Abstract
Neuronal cells are highly sensitive to hypoxia and may be subjected to apoptosis when exposed to hypoxia. Several apoptosis-related genes and miRNAs involve in hypoxia-induced apoptosis. This study aimed to examine the role of HIF1α-miR-204-BCL-2 pathway in hypoxia-induced apoptosis in neuronal cells. Annexin V/propidium iodide assay was performed to analyze cell apoptosis in AGE1.HN and PC12 cells under hypoxic or normoxic conditions. The expression of BCL-2 and miR-204 were determined by Western blot and qRT-PCR. The effects of miR-204 overexpression or knockdown on the expression of BCL-2 were evaluated by luciferase assay and Western blot under hypoxic or normoxic conditions. HIF-1α inhibitor YC-1 and siHIF-1α were employed to determine the effect of HIF-1α on the up-regulation of miR-204 and down-regulation of BCL-2 induced by hypoxia. Apoptosis assay showed the presence of apoptosis induced by hypoxia in neuronal cells. Moreover, we found that hypoxia significantly down-regulated the expression of BCL-2, and increased the mRNA level of miR-204 in neuronal cells than that in control. Bioinformatic analysis and luciferase reporter assay demonstrated that miR-204 directly targeted and regulated the expression of BCL-2. Specifically, the expression of BCL-2 was inhibited by miR-204 mimic and enhanced by miR-204 inhibitor. Furthermore, we detected that hypoxia induced cell apoptosis via HIF-1α/miR-204/BCL-2 in neuronal cells. This study demonstrated that HIF-1α-miR-204-BCL-2 pathway contributed to apoptosis of neuronal cells induced by hypoxia, which could potentially be exploited to prevent spinal cord ischemia–reperfusion injury.
Keywords: Spinal cord ischemia–reperfusion injury, neuronal cells, hypoxia, hypoxia-inducible factor-1α, microRNA-204, B-cell lymphoma-2
Introduction
Spinal cord ischemia–reperfusion injury (SCII) is a momentous complication of thoracoabdominal surgery and may lead to paraplegia.1 To date, it has been suggested that various methods to protect spinal cord during the ischemia–reperfusion period, such as partial bypass, hypothermia, cerebrospinal fluid drainage, ischemic preconditioning, and pharmacologic interventions.2 However, the exact mechanisms of SCII are not fully understood.
SCII can induce hypoxia and threaten to end organ viability. In particular, neural tissues and neuronal cells are highly sensitive to hypoxia and reperfusion stress.3 Mounting evidence indicated that hypoxia may lead to tissue injury and cell apoptosis. It has been reported that motor neurons might be apoptosis following transient spinal cord ischemia. In addition, apoptosis of spinal neurons was also showed after SCII.1 Savas et al. reported that apoptotic changes of rabbit motor neurons played critical roles in delayed paraplegia.4 Hypoxia-inducible factor (HIF)-1 belongs to heterodimeric transcription factor which contained HIF-1 and HIF-1α. HIF-1α was important in cellular oxygen homeostasis and has been shown to mediate hypoxia-induced apoptosis.5 However, the exact mechanism of hypoxia-induced apoptosis needs further elucidation.
MicroRNAs, as a well-conserved small RNAs, regulate the biological processes of several diseases and are employed as diagnostic and prognostic biomarkers in cancers. Recent study reported that up-regulation of miR-204 was observed in rats with SCII.6 In addition, Gao et al. reported that miR-204 promoted Schwann cell apoptosis induced by oxidative stress through inhibiting neuritin expression.7 Moreover, up-regulation of miR-204 led to human trabecular meshwork cell apoptosis under oxidative stress, and anti-apoptotic proteins BCL-2 and cIAP1 were regulated by miR-204, which indicated that miR-204 could regulate the expression of several anti-apoptotic proteins.8,9
This study investigated the mechanism of hypoxia-induced apoptosis in human neuronal cell lines AGE1.HN and rat pheochromocytoma cells lines PC12. We found that hypoxia stimulated the expression of miR-204 and down-regulated BCL-2 expression in neuronal cells. Moreover, we identified that hypoxia induced cell apoptosis via HIF-1α/miR-204/BCL-2 in neuronal cells.
