17 | gi|226246531 | phosphoglycerate kinase 2 [Mus musculus] | 1.42 |
| 18 | gi|161172175 | Chain A, Crystal Structure Of Phosphoglycerate Kinase-2 | 1.57 |
| 19 | gi|8850219 | haptoglobin precursor [Mus musculus] | -1.66 |
| 20 | gi|134035924 | RecName: Full = Alpha-2-macroglobulin; Short = Alpha-2-M; AltName: Full = Pregnancy zone protein; Contain | -1.43 |
| 21 | gi|22128627 | sorbitol dehydrogenase [Mus musculus] | 1.56 |
| 22 | gi|293597567 | fructose-bisphosphate aldolase A isoform 1 [Mus musculus] | 1.52 |
| 23 | gi|6996913 | annexin A2 [Mus musculus] | -1.56 |
| 24 | gi|33563282 | proteasome subunit alpha type-1 [Mus musculus] | 1.86 |
| 25 | gi|157951659 | cysteine-rich secretory protein 1 precursor [Mus musculus] | 1.78 |
| 26 | gi|157951659 | cysteine-rich secretory protein 1 precursor [Mus musculus] | 1.63 |
| 27 | gi|15029854 | Cysteine-rich secretory protein 1 [Mus musculus] | 1.57 |
| 28 | gi|6755965 | voltage-dependent anion-selective channel protein 2 [Mus musculus] | -3.95 |
| 29 | gi|31982861 | carbonic anhydrase 3 [Mus musculus] | -1.63 |
| 30 | gi|84794552 | phosphatidylethanolamine-binding protein 1 [Mus musculus] | 1.44 |
| 31 | gi|148693731 | apolipoprotein A-I, isoform CRA_b [Mus musculus] | -1.51 |
| 32 | gi|311771688 | adenylate kinase isoenzyme 1 isoform 2 [Mus | 1.49 |
| 33 | gi|2317286 | prostaglandin D synthetase [Mus musculus] | -1.67 |
| 34 | gi|2317286 | prostaglandin D synthetase [Mus musculus] | -1.83 |
| 35 | gi|2317286 | prostaglandin D synthetase [Mus musculus] | -1.95 |
| 36 | gi|3241966 | mE-RABP minor form protein [Mus musculus] | -2.14 |
| 37 | gi|4097810 | epididymal retinoic acid binding protein [Mus musculus] | -2.13 |
| 38 | gi|4097810 | epididymal retinoic acid binding protein [Mus musculus] | -2.42 |
| 39 | gi|306440452 | Chain A, Mouse Sod1 | 1.50 |
| 40 | gi|201102 | seminal vesicle secretory protein IV (SVS IV) precursor [Mus musculus] | -7.07 |
| 41 | gi|145046248 | seminal vesicle secretory protein 6 precursor [Mus musculus] | -1.73 |
| 42 | gi|6094379 | RecName: Full = Seminal vesicle secretory protein 6; AltName: Full = SVSP99; AltName: Full = Seminal vesi | -4.13 |
| 43 | gi|22164798 | selenium-binding protein 1 [Mus musculus] | -3.22 |
| 44 | gi|148704970 | dihydrolipoamide dehydrogenase [Mus musculus] | 1.65 |
| 45 | gi|31981562 | pyruvate kinase isozymes M1/M2 [Mus musculus] | 1.79 |
| 46 | gi|309265176 | PREDICTED: alpha-enolase-like isoform 7 [Mus musculus] | 1.49 |
| 47 | gi|8850219 | haptoglobin precursor [Mus musculus] | 1.77 |
| 48 | gi|12805431 | Pdhb protein [Mus musculus] | -1.86 |
| 49 | gi|22128627 | sorbitol dehydrogenase [Mus musculus] | 1.67 |
| 50 | gi|148704778 | mCG22349 [Mus musculus] | 1.53 |
| 51 | gi|229092064 | carcinoembryonic antigen-related cell adhesion molecule 10 precursor [Mus musculus] | 1.60 |
Protein ratio of densitometry data. “-” represents that the densitometry value of Ctrl is lower than the Clock group.
We then performed cluster analysis using a public bioinformatics tool available from the NIH called the Database for Annotation, Visualization and Integrated Discovery (DAVID). At the exact P value < 0.05 as cutoff, these proteins were grouped into two clusters under the highest stringency, serine protease inhibitor (P < 0.001, Bonferroni = 0.034) and glycolysis (P < 0.001, Bonferroni > 0.05) (Table 2). Although only serine protease inhibitor survived the Bonferroni test, we decided to investigate the members of these two clusters with the accession numbers of 18044689, 14602605, 84794552, 16741103, 6678083, 134035924, 12805431, 31981562, and 226246531. The corresponding genes of these proteins are serine (or cysteine) peptidase inhibitor, clade A, member 3K (Serpina3k), Serine (or cysteine) peptidase inhibitor, clade A, member 1C (Serpina1c), alpha-1-antitrypsin 1-3 (Serpina1c), Alpha-2-macroglobulin (A2m), phosphatidylethanolamine-binding protein 1 (Pebp1), phosphoglycerate kinase 2 (Pgk2), pyruvate kinase isozymes M1/M2 (Pkm), and Pdhb protein (Pdhb).
