Abstract
Chitinase 3-like 1 (CHI3L1) and intelectin 1 (ITLN-1) recognize microbial N-acetylglucosamine polymer and galactofuranosyl carbohydrates, respectively. Both lectins are highly abundant in plasma and seem to play pro- and anti-inflammatory roles, respectively, in obesity and inflammatory-related illnesses. The aim of this study was to examine whether plasma levels of these lectins in obese subjects are useful for monitoring inflammatory conditions immediately influenced by acute aerobic exercise. Plasma interleukin-6, a pro-inflammatory cytokine, was also examined. Twenty-two (11 obese and 11 normal-weight) healthy subjects, ages 18–30 years, were recruited to perform a 30 min bout of acute aerobic exercise at 75% VO2max. We confirmed higher baseline levels of plasma CHI3L1, but lower ITLN-1, in obese subjects than in normal-weight subjects. The baseline levels of CHI3L1 were negatively correlated with cardiorespiratory fitness (relative VO2max). However, when controlled for BMI, the relationship between baseline level of CHI3L1 and relative VO2max was no longer observed. While acute aerobic exercise elicited an elevation in these parameters, we found a lower ITLN-1 response in obese subjects compared to normal-weight subjects. Our study clearly indicates that acute aerobic exercise elicits a pro-inflammatory response (e.g. CHI3L1) with a lower anti-inflammatory effect (e.g. ITLN-1) in obese individuals. Furthermore, these lectins could be predictors of outcome of exercise interventions in obesity-associated inflammation.
Keywords: Obesity, exercise, chitinase-3 protein 1, intelectin-1, interleukin-6, inflammation
Introduction
Obesity is considered a chronic inflammatory condition that enhances the risk of numerous inflammatory diseases, including type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).1,2 Emerging research indicates that physical activity provides health-related benefits in obesity-associated inflammatory diseases.3,4 However, reliable biomarkers predicting outcome of exercise treatments to prevent or delay obesity-associated inflammatory disease development remain to be studied.
Previously, we have studied plasma chitinase 3-like 1 (CHI3L1) and intelectin 1 (ITLN-1) binding to chitin- and galactofuranosyl carbohydrate-containing microbes, respectively. Chitin, a non-antigenic N-acetylglucosamine polymer and a substrate of CHI3L1, exists in the cell wall of fungi and shells of crustaceans (e.g. crab, shrimp, and lobster). Although mammalians do not possess chitin as a structural component, our laboratory and others have reported elevated plasma levels of CHI3L1 in obese individuals5–7 as well as other inflammatory-related illnesses in humans, such as T2DM, inflammatory bowel disease (IBD), colon cancer, breast cancer, rheumatoid arthritis, bronchial asthma, coronary artery disease, and Alzheimer's disease.8–13 It is particularly important that this CHI3L1 marker has been studied for monitoring weight loss intervention in patients with morbid obesity and obesity-associated T2DM.14,15 In this regard, local inflamed tissues such as intestinal mucosa in IBD10 and adipose tissues in T2DM11 produce CHI3L1. Particularly, activated immune cells including tissue macrophages are considered as major CHI3L1 producers.10 However, our previous study did not show any difference in the quantity of chitin-induced CHI3L1 production by peripheral blood mononuclear cells ex vivo between obese and normal-weight individuals.7
In addition, we have demonstrated that obese individuals present with lower levels of plasma ITLN-1.7 ITLN-1 is mainly expressed by visceral, not subcutaneous, adipose tissue16 and intestinal Paneth cells recognizing bacterial cell wall.17 ITLN-1 not only plays a role of host defense against pathogenic bacteria,18 but also an anti-inflammatory effect of inhibiting tumor necrosis factor-alpha (TNF-α)-mediated inflammation in vascular smooth muscle cells.19 It is important to note that following weight loss, plasma ITLN-1 concentrations tend to normalize (increase) to concentrations observed in normal-weight individuals.20
While increased cardiorespiratory fitness has been shown to provide anti-inflammatory and cardioprotective actions to prevent the risk of CVD,3,21,22 acute intense exercise enhances the activation of inflammatory signaling pathways (e.g. nuclear factor-kappa B [NF-kB]) and the production of inflammatory cytokines (e.g. interleukin-6 [IL-6]).23 Interestingly, acute intense exercise may be more effective to enhance IL-6 and other pro-inflammatory cytokines in obese individuals than normal-weight individuals.24 However, there is limited information regarding the effect of acute exercise on plasma concentrations of CHI3L1 and ITLN-1 in obese populations. In the present study, therefore, we attempted to clarify whether plasma CHI3L1 and ITLN-1 levels would be altered immediately in response to acute aerobic exercise. These lectin levels were also compared between obese and normal-weight subjects. We further determined whether the changes of lectin levels would be associated with plasma IL-6 levels.7,25,26
Material and methods
Subjects
Twenty-two (11 obese [4 men and 7 women] and 11 normal-weight [5 men and 6 women]) untrained healthy subjects ages 18–30 years old were recruited to participate in this study. Subjects with a BMI above 30 kg/m2 comprised the obese group, and those with a BMI between 18.5 and 24.9 kg/m2 comprised the normal-weight group. All subjects completed the informed consent process and a medical history questionnaire prior to data collection. Experimental procedures were approved by Florida Atlantic University's Institutional Review Board.