Materials and methods
Patients and sample collection
Forty patients were recruited in this study, who had clinical and radiologic signs of cervical spondylotic myelopathy for half year. The patients accorded with the following condition were excluded: older than 75 years, with heart, hepatic, renal or pulmonary diseases, and those with peripheral vascular diseases affecting the upper limbs. Before induction of anesthesia, patient were randomly divided into either a control SCII group (n = 20) or a SCII group (n = 20). This study was approved by the hospital board of ethics and informed written consent was obtained from all of the subjects. Anterior decompression was performed by an experienced team of orthopedic surgeons. SCII performed with right upper arm ischemia for three 5-min cycles, which inflated to 200 mm Hg using an automated cuff-inflator, with an intervening 5 min of reperfusion during, which the cuff was deflated. A deflated cuff was placed on the right upper arm of control patients for 30 min. Blood samples for measurements of miR-204 were collected before the induction of anesthesia, before decompression, after decompression, 6 h, 12 h, and 24 h after surgery. Serum samples were obtained and stored at −80℃ for further analysis.
Cell culture
Human neuronal cell lines AGE1.HN were propagated in maintenance medium (1:1 Dulbecco’s modified Eagle’s medium: Ham’s F-12 mix, 5% fetal bovine serum (FBS) (Gibco), 1% antibiotic-antimycotic (AB) (Gibco), containing 10 µg/mL human transferring and 30 mmol/L sodium selenite (Sigma-Aldrich, St Louis, MO) for 48 h. Rat pheochromocytoma cell lines, PC12, were grown on rat-tail collagen-coated dishes in RPMI 1640 medium containing 5% FBS and 10% heat-inactivated horse serum. Cells were maintained in Anoxomat hambers (Mart Microbiology, Lichtenvoorde, Netherlands) for physiological hypoxia (1% O2) or normoxia (20% O2) at 37℃.
Apoptosis assay
Flow cytometry was performed to measure the percentage of cells actively undergoing apoptosis with Annexin V/PI Apoptosis Detection Kit (Roche, Shanghai, China). Cells were collected and resuspended in binding buffer after cultured at 37℃ in the 5% CO2 incubator for 48 h with reagents. Cells were mixed with Annexin V (1:20) for 5 min, and then incubated with propidium iodide (PI, 1 mg/mL) for 15 min and immediately analyzed with a FACScanto flow cytometer (Becton Dickinson, Erembodegem, Belgium). Each measurement was carried out in triplicate.
Western blot
Protein lysate were separated by 10% SDS-polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride (PVDF) membranes. The primary antibodies against BCL-2 (1:2000; Abcam, Shanghai, China) and HIF-1α (1:2000; Abcam) were used, with GAPDH antibody as an internal control. The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies at room temperature for 1 h. Labworks Image Acquisition and Analysis Software (UVP, Upland, CA) was used for densitometric analysis.
Quantitative real-time PCR
RNA was extracted from cells using RNeasy kits (Takara, Dalian, China). MiRNAs was isolated using mirVANA miRNA isolation kit (Takara). The expression of miR-204 was detected by the mirVANA qRT-PCR miRNA Detection Kit (Ambion, Austin, TX) and qRT-PCR Primer Sets. qRT-PCR was performed on Light Cycler 2.0 (Roche, Darmstadt, Germany). The U6 small nuclear RNA was used as an endogenous reference of expression of miR-204. β-Actin was used as the internal control for the expression of BCL-2. Data were analyzed by using ΔΔCt method as relative quantification.
Cell transfection and luciferase reporter assay
Cells were transfected by Lipofectamine 2000 (Invitrogen) with miR-204 inhibitor (or mimics) or miR-204 inhibitor control (or mimics control) (GenePharma). siRNAs specific for human HIF-1α siRNA (sense: 5′-AGAGGTGGATATGTGTGGGdTdT-3′ and antisense: 5′-TGTATTTGTTCACATTATCdTdT-3′), rat HIF-1α siRNA (sense: 5′-TGGATTCTTCGCTTCTGTGdTdT-3′ and antisense: 5′-CGTTGCTGACTTGATGTTCdTdT-3′), human HIF-2α siRNA (sense: 5′-ATCTGCTGGTCAGCTTCGGdTdT-3′ and antisense: 5′-GGAGCAGCTGCTGCTGCTG dTdT-3′), RAT HIF-2α siRNA (sense: 5′-GGCAATGAAGCCCTCCAAGdTdT-3′ and antisense: 5′-GGCCAGGCGCATGATGGAGdTdT-3′) were synthesized (Ribobio, Guangzhou, China) and transfected into cells.