Table 2.
Functional annotation clustering of putative CLOCK targets using DAVID tools
| Category | Term | Count | P | Bonferroni | ||
|---|---|---|---|---|---|---|
| Functional Group 1 | Enrichment Score: 3.248190161694644 | |||||
| SP_PIR_KEYWORDS | Serine protease inhibitor | 4 | 2.82 × 10−4 | 0.03350025 | ||
| SP_PIR_KEYWORDS | protease inhibitor | 4 | 6.49 × 10−4 | 0.07552948 | ||
| GOTERM_MF_FAT | GO:0004867∼serine-type endopeptidase inhibitor activity | 4 | 9.86 × 10−4 | 0.09836285 | ||
| Functional Group 2 | Enrichment Score: 2.2043643678201708 | |||||
| SP_PIR_KEYWORDS | glycolysis | 3 | 0.00196 | 0.21173885 | ||
| GOTERM_BP_FAT | GO:0006096∼glycolysis | 3 | 0.00227 | 0.52140424 | ||
| GOTERM_BP_FAT | GO:0006007∼glucose catabolic process | 3 | 0.00316 | 0.64131271 | ||
| GOTERM_BP_FAT | GO:0019320∼hexose catabolic process | 3 | 0.00316 | 0.64131271 | ||
| GOTERM_BP_FAT | GO:0046365∼monosaccharide catabolic process | 3 | 0.0034 | 0.66861194 | ||
| GOTERM_BP_FAT | GO:0044275∼cellular carbohydrate catabolic process | 3 | 0.00418 | 0.742992 | ||
| GOTERM_BP_FAT | GO:0046164∼alcohol catabolic process | 3 | 0.00489 | 0.79594832 | ||
| GOTERM_BP_FAT | GO:0016052∼carbohydrate catabolic process | 3 | 0.0075 | 0.91288838 | ||
| GOTERM_BP_FAT | GO:0006006∼glucose metabolic process | 3 | 0.02128 | 0.99905861 | ||
| GOTERM_BP_FAT | GO:0019318∼hexose metabolic process | 3 | 0.03018 | 0.99995127 | ||
| GOTERM_BP_FAT | GO:0005996∼monosaccharide metabolic process | 3 | 0.03776 | 0.99999617 | ||
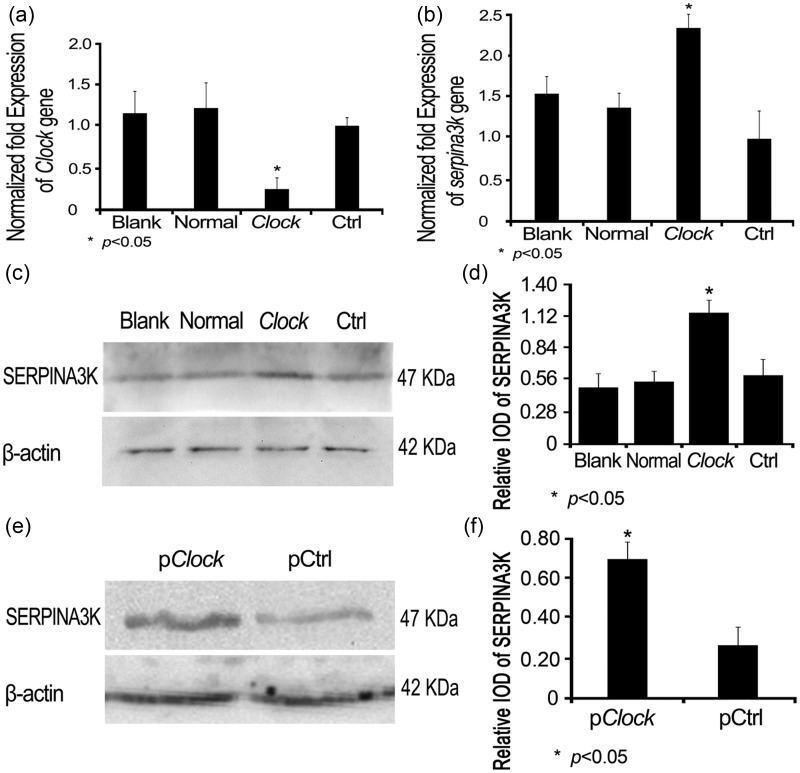
Considering that Clock might probably regulate those candidates before the maturation of spermatozoa, we believed that the sperm we obtained from caudae epididymal is not suitable for what we explore. So we continued our study with a cell line instead. To better verify the relationship between CLOCK knockdown and the mRNA expression of those differentially expressed proteins, we altered the Clock knock down strategy with small interfering RNA (Clock.siRNA). MS1 cells were first transfected with this Clock.siRNA to verify the knockdown efficiency. A comparison of the cells treated with transfection reagent only (Blank control), untransfected cells (Normal control), or the cells transfected with a control siRNA (Ctrl.siRNA) showed that Clock.siRNA transfection resulted in a significant decrease of Clock expression (Figure 2(A)). Next, the Clock.siRNA was introduced into MS1 cells, along with other controls as described above, and mRNA levels of the differentially expressed proteins were determined. Real-time PCR suggested that CLOCK knockdown was only associated with the up-regulation of SERPINA3K mRNA (P < 0.05, Figure 2(B)) among the seven genes (data not shown). Western blotting analysis was performed to confirm the effect of CLOCK knockdown on the SERPINA3K (p < 0.05, Figure 2(C) and (D)). The level of SERPINA3K was verified to increase in Clock-knockdown sperm by Western blot analysis (P < 0.05, Figure 2(E) and (F)).