Subjects were excluded from the study if they possessed any known inflammatory diseases/conditions (e.g. CVD, chronic kidney or liver disease, diabetes) or were currently under medication that may affect the laboratory test results. Subjects were also excluded from the study if they were users of tobacco products (cigarettes, cigars, chewing tobacco), or if they consumed an average of 10 or more alcoholic beverages per week. Subjects were instructed to undergo an overnight fast for at least 8 h and to abstain from alcohol, caffeine intake, and intense physical activity for at least 24 h prior to each laboratory visit. To limit the effect of training on physiological response to acute exercise, those who reported more than 150 min of moderate and high physical activity levels per week were excluded from participation.27 Finally, women who were pregnant, nursing, or taking hormone replacement therapy also were excluded from the study because of the potential effects on immune responses.28
Experimental protocol
Two testing sessions comprised the data collection. Subjects were asked to arrive at the laboratory between 7:00 AM and 9:00 AM for the testing sessions. The first session consisted of informing participants of procedures and obtaining consent to participate, familiarization with all instruments and procedures, assessment of anthropometric parameters, and an assessment of maximal oxygen consumption (VO2 max). A maximal graded exercise test on a treadmill to assess VO2max was then administered beginning with a 3 min warm-up at 3 mph with 0% grade. Speed was subsequently increased to elicit 80% ± 5 bpm of the subject's age-predicted maximal heart rate (HR). After 4 min, grade was increased 2% every 2 min while speed remained constant until voluntary exhaustion resulted within 12–15 min.
HR was assessed while rating of perceived exertion (RPE) was obtained once every exercise stage during maximal exercise testing. Criteria for attaining VO2max included a plateau in O2 consumption and two of the following secondary criteria: respiratory exchange ratio ≥ 1.15, HR within 10 beats/min of subject's age-predicted maximum HR (220-age), and an RPE ≥ 19. HR and blood pressure (BP) were assessed by HR monitors (Polar T31, Polar Electro, Kempele, Finland) and sphygmomanometer (752M-Mobile Series, American Diagnostic Corporation, Hauppauge, NY) prior to exercise and during recovery.
After one week following completion of the first exercise testing session, subjects participated in a 30-min continuous exercise on a treadmill at 75% VO2max as determined during session one, with HR and BP assessment prior to and immediately postexercise. Blood draws were performed by a trained phlebotomist in accordance with institutional review board standard operating procedure prior to, immediately post, and after the conclusion of aerobic exercise at 75% VO2max (recovery 1 h [R1h]). A 10 mL sample of whole blood was collected from the antecubital vein of each subject using a 21G butterfly needle into a tube containing K2 ethylenediaminetetraacetic acid (K2EDTA) (BD Vacutainer, Franklin Lakes, NJ). Blood samples were immediately centrifuged at 1,000×g for 20 min at room temperature. Immediately thereafter, appropriate sample volumes were collected into specific collection tubes for subsequent analysis.
Metabolic measurements
A 5 mL blood sample was collected prior to, immediately post, and R1h in a tube containing K2EDTA for plasma glucose and insulin analyses, and centrifuged for 15 min at 2,000×g at 4℃. All samples were stored at −80℃ for further analyses. Plasma insulin was assayed in duplicate with enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics, Salem, NH). The glucose concentrations were quantified using colorimetric assay kits (Cayman Chemical, Ann Arbor, MI). Subsequently, insulin resistance was evaluated by the homeostasis model of assessment (HOMA-IR) index according to the following formula29: (fasting insulin [μIU/mL] × fasting glucose [mg/dL]) / 405.
Assessment of CHI3L1, ITLN-1, and IL-6
CHI3L1 was assayed in plasma in duplicate with ELISA kits (R&D Systems, Minneapolis, MN). Plasma ITLN-1 was analyzed using ELISA kits (BioVendor, LLC, Candler, NC). Furthermore, plasma concentrations of IL-6 were measured using high sensitivity ELISA kits (R&D Systems, Minneapolis, MN). All analyses were conducted according to the manufacturer's instructions.