To examine the effects of miRNAs on BCL-2, a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) was used. Briefly, cells were co-transfected with pGL3CM reporter plasmids (100 ng) and miR-204 (1 mL) or control using lipofectamine 2000. After 48 h, cells were collected for luciferase assay and were lysed with 100 µL lysis buffer (Promega) for 15 min. Firefly luciferase was measured on a Veritas luminometer. Then Stop & Glo Reagent (30 mL) was added and mixed to detect renilla luciferase.
Statistical analyses
All data were expressed as mean ± standard deviation (mean ± SD) and were analyzed with SPSS software. All variables measured in this study were normally distributed, and the groups were compared with one-way analysis of variance (ANOVA). A P value less than 0.05 was statistically significant.
Results
Hypoxia induces apoptosis in neuronal cells
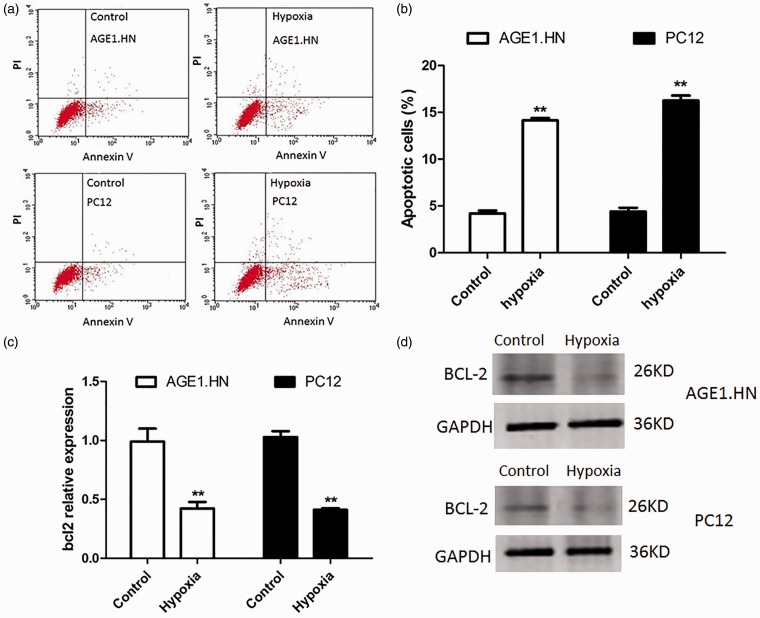
Hypoxia was suggested to relate to apoptotic and pro-apoptotic factors.5 To detect the effect of hypoxia on neuronal cells, Annexin V and PI binding assay was performed to analyze cell apoptosis in AGE1.HN and PC12 cells under hypoxic or normoxic conditions. As shown in Figure 1a and b, flow cytometry revealed that the amount of apoptotic cells in the cells exposed to hypoxia was greatly increased, and the AGE1.HN cells group had apoptotic cells up to 14.3% of the total and 15.8% for the PC12 cells group.
Figure 1.
Hypoxia induces apoptosis in neuronal cells and decreases the expression of BCL-2. (a) Exposed to hypoxia or normoxia for 18 h, cell apoptosis was tested by Annexin V/PI flow cytometry in AGE1.HN and PC12 cells. (b) Graphical representation of the data shown in a. (c) qRT-PCR analyses were performed to examine the mRNA expression of BCL-2 in AGE1.HN and PC12 cells. (d) Western blotting was performed to determine BCL-2 protein levels in AGE1.HN and PC12 cells. The data are the mean ± SD. **P < 0.01 vs. control. (A color version of this figure is available in the online journal.)
As BCL-2 are thought to play regulatory roles in the apoptotic execution of cells, we analyzed the mRNA and protein levels of BCL-2 in AGE1.HN and PC12 cells under hypoxic or normoxic conditions. The results of qRT-PCR and Western blot showed that the mRNA and protein levels of BCL-2 in AGE1.HN and PC12 cells exposed to hypoxia were significantly lower than that in control (Figure 1c and d).