Figure 2.
The effect of Clock knockdown on Clock and Serpina3k expressions. MS1 cells were grouped and first transfected with Clock.siRNA to verify the efficiency of knockdown. The cells treated with transfection reagent only (Blank control), untransfected cells (Normal control), and the cells transfected with a control siRNA (Ctrl.siRNA) were set as controls. MS1 cells were harvested 48 h post transfection for Clock (A) and Serpina3k (B) gene expression by real-time PCR, while other MS1 wells were harvested 72 h post transfection for SERPINA3K protein expression by Western blot using an anti-SERPINA3K antibody (C). The SERPINA3K protein levels were calculated using β-actin as a reference (D). The matured male mice were grouped and their testes were respectively transfected with pClockshRNA plasmid and the pCtrl plasmid. Total sperm samples were obtained 18 days post transfection for SERPINA3K protein expression by Western blot using an anti-SERPINA3K antibody (E). The SERPINA3K protein levels were calculated using β-actin as a reference (F). β-actin was used as a control for Western blotting, while Gapdh was used as a control for qRT-PCR
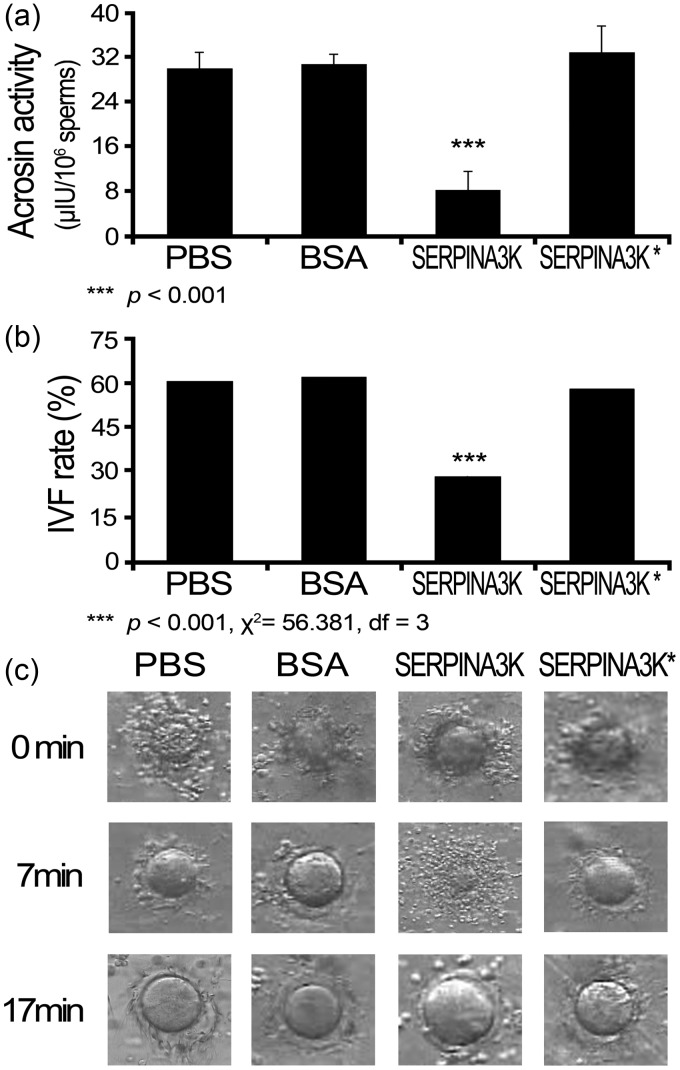
To determine the possibility that SERPINA3K up-regulation is responsible for the reduced acrosin activity and lower IVF rate in CLOCK knockdown sperm, recombinant SERPINA3K protein was expressed and purified. The acrosin activity of normal sperm was assayed in the presence of the purified recombinant SERPINA3K protein. Co-incubation of normal sperm with the recombinant SERPINA3K protein led to an enhanced acrosin activity of 8.01 ± 3.50 μIU/106 sperm (Figure 3(A)), which was significantly lower than the PBS and BSA controls (P < 0.001). Importantly, this inhibition was almost completely reversed by inactivation of this protein (SERPINA3K* in Figure 3(A)). Similarly, the IVF rate was determined in the presence of the recombinant SERPINA3K protein. As assessed by the percentage of ova in Metaphase II that progressed to the two-cell stage, the IVF rate was 28.4% (50/176) in the SERPINA3K protein-treated group (Figure 3(B)), which was significantly lower than the PBS, BSA, and inactivated SERPINA3K protein control groups (χ2 = 56.381, df = 3, P < 0.001), while the IVF rates of the three controls did not have significant differences (χ2 = 0.518, df = 2, P = 0.772). Of note that there was a delay of about 10 min in dispersing cumulus cells in this group (Figure 3(C)), which was similar as we observed in the sperm of pClock.shRNA mice.22 Again, the heat-inactivated recombinant SERPINA3K completely reversed the inhibitory effect. Taken together, these results demonstrated that exogenous supplement of SERPINA3K led to a lower IVF rate and reduced acrosin activity of the sperm.