Statistical analyses
Data analyses were performed with the Statistical Package for the Social Sciences (SPSS version 20.0). A two group (obese and normal-weight) × three time points (prior to, immediately postexercise, and recovery 1 h) repeated measures analyses of variance (ANOVA) and Bonferroni post hoc comparisons were used to examine the effect of acute aerobic exercise on plasma levels of CHI3L1, ITLN-1, and IL-6. The Greenhouse–Geisser correction of degrees of freedom was used when sphericity assumptions were violated. Significant effects were further analyzed with Bonferroni post hoc comparisons. Independent t-tests were conducted to compare the baseline levels and percent change (baseline to immediately postexercise) on all variables between obese and normal-weight groups. Finally, Pearson product-moment correlations were used to examine the relationships between CHI3L1, ITLN-1, IL-6, and cardiorespiratory fitness level (relative VO2max). Statistical significance was defined as a P value < 0.05. All graphs were generated using Prism software (version 6.0).
Results
Anthropometric and metabolic measurements of the study participants
Baseline anthropometric and metabolic characteristics of obese and non-obese participants are reported in Table 1. Differences between obese and normal-weight groups at baseline were statistically significant for weight, BMI, VO2max, systolic and diastolic BPs, HR, waist/hip circumferences, plasma fasting glucose, and HOMA-IR index. In addition, no differences were found in any outcome variables between men and women.
Table 1.
Participant anthropometric and metabolic characteristics
| Variable | Obese (N =11) | Normal-weight (N =11) | P value |
|---|---|---|---|
| Age (years) | 21.55 ± 0.59 | 23.17 ± 0.68 | 0.069 |
| Gender (M/F) | 4/7 | 5/6 | 0.394 |
| Height (cm) | 166.68 ± 0.03 | 169.64 ± 0.04 | 0.527 |
| Weight (kg) | 97.26 ± 5.11 | 64.21 ± 3.84 | * < 0.001 |
| BMI (kg/m2) | 34.81 ± 1.07 | 22.08 ± 0.52 | * < 0.001 |
| Absolute VO2max (L/min) | 3.06 ± 0.24 | 3.05 ± 0.31 | 0.976 |
| Relative VO2max (mL/kg/min) | 31.18 ± 1.56 | 46.66 ± 2.43 | * < 0.001 |
| Systolic blood pressure (mmHg) | 126.55 ± 2.91 | 109.27 ± 2.65 | * < 0.001 |
| Diastolic blood pressure (mmHg) | 82.00 ± 2.09 | 72.27 ± 1.77 | * 0.001 |
| Heart rate | 74.64 ± 2.76 | 66.36 ± 1.60 | * 0.017 |
| Waist circumference (cm) | 97.09 ± 2.57 | 71.73 ± 2.19 | * < 0.001 |
| Hip circumference (cm) | 116.17 ± 2.66 | 95.27 ± 1.26 | * < 0.001 |
| Plasma fasting glucose (mg/dL) | 98.77 ± 1.51 | 89.87 ± 2.09 | * 0.002 |
| Plasma fasting insulin (μlU/mL) | 16.92 ± 4.38 | 7.34 ± 1.81 | 0.057 |
| HOMA-IR index | 4.11 ± 1.06 | 1.67 ± 0.44 | * 0.047 |
The * indicates the difference between obese and normal-weight groups. Data are presented as means ± standard error of mean (SEM).
Measurements of circulating CHI3L1, ITLN-1, and IL-6
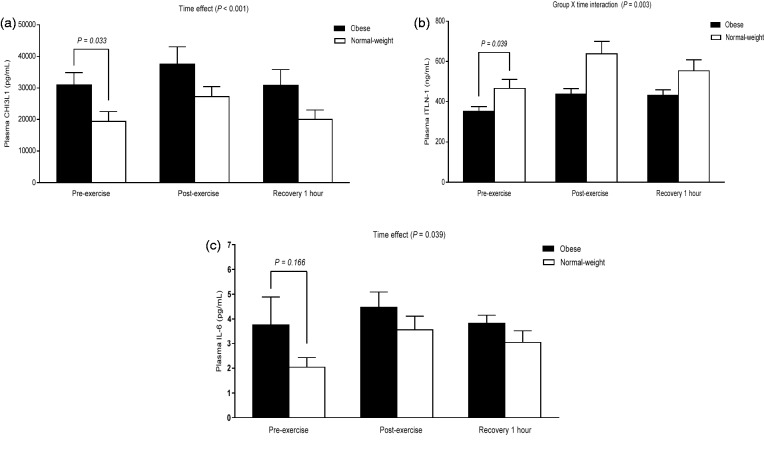
At baseline, obese subjects showed significantly higher levels of plasma CHI3L1 [t(20) = −2.290, P = 0.033], whereas the concentrations of plasma ITLN-1 were lower [t(20) = 2.214, P = 0.039] compared to normal-weight subjects (Figure 1(a) and (b)). Although our analysis did not show the difference in plasma IL-6 at baseline between two groups (Figure 1(c)), a positive correlation with BMI was observed (r = 0.453, P = 0.034).