Hypoxia stimulates the expression of miR-204 in neuronal cells
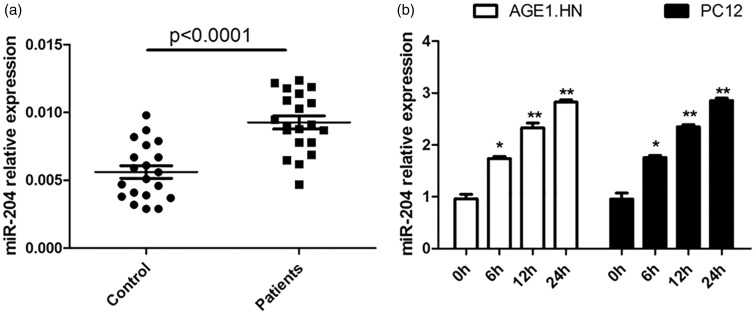
To detect the effect of hypoxia on the expression of miR-204, we employed the qRT-PCR method to compare the expression of miR-204 in peripheral plasma of patients with spinal cord ischemia–reperfusion and healthy controls. As shown in Figure 2a, high mRNA level of miR-204 was detected in peripheral plasma of patients with SCIR. To further verify the expression difference, we detected the miR-204 expression in AGE1.HN and PC12 cells exposed to hypoxia or normoxia for 0, 6, 12, and 24 h. The expression of miR-204 appeared to be gradually up-regulated in a time-dependent manner (Figure 2b). These results suggested that miR-204 was up-regulated in hypoxic neuronal cells, which might be involved in hypoxia-induced apoptosis development.
Figure 2.
Hypoxia increases the expression of miR-204 in neuronal cells. (a) The expression of miR-204 in peripheral plasma of patients with spinal cord ischemia–reperfusion. (b) qRT-PCR analyses were performed to examine the expression of miR-204 in AGE1.HN and PC12 cells exposed to hypoxia or normoxia for different duration. The data are the mean ± SD. *P < 0.05 vs. control, **P < 0.01 vs. control
BCL-2 is the target of miR-204
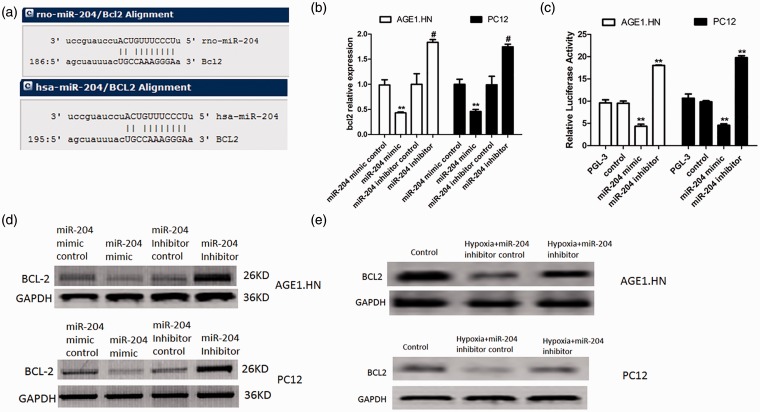
In order to elucidate the underlying molecular mechanism, we performed a bioinformatic analysis using mircoRNA.org. to predict the relationship between miR-204 and BCL-2. We found that BCL-2 gene from human and rats contained theoretical miR-204 binding sites in its 3′-UTR (Figure 3a). To examine the regulation of miR-204 on the expression of BCL-2, qRT-PCR was performed to examine the expression of BCL-2 in the AGE1.HN and PC12 cells transfected with miR-204 mimic or inhibitor or corresponding controls. As shown in Figure 3b, significantly lower mRNA level of BCL-2 was detected in the cells transfected with miR-204 mimic. To verify whether BCL-2 is a target of miR-204, dual-luciferase reporter assay was performed as described in “Materials and methods” section. As shown in Figure 3c, miR-204 mimic suppressed the luciferase activity of the reporter containing BCL-2 3′-UTR sequence, and miR-204 inhibitor increased the luciferase activity. Furthermore, the expression of BCL-2 protein level was also suppressed by miR-204 mimic and enhanced by miR-204 inhibitor (Figure 3d). To explore whether miR-204 regulated BCL-2 under conditions of hypoxia in these cells, BCL-2 protein levels were detected in AGE1.HN and PC12 cells which were transfected with miR-204 inhibitor control or miR-204 inhibitor and then cultured under conditions of hypoxia. Consistent with the results in cells cultured under normal oxygen conditions, the protein level of BCL-2 was enhanced in cells transfected with miR-204 inhibitor comparing to that in control (Figure 3e). These results demonstrated that BCL-2 is targeted and regulated by miR-204.
Figure 3.