Figure 3.
The effect of recombinant SERPINA3K on acrosin activity and in vitro fertilization. Sperm were collected from the sexually mature normal male mice, and 1 mL of 108 sperm was incubated at 37℃ with various treatments, and then the acrosin activity an IVF rate of the sperm were determined. The treatments were recombinant His-tagged SERPINA3K protein (2.5 mg/mL), and as controls, an equal amount of the vehicle (PBS), BSA, and inactivated recombinant His-tagged SERPINA3K protein that was heated at 100℃ for 20 min (SERPINA3K*). (A) The acrosin activity data are mean ± SEM of at least three independent experiments. (B) The capitalized normal sperm was co-incubated with recombinant SERPINA3K for 30 min before they were added to the oocyte. (C) The moment when the sperm was added to oocyte was recorded as 0 min, the duration for sperm dispersing cumulus cells was recorded. PBS: phosphate-buffered saline; BSA: bovine serum albumin; IVF: in vitro fertilization
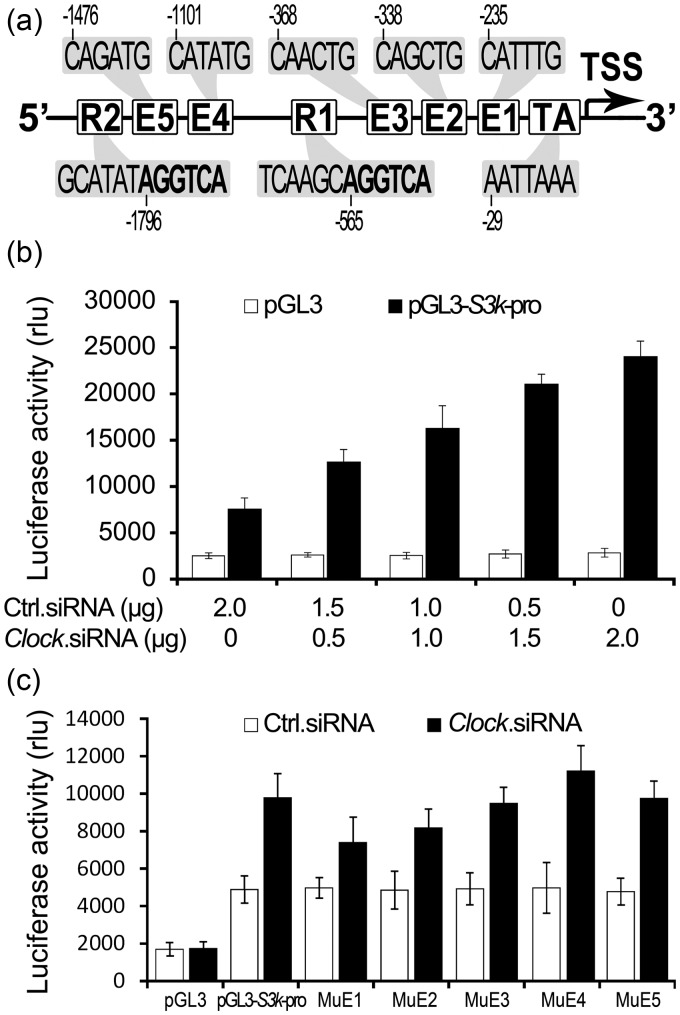
CLOCK contributes to the direct regulation of many genes through forming complexes with BMAL1 and subsequent binding of the heterodimers to E-Box elements in the promoter region of responsive genes.10,30,31 The transcription of orphan receptor Rev-erbα is activated by CLOCK and BMAL1, while REV-ERBα acts as a repressor in participation of the regulation of circadian Bmal1 and Clock expressions through RORE in the promoter region of Bmal1.32 Thus, we analyzed the promoter region of Serpina3k for the presence of E-Boxes and ROREs. Five putative E-boxes (CAN NTG) and two putative ROREs ([A/T]A[A/T] NT[A/G] GGT CA) upstream of the TATA box of the Serpina3k gene were identified (Figure 4(A)). However, in additional to typical CLOCK:BMAL1-binding sequence CAC GTG, three noncanonical sequences were identified.4,33–35 The E-boxes in the Serpina3k promoter are all divergent from the established “circadian E-box” sequences (Figure 4(A)) (CAG ATG: -1476 to -1471; CAT ATG: -1101 to -1096; CAA CTG: -368 to -363; CAG CTG: -338 to -333; CAT TTG: -235 to -230). Compared to the natural response elements,36 the recognized ROREs also had some diversities in the sequence prior to the core sequence of [A/G]GG TCA (Figure 4(A)) (GCA TAT AGG TCA: -1802 to -1791; TCA AGC AGG TCA: -571 to -560).
Figure 4.
The effect of CLOCK knockdown on the SERPINA3K promoter-driven luciferase reporter gene expression. (A) Five putative noncanonical E-boxes and ROREs were identified within the promoter region—2000 bp upstream of the transcription start site (TTS) of the Serpina3k gene. (B) MS1 cells were transfected with a Serpina3k promoter-driven luciferase reporter gene pGL3-s3k-pro and increasing amounts of Clock.siRNA. Cells were harvested and lysed for luciferase activity assay 48 h post transfection. Cloning backbone vectors pGL3 and Ctrl.siRNA were included as controls for pGL3-s3k-pro and Clock.siRNA, respectively. pSV-β-Galactosidase control vector was included to normalize the transfection efficiencies among the transfections. (C) MS1 cells were co-transfected with one of the five E-box mutant pGL3-s3k-pro plasmids (MuE1, MuE2, MuE3, MuE4, and MuE5) and 0.5 µg of pClock.shRNA. Cells were harvested and lysed for the luciferase activity assay 48 h post transfection. Cloning backbone vectors pGL3, pGL3-s3k-pro, and Ctrl.siRNA were included as controls. pSV-β-Galactosidase control vector was included to normalize the transfection efficiencies among the transfections
To address the possibility that CLOCK regulates SERPINA3K expression at the transcriptional level, the putative Serpina3k promoter was cloned from mouse genomic DNA into the promoter-less luciferase reporter pGL3-basic vector to obtain a Serpina3k promoter-driven luciferase reporter gene, named pGL3-S3k-pro. Co-transfection of pGL3-S3k-pro with Clock.siRNA into MS1 cells led to a dose-dependent activation of Serpina3k promoter upon CLOCK knockdown (Figure 4(B)). To verify whether CLOCK could interact with one of these five putative E-boxes, the core sequence of each E-boxes was mutated in the pGL3-S3k-pro plasmid, and the luciferase activity was determined after co-transfection of the mutant plasmid and 0.5 μg of Clock.siRNA plasmid. A decrease of luciferase activity was found in cells transfected with MuE1 or MuE2 E-box mutant plasmid, but with no statistical significance (P > 0.05, Figure 4(C)). Cells co-transfected with the mutant plasmid and 0.5 μg of Ctrl.siRNA demonstrated that the basal promoter activity was not affected by the mutation.
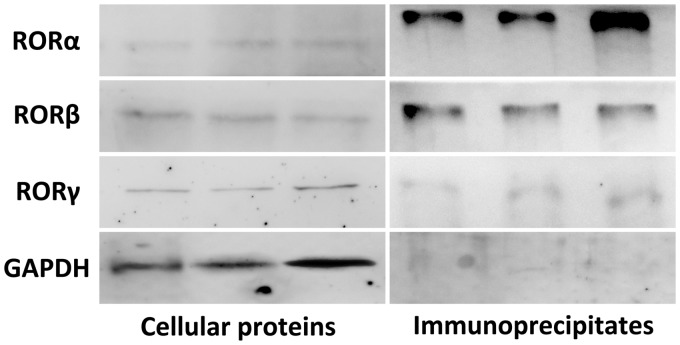
To test whether SERPINA3K protein interacts with RORs, normal lysed MS1 cells proteins were used for Co-IP. Western blot analysis on the whole cell lysates presented an expression of RORα, RORβ, and RORγ in normal MS1 cells (left panel, Figure 5). The anti-SERPINA3K immunoprecipitates demonstrated that RORα and RORβ could physiologically interact with SERPINA3K in intact cells but rarely RORγ (right panel, Figure 5).
Figure 5.