BCL-2 is the target of miR-204. (a) Sequence alignment of miR-204 and 3′-UTR of BCL-2 using mirco-RNA.org. (b) AGE1.HN and PC12 cells were transfected with miR-204 mimic or miR-204 inhibitor or controls under normal oxygen conditions. The expression of BCL-2 was detected with qRT-PCR. (c) Relative luciferase activity in cells transfected with miR-204 mimics (or miR-204 inhibitor) and luciferase expression vector of BCL-2 under normal oxygen conditions. (d) AGE1.HN and PC12 cells were transfected with miR-204 mimic or miR-204 inhibitor or controls under normal oxygen conditions. The protein expression of BCL-2 was detected with Western blotting. (e) AGE1.HN and PC12 cells were transfected with miR-204 inhibitor or controls and then exposed to hypoxia. The protein expression of BCL-2 was detected with Western blotting. The data are the mean ± SD. **P < 0.01 vs. control; #P < 0.05 vs. inhibitor control. (A color version of this figure is available in the online journal.)
Hypoxia increases the expression of miR-204 and reduced the expression of BCL-2 via HIF-1α pathway
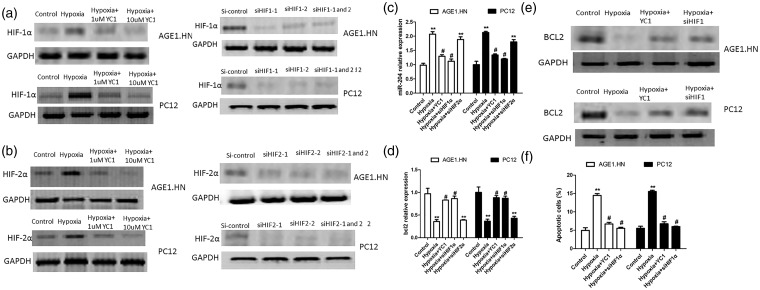
HIF-1α is a key transcription factor and plays pivotal roles in hypoxia. As reported previously, YC-1 (Sigma, China) reduced the protein levels of both HIF-1α and HIF-2α under hypoxic conditions in a dose-dependent manner.10 To determine the role of HIF-1α in hypoxia induced up-regulation of miR-204 and down-regulation of BCL-2, we employed YC-1 and siRNAs which specifically targeted HIF-1α or HIF-2α to treat AGE1.HN and PC12 cells. Cells were treated with 1 µmol/L and 10 µmol/L YC-1 for 6 h, then exposed to hypoxia for 24 h. As the concentration of YC-1 in previous reports was in the range from 3 µm to 200 µm, the concentration of YC-1 in this study might be below non-specific and toxic levels.10 The results of Western blot indicated that YC-1 effectively suppressed the protein level of HIF-1α and HIF-2α in cells exposed to hypoxia. Moreover, the use of siRNAs which specifically target HIF-1α or HIF-2α further verified these results (Figure 4a and b). Next, the expression of miR-204 and BCL-2 were detected in cells transfected with YC-1 or siHIF-1α or siHIF-2α and exposed to hypoxia. As shown in Figure 4c and d, YC-1 and siHIF-1α reversed the effect of hypoxia on the expression of miR-204 and BCL-2. They decreased the mRNA level of miR-204 and increased the mRNA level of BCL-2, while siHIF-2α has no effect on the expression of miR-204 and BCL-2. We further detected the protein level of BCL-2 in cells transfected with YC-1 or siHIF-1α under hypoxia. As shown in Figure 4e, YC-1 and siHIF-1α reversed the effect of hypoxia on the protein level of BCL-2. In addition, the levels of apoptosis were measured with flow cytometry in AGE1.HN and PC12 cells transfected with YC-1 or siHIF-1α under hypoxia. It has been shown that YC-1 and siHIF-1α reversed the percentage of apoptotic cells induced by hypoxia (Figure 4f). Taken together, these findings indicated that hypoxia stimulates the expression of miR-204 and reduced the expression of BCL-2 via HIF-1α pathway in neuronal cells.
Figure 4.