The interaction between SERPINA3K and RORs. Proteins were extracted from normally cultured MS1 cells. Western blot analysis used the whole cellular protein was conducted to verify the expression of RORs (left panel). Anti-SERPINA3K polyclonal antibody was added into the cellular proteins to produce protein-antibody immunoprecipitates, which were collected by protein A/G PLUS-Agarose. Western blot was applied using this protein-anti-SERPINA3K immunoprecipitates, and found apparent straps of RORα and RORβ, while the strap of RORγ was hardly to see (right panel)
Discussion
In our previous study, we used the small hairpin RNA strategy to knock down CLOCK expression in mouse testes and found a significant decrease in the offspring produced by these male mice, which would possibly due to the decreased acrosin activity in the CLOCK knockdown sperm.22 To identify the possible Clock gene targets and its regulatory pathway for acrosin activity in the testes, a proteomics approach was performed to define the alterations in protein expression resulting from CLOCK knockdown. The proteomics analysis revealed 51 protein spots with significantly different expression in sperm of CLOCK knockdown mice (Figure 1 and Table 1). Cluster analysis narrowed the list to the serine protease inhibitor cluster (P < 0.001, Bonferroni = 0.034) and the glycolysis cluster (P = 0.002, Bonferroni = 0.212) (Table 2). Real-time PCR detected the relationship between CLOCK knockdown and each of the eight proteins in the two clusters at transcriptional level in vitro, and finally focused on serine (or cysteine) peptidase inhibitor, clade A, member 3 K (Serpina3k). It appeared that CLOCK knockdown was responsible for the up-regulation of serine protease inhibitor SERPINA3K at the transcriptional and translational levels (Figure 2 to F). The notable role of SERPINA3K in controlling acrosin activity and the IVF rate was evaluated using the recombinant SERPINA3K protein (Figure 3). We verified that acrosin activity and IVF rate were reduced in SERPINA3K-treated sperm, and there was a decrease in dispersing cumulus cells, producing a pattern of phenotypes very similar to that previously observed in the CLOCK knockdown sperm.22
As we know, acrosin is a serine protease,37 and preproacrosin is suggested to be closely related to the serine proteinase subfamily containing trypsin and kallikrein according to the exon-intron structure38 while SERPINA3K is first identified as a specific inhibit of tissue kallikrein, and thus is also known as kallikrein-binding protein.35 SERPINA3K specifically binds with tissue kallikrein to form a covalent complex and inhibits its proteolytic activities. So far, there have been no studies indicating roles of SERPINA3K in the circadian system or in male reproduction. Nevertheless, serine protease inhibitor (SERPIN) is a group of proteins with similar structures that were first identified to inhibit proteases, many of which inhibit chymotrypsin-like serine proteases.39,40 They have been shown to play important roles in both male and female reproduction. Serine protease inhibitor protein C inhibitor (PCI) inhibits acrosin, and absence of PCI induces infertility, probably due to destruction of the Sertoli cell barrier or unopposed proteolytic activity41; serine protease inhibitor SPINKL could also inhibit sperm capacitation reaction.42 Therefore, we believe this structural relationship between SERPINA3K and acrosin might explain the previous findings that why the activity of acrosin was greatly decreased in the CLOCK knockdown sperm, but there was no corresponding decrease in the activity of hyaluronidase.22 Mice with sperm lacking the acrosin protease activity exhibit a delay in penetration of the zona pellucida, suggesting that acrosin plays a major role in acceleration of the dispersal of acrosomal proteins.43 The lower acrosin activity in SERPINA3K-treated sperm may also account for the 10-min delay of SERPINA3K-treated sperm in dispersing cumulus cells.
Considering the increased expression of SERPINA3K when Clock was knockdown, we expected to find a connection between Clock and Sperpina3k. Generally, the Clock gene regulates its targets through heterodimerizing with BMAL1 and binding to E-box elements in the promoters of target genes.8,9 Clock gene expression has been detected exclusively in round spermatids20; therefore, the regulation process must happen before the maturation of spermatozoa. Then, a cell line would be a better model to explore than the sperm we obtained from caudae epididymal. We indeed found five putative core sequences of E-box in the promoter region of Sperpina3k via sequence analysis (Figure 4(A)) and proved a Clock.siRNA-dose-dependent activation of Serpina3k promoter through Serpina3k promoter-driven reporter gene assay (Figure 4(B)). But to our surprise, the following mutation of those putative E-boxes could not break the activation triggered by CLOCK knockdown (Figure 4(C)). Combining this disappointing result, it indicates that CLOCK (possibly by forming CLOCK/BMAL1 heterodimer) inhibits Serpina3k expression, but its regulation of Serpina3k seems under some other mechanism rather than the classical transcriptional activation through E-Box.
The DNA sequence analysis also revealed two potential ROREs within the promoter region of Serpina3k (Figure 4(A)). ROREs are specific response elements of REV-ERBs and RORs13 with the consensus sequences of a 6-bp AT-rich sequence preceding a single core sequence of [A/G]GG TCA.36,44 The preceding sequences have many variants,36 the ROREs are usually recognized as [A/T]A[A/T] NT[A/G] GGT CA.45 Accordingly, both these “ROREs” in Serpina3k promoter have the canonical core sequence of AGG TCA followed variant preceding sequences. Therefore, we wondered whether RORs and REV-ERBs could really interact with Serpina3k in intact cells. We then used Co-IP to investigate the normal protein–protein interaction between SERPINA3K and RORs in cells and found the physiological combination of RORα-SERPINA3K and RORβ-SERPINA3K in normally cultured cells (right panel, Figure 5). As REV-ERBα and RORα competitively binds to RORE,46 we presume the natural combination of REV-ERBα and SERPINA3K is also existed in cells. As we know, Rev-erbα is a negative component of the circadian network.47 An example of this type of regulation occurs for Bmal1: the predominant regulation is inhibitory, mediated by Rev-erbα.4,18,48 Disruption of Clock expression leads to reduced Rev-erbα, and an increase in expression of Bmal1 due to loss of the inhibitory tone mediated by ROR elements in the BMAL1 promoter.49 It is possible that Rev-erbα is an inhibitor for Serpina3k expression, and its regulatory mechanism might be like in the regulation of Bmal1.
In summary, we have shown that CLOCK knockdown leads to an up-regulation of SERPINA3K in the sperm and co-incubation with recombinant SERPINA3K would reduce the acrosin activity of sperm and their IVF rate. In MS1 cells, we demonstrated a normal protein–protein interaction between SERPINA3K and RORs (RORα and RORβ), indicating a natural combination of REV-ERBα and SERPINA3K in intact cells. It is possible that Rev-erbα is an inhibitor of Serpina3k expression, and its regulatory mechanism might be just like in the regulation of Bmal1. We suppose it mechanistically that the decreased acrosin activity was directly linked to SERPINA3K up-regulation, which was indirectly due to the CLOCK knockdown through RORE.
Acknowledgements
The authors wish to acknowledge membership within and support from the National Nature Science Foundation of China (No. 41074131 to ZW), China Medical Board (No. 88-486 to ZW), and the Scientific Research Foundation for New Teachers of Sichuan University (No. 2013SCU11007). We thank David Weaver (UMass Medical School, Worcester, MA, USA) and Johnny He (UNT Health Science Center, Fort Worth, TX, USA) for comments and for editing the manuscript.
Author contributions
Conception and design: SC, XL, ZJ, YW; Analysis and interpretation of data: ZJ, YW, SC; Acquisition of data: SC, XL, WH, SL; Drafting the article: SC; Critical revision of the article: JZ, YL Final approval of the article: ZW; Statistical analysis: YL, SC; Obtained funding: ZW.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lim FL, Currie RA, Orphanides G, Moggs JG. Emerging evidence for the interrelationship of xenobiotic exposure and circadian rhythms: a review. Xenobiotica 2006; 36: 1140–51. [DOI] [PubMed] [Google Scholar]
- 2.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 2001; 63: 647–76. [DOI] [PubMed] [Google Scholar]
- 3.Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol 2013; 217: 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 2005; 37: 187–92. [DOI] [PubMed] [Google Scholar]
- 5.Roenneberg T, Merrow M. The network of time: understanding the molecular circadian system. Curr Biol 2003; 13: R198–207. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto T, Noshiro M, Sato F, Maemura K, Takeda N, Nagai R, Iwata T, Fujimoto K, Furukawa M, Miyazaki K, Honma S, Honma K, Kato Y. A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem Biophys Res Commun 2004; 313: 117–24. [DOI] [PubMed] [Google Scholar]
- 7.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 2003; 421: 177–82. [DOI] [PubMed] [Google Scholar]
- 8.Munoz E, Baler R. The circadian E-box: when perfect is not good enough. Chronobiol Int 2003; 20: 371–88. [DOI] [PubMed] [Google Scholar]
- 9.Kyriacou CP, Rosato E. Squaring up the E-box. J Biol Rhythms 2000; 15: 483–90. [DOI] [PubMed] [Google Scholar]
- 10.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998; 280: 1564–9. [DOI] [PubMed] [Google Scholar]
- 11.Oishi K, Shirai H, Ishida N. Identification of the circadian clock-regulated E-box element in the mouse plasminogen activator inhibitor-1 gene. J Thromb Haemost 2007; 5: 428–31. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy WP, Kaminski JM, Van der Ven HH, Jeyendran RS, Reid DS, Blackwell J, Bielfeld P, Zaneveld LJ. A simple, clinical assay to evaluate the acrosin activity of human spermatozoa. J Androl 1989; 10: 221–31. [DOI] [PubMed] [Google Scholar]
- 13.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 2005; 20: 391–403. [DOI] [PubMed] [Google Scholar]
- 14.Yin L, Joshi S, Wu N, Tong X, Lazar MA. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erb alpha. Proc Natl Acad Sci U S A 2010; 107: 11614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev 1999; 20: 689–725. [DOI] [PubMed] [Google Scholar]