Hypoxia increases the expression of miR-204 and reduces the expression of BCL-2 via HIF1-α pathway. (a) AGE1.HN and PC12 cells were treated with 1 µmol/L and 10 µmol/L YC-1 for 6 h and then exposed to hypoxia for 24 h or transfected with siHIF-1α. The protein expression of HIF1α was detected with Western blotting. (b) AGE1.HN and PC12 cells were treated with 1 µmol/L and 10 µmol/L YC-1 for 6 h and then exposed to hypoxia for 24 h or transfected with siHIF-2α. The protein expression of HIF-2α was detected with Western blotting. (c) The mRNA level of miR-204 was detected in cells transfected with YC-1 or siHIF-1α or siHIF-2α and exposed to hypoxia. (d) The mRNA level of BCL-2 was detected in cells transfected with YC-1 or siHIF-1α or siHIF-2α and exposed to hypoxia. (e) The protein level of BCL-2 was detected in AGE1.HN and PC12 cells transfected with YC-1 and siHIF-1α and exposed to hypoxia. (f) The percentage of apoptotic cells was detected by flow cytometry in AGE1.HN and PC12 cells transfected with YC-1 or siHIF-1α. The data are the mean ± SD. **P < 0.01 vs. control; #P < 0.05 vs. hypoxia
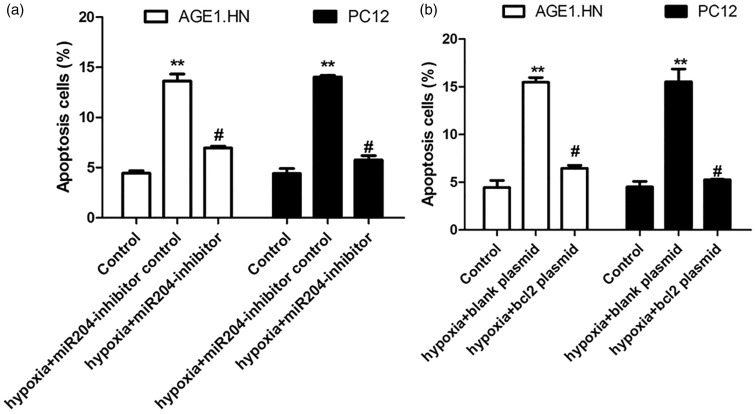
Hypoxia induced cell apoptosis via miR-204 and BCL-2
To investigate the role of miR-204 and BCL-2 in cell apoptosis induced by hypoxia, AGE1.HN and PC12 cells were transfected with miR-204 inhibitor (or control) or BCL-2 plasmid (or control) and exposed to hypoxia. Apoptotic cells were detected in these cells. As shown in Figure 5, the percentage of apoptotic cells was significantly decreased by miR-204 inhibitor and enhanced by BCL-2 plasmid. These data indicated that hypoxia induced cell apoptosis via miR-204 and BCL-2.
Figure 5.
Hypoxia induces cell apoptosis via miR-204 and BCL-2. (a) AGE1.HN and PC12 cells were transfected with miR-204 inhibitor or controls and then exposed to hypoxia. Flow cytometry was performed to detect the levels of apoptosis. (b) AGE1.HN and PC12 cells were transfected with BCL-2 plasmid or controls and then exposed to hypoxia. Flow cytometry was performed to detect the levels of apoptosis. The data are the mean ± SD. **P < 0.01 vs. control; #P < 0.05 vs. hypoxia
Discussion
In this study, we verified the presence of apoptosis induced by hypoxia in neuronal cells. Moreover, we found that hypoxia significantly down-regulated the expression of BCL-2, and increased the mRNA expression of miR-204 in neuronal cells than that in control. Bioinformatic analysis and luciferase reporter assay demonstrated that the expression of BCL-2 was directly regulated by miR-204 under normal oxygen and hypoxic conditions. Specifically, the expression of BCL-2 was inhibited by miR-204 mimic and enhanced by miR-204 inhibitor. Furthermore, we detected that hypoxia induced cell apoptosis via HIF-1α/miR-204/BCL-2 pathway in neuronal cells.
The ischemia/reperfusion (I/R) injury of the spinal cord is the main contributor to severe neurological injury and still represents one of the most catastrophic complications after thoracoabdominal aneurysm surgery. The mechanisms that underline I/R injury are multifactorial and complex. Most important mechanisms that lead to neuronal damage after spinal cord I/R injury appear to be hypoxia with energy failure, excitotoxicity, oxidative stress, inflammation, lipid peroxidation, and apoptosis.11 Neuronal cell is comparatively sensitive to hypoxia resulted from ischemia and then subjects to cell apoptosis, which is supposed to the major cause of neuron death. In the present study, we also found that the amount of apoptotic cells in the neuronal cells exposed to hypoxia was significantly elevated than the cells in normoxic conditions.