- 16.Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, Collins J, Farrow S, Donn R, Ray D, Loudon A. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci 2008; 121: 3629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzoccoli G, Cai Y, Liu S, Francavilla M, Giuliani F, Piepoli A, Pazienza V, Vinciguerra M, Yamamoto T, Takumi T. REV-ERBalpha and the clock gene machinery in mouse peripheral tissues: a possible role as a synchronizing hinge. J Biol Regul Homeost Agents 2012; 26: 265–76. [PubMed] [Google Scholar]
- 18.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004; 43: 527–37. [DOI] [PubMed] [Google Scholar]
- 19.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 1997; 389: 512–6. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod 2003; 69: 81–91. [DOI] [PubMed] [Google Scholar]
- 21.Hodzic A, Ristanovic M, Zorn B, Tulic C, Maver A, Novakovic I, Peterlin B. Genetic variation in circadian rhythm genes CLOCK and ARNTL as risk factor for male infertility. PLoS One 2013; 8: e59220–e59220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, Cheng S, Jiang X, He X, Wang Y, Jiang Z, Hou W, Li S, Liu Y, Wang Z. The noncircadian function of the circadian Clock gene in the regulation of male fertility. J Biol Rhythms 2013; 28: 208–17. [DOI] [PubMed] [Google Scholar]
- 23.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sgouras D, Duncan R. Methods for the evaluation of biocompatibility of soluble synthetic polymers which have potential for biomedical use: 1 — use of the tetrazolium-based colorimetric assay (MTT) as a preliminary screen for evaluation ofin vitro cytotoxicity. J Mater Sci: Mater Med 1990; 1: 61–8. [Google Scholar]
- 26.Toshimori K, Saxena DK, Tanii I, Yoshinaga K. An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol Reprod 1998; 59: 22–9. [DOI] [PubMed] [Google Scholar]
- 27.Saxena DK, Tanii I, Yoshinaga K, Toshimori K. Role of intra-acrosomal antigenic molecules acrin 1 (MN7) and acrin 2 (MC41) in penetration of the zona pellucida in fertilization in mice. J Reprod Fertil 1999; 117: 17–25. [DOI] [PubMed] [Google Scholar]
- 28.Balakier H, Casper RF. A morphologic study of unfertilized oocytes and abnormal embryos in human in vitro fertilization. J In Vitro Fertil Embryo Transf 1991; 8: 73–9. [DOI] [PubMed] [Google Scholar]
- 29.Huang FJ, Chang SY, Tsai MY, Lin YC, Kung FT, Wu JF, Lu YJ. Relationship of the human cumulus-free oocyte maturational profile with in vitro outcome parameters after intracytoplasmic sperm injection. J Assist Reprod Genet 1999; 16: 483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 1999; 96: 57–68. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J 2005; 386: 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preitner N, Damiola F, Luis Lopez M, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERB ± controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002; 110: 251–60. [DOI] [PubMed] [Google Scholar]
- 33.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 2004; 101: 5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev 2000; 14: 679–89. [PMC free article] [PubMed] [Google Scholar]
- 35.Chao J, Tillman DM, Wang MY, Margolius HS, Chao L. Identification of a new tissue-kallikrein-binding protein. Biochem J 1986; 239: 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrader M, Danielsson C, Wiesenberg I, Carlberg C. Identification of natural monomeric response elements of the nuclear receptor RZR/ROR. They also bind COUP-TF homodimers. J Biol Chem 1996; 271: 19732–6. [DOI] [PubMed] [Google Scholar]
- 37.Klemm U, Muller-Esterl W, Engel W. Acrosin, the peculiar sperm-specific serine protease. Hum Genet 1991; 87: 635–41. [DOI] [PubMed] [Google Scholar]
- 38.Keime S, Adham IM, Engel W. Nucleotide sequence and exon-intron organization of the human proacrosin gene. Eur J Biochem 1990; 190: 195–200. [DOI] [PubMed] [Google Scholar]
- 39.Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem 2010; 285: 24299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Huntington JA. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J Biol Chem 2010; 285: 24307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhrin P, Dewerchin M, Hilpert M, Chrenek P, Schofer C, Zechmeister-Machhart M, Kronke G, Vales A, Carmeliet P, Binder BR, Geiger M. Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J Clin Invest 2000; 106: 1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin M-H, Lee RK-K, Hwu Y-M, Lu C-H, Chu S-L, Chen Y-J, Chang W-C, Li S-H. SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction 2008; 136: 559–71. [DOI] [PubMed] [Google Scholar]
- 43.Yamagata K, Murayama K, Okabe M, Toshimori K, Nakanishi T, Kashiwabara S, Baba T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J Biol Chem 1998; 273: 10470–4. [DOI] [PubMed] [Google Scholar]
- 44.Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev 1994; 8: 538–53. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 2004; 5: 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem 2010; 285: 35386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 2007; 318: 1786–9. [DOI] [PubMed] [Google Scholar]
- 48.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol 2007; 5: e34–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 2006; 50: 465–77. [DOI] [PubMed] [Google Scholar]