Apoptosis-related genes and several miRNAs played a critical role for apoptosis and preponderance of reports showed that these apoptosis-related genes and miRNAs involved hypoxia-induced apoptosis.12–14 To clarify the potential mechanisms of the apoptosis induced by hypoxia, we detected the expression of BCL-2 and miR-204 in neuronal cells exposed to hypoxia. BCL-2 is the major member of BCL-2 family and essentially studied for its role in apoptosis.15 Anti-apoptotic (Bcl-2, Bcl-XL and Bcl-w) and pro-apoptotic (Bax, Bcl-Xs, Bak and Bad) proteins constitute the BCL-2 family.16 We also found BCL-2 was down-regulated in AGE1.HN and PC12 cells exposed to hypoxia, which was consistent with the anti-apoptotic character of BCL-2. Mounting evidence showed that miRNAs involved in post-transcriptional gene regulation through binding to the untranslated region of mRNA.17,18 Li et al. reported that miR-204 could bind to BCL-2 and induced cell apoptosis.19 Here, luciferase reporter assay further verified this result and detected that miR-204 mimic could down-regulate the expression of BCL-2 under normal oxygen and hypoxic conditions. In addition, hypoxia resulted in the up-regulation of miR-204 and down-regulation in neuronal cells. MiR-204 is involved in several cellular functions through regulating the expression of target genes. In addition, miR-204 may regulate the expression of the targeted tyrosine kinase receptor NTRK2/TrkB of BDNF.20 MiR-204 also participated in the axonal structure and function and the growth and development of neuronal.21 MiR-204 still involved in promoting apoptosis in rat Schwann cells induced by oxidative stress.7 We also found that miR-204 was related to the apoptosis induced by hypoxia.
HIF-1α is the most important hypoxia inducible factor and reported to mediate hypoxia-induced growth arrest and apoptosis.22 HIF-1α exerts its effects through regulating its target genes, such as vascular endothelial growth factor, erythropoietin, and the glucose transporter, which may have neuro-protective functions. HIF-1α also could target to BCL-2 family members.23 Dong et al. reported that hippocampal apoptosis was aggravated by YC-1, which was an inhibitor of HIF-1α and HIF-2α.24,25 We found YC-1 effectively suppressed the protein level of HIF-1α and HIF-2α in AGE1.HN and PC12 cells exposed to hypoxia. When AGE1.HN and PC12 cells transfected with YC-1 or siHIF-1α or siHIF-2α and exposed to hypoxia, we found that YC-1 and siHIF-1α reversed the effect of hypoxia on the expression of miR-204 and BCL-2. While, siHIF-2α has no effect on the expression of miR-204 and BCL-2. These data indicated that miR-204 and BCL-2 were regulated by HIF-1α but not HIF-2α. Finally, flow cytometry analysis suggested that YC-1 and siHIF-1α reversed the percentage of apoptotic cells induced by hypoxia as well as miR-204 inhibitor and BCL-2 plasmid.
In conclusion, our findings showed that hypoxia resulted in apoptosis in neuronal cells and stimulated the expression of miR-204, while BCL-2 was down-regulated in neuronal cells exposed to hypoxia. MiR-204 regulated the expression of BCL-2 in the neuronal lines (AGE1.HN and PC12). We also identified that hypoxia induced cell apoptosis via HIF-1α/miR-204/BCL-2 pathway. The present findings reveal a novel mechanism of HIF1α-miR-204-BCL-2 pathway involved in apoptosis of neuronal cells induced by hypoxia, which can potentially be exploited to prevent SCII.
Author contributions
All authors participated in the design and interpretation of the studies. They also analyzed the data and reviewed the manuscript. XW carried out the experiments, DW and JL supplied critical reagents, XB wrote the manuscript, and JL contributed the operation, conceived of the study, and helped to draft the manuscript.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Li Y, Gu J, Liu Y, Long H, Wang G, Yin G, Fan J. iNOS participates in apoptosis of spinal cord neurons via p-BAD dephosphorylation following ischemia/reperfusion (I/R) injury in rat spinal cord. Neurosci Lett 2013; 545: 117–22. [DOI] [PubMed] [Google Scholar]
- 2.Nazli Y, Colak N, Namuslu M, Erdamar H, Haltas H, Alpay MF, Aksoy ON, Akkaya IO, Cakir O. Cilostazol attenuates spinal cord ischemia-reperfusion injury in rabbits. J Cardiothorac Vasc Anesth. Epub ahead of print 2014. [DOI] [PubMed] [Google Scholar]
- 3.Yavuz C, Demirtas S, Guclu O, Karahan O, Caliskan A, Yazici S, Mavitas B. Rosuvastatin may have neuroprotective effect on spinal cord ischemia reperfusion injury. CNS Neurol Disord Drug Targets 2013; 12: 1011–6. [DOI] [PubMed] [Google Scholar]
- 4.Savas S, Delibas N, Savas C, Sutcu R, Cindas A. Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord 2002; 40: 224–9. [DOI] [PubMed] [Google Scholar]
- 5.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 2004; 57: 1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J-R, Lv G-H, Yin B-L. Altered microRNA expression in the ischemic–reperfusion spinal cord with atorvastatin therapy. J Pharmacol Sci 2013; 121: 343–6. [DOI] [PubMed] [Google Scholar]
- 7.Gao R, Wang L, Sun J, Nie K, Jian H, Gao L, Liao X, Zhang H, Huang J, Gan S. MiR-204 promotes apoptosis in oxidative stress-induced rat Schwann cells by suppressing neuritin expression. FEBS Lett 2014; 588: 3225–32. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Yan H-X, Yang W, Hu L, Yu L-X, Liu Q, Li L, Huang D-D, Ding J, Shen F. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol 2009; 50: 358–69. [DOI] [PubMed] [Google Scholar]
- 9.Wu W, Lin Z, Zhuang Z, Liang X. Expression profile of mammalian microRNAs in endometrioid adenocarcinoma. Eur J Cancer Prev 2009; 18: 50–5. [DOI] [PubMed] [Google Scholar]
- 10.Chun YS, Yeo EJ, Choi E, Teng CM, Bae JM, Kim MS, Park JW. Inhibitory effect of YC-1 on the hypoxic induction of erythropoietin and vascular endothelial growth factor in Hep3B cells. Biochem Pharmacol 2001; 61: 947–54. [DOI] [PubMed] [Google Scholar]
- 11.Gürer B, Kertmen H, Kasim E, Yilmaz ER, Kanat BH, Sargon MF, Arikok AT, Ergüder BI, Sekerci Z. Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury. Epub ahead of print 2014. [DOI] [PubMed] [Google Scholar]
- 12.Boldin M, Chang K. NF-kappaB· dependent induction of microRNA miR-146. an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006; 103: 12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Dong L-Y, Li Y-J, Hong Z, Wei W-S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J Neuroinflamm 2012; 9: 211–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aukkarasongsup P, Haruyama N, Matsumoto T, Shiga M, Moriyama K. Periostin inhibits hypoxia-induced apoptosis in human periodontal ligament cells via TGF-β signaling. Biochem Biophys Res Commun 2013; 441: 126–32. [DOI] [PubMed] [Google Scholar]
- 15.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature Rev Mol Cell Biol 2014; 15: 49–63. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Qiu J, Grafe MR, Rea HC, Rassin DK, Perez-Polo JR. Bcl-2 family members make different contributions to cell death in hypoxia and/or hyperoxia in rat cerebral cortex. Int J Dev Neurosci 2003; 21: 371–7. [DOI] [PubMed] [Google Scholar]
- 17.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 2005; 6: 376–85. [DOI] [PubMed] [Google Scholar]
- 18.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 2001; 294: 853–8. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Investig Ophthalmol Vis Sci 2011; 52: 2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan J, Tivnan A, Fay J, Bryan K, Meehan M, Creevey L, Lynch J, Bray I, O'Meara A, Davidoff A. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br J Cancer 2012; 107: 967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natera-Naranjo O, Aschrafi A, Gioio AE, Kaplan BB. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA. Epub ahead of print 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Qu Y, Li J, Xiong Y, Mao M, Mu D. Relationship between HIF-1α expression and neuronal apoptosis in neonatal rats with hypoxia–ischemia brain injury. Brain Res 2007; 1180: 133–9. [DOI] [PubMed] [Google Scholar]
- 23.Sendoel A, Hengartner MO. Apoptotic cell death under hypoxia. Physiology (Bethesda, MD) 2014; 29: 168–76. [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Li Y, Feng D, Wang J, Wen H, Liu D, Zhao D, Liu H, Gao G, Yin Z. Protective effect of HIF-1α against hippocampal apoptosis and cognitive dysfunction in an experimental rat model of subarachnoid hemorrhage. Brain Res 2013; 1517: 114–21. [DOI] [PubMed] [Google Scholar]
- 25.Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1{alpha}. Mol Cancer Ther 2008; 7: 3729–38. [DOI] [PubMed] [Google Scholar]