Figure 1.
The percent change of CHI3L1, ITLN-1, and IL-6 in response to acute exercise. A significant elevation in plasma CHI3L1 and IL-6 expression by repeated measures ANOVA was found immediately following acute aerobic exercise in both obese and normal-weight groups (panel a and c). However, the obese group elicited a significantly lower plasma ITLN-1 response to acute aerobic exercise compared to the normal-weight group (panel b). Data are presented as means ± SEM
Immediately following exercise, a significant elevation in plasma CHI3L1 and IL-6 was found in both obese and normal-weight groups (F([2, 40]) = 31.695, P < 0.001; F ([1.166, 23.312]) = 4.506, P = 0.039, respectively); these increases returned to baseline at recovery 1 h (Figure 1(a) and (c)). Furthermore, acute exercise elicited a lower ITLN-1 plasma response in obese subjects compared to normal-weight subjects (F([2, 40]) = 6.593, P = 0.003) (Figure 1(b)).
Correlation analyses
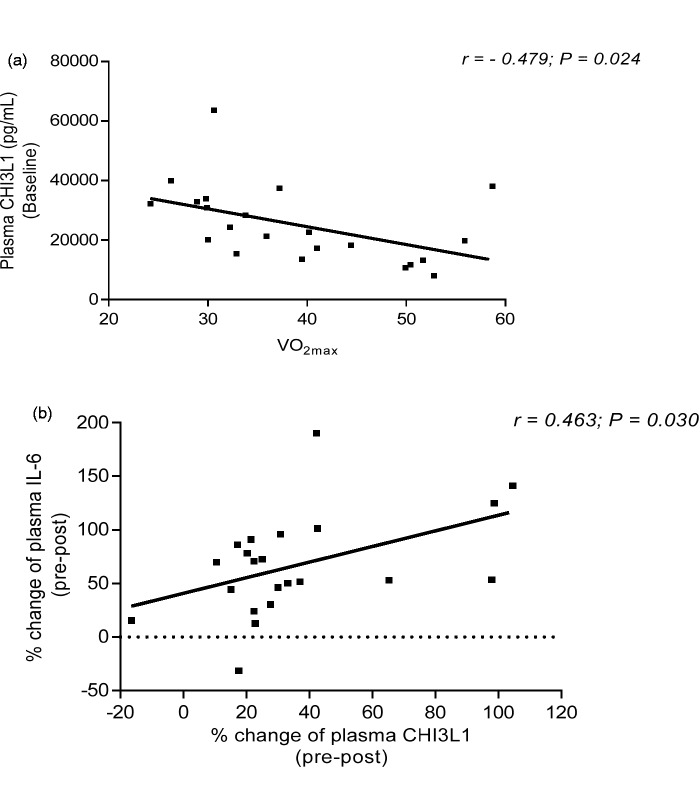
At baseline, our analyses did not observe any correlation among CHI3L1, ITLN-1, and IL-6. Furthermore, the baseline level of plasma CHI3L1 was negatively correlated with cardiorespiratory fitness level (relative VO2max) (r = −0.479; P = 0.024; Figure 2(a)). However, when controlled for BMI, the relationship between baseline level of CHI3L1 and relative VO2max was no longer observed. In response to acute aerobic exercise, the percent change (baseline to immediately postexercise) of plasma CHI3L1 was positively correlated with the percent change of plasma IL-6 (r = 0.463; P = 0.030; Figure 2(b)), but this relationship did not exist after controlling for BMI. Finally, plasma ITLN-1 was not correlated with either plasma CHI3L1 or IL-6 following exercise.
Figure 2.
The relationship of CH13L1 with VO2max and IL-6. At baseline, the concentration of plasma CHI3L1 was negatively correlated with cardiovascular fitness levels (relative VO2max; panel a). In response to acute aerobic exercise, the percent change of CHI3L1 was positively correlated with the percent change of IL-6 in plasma (panel b). However, these relationships were no longer observed after controlling for BMI
Discussion
This study confirmed previous observations5,7,16 that obese subjects have higher baseline levels of plasma CHI3L1, but lower ITLN-1, compared to normal-weight subjects. We further demonstrate, for the first time, that acute aerobic exercise immediately increased all these plasma mediators, and these elevations returned to baseline at recovery 1 h. Additionally, the baseline levels of CHI3L1 were negatively correlated with cardiorespiratory fitness (relative VO2max). Nonetheless, when controlled for BMI, this relationship was no longer observed. These findings suggest that circulating pro-inflammatory CHI3L1 and anti-inflammatory ITLN-1 could be biomarkers for monitoring the effectiveness of exercise programs in obese individuals.
Previous research has shown the role of CHI3L1 in promoting the development of pro-inflammatory mediators (e.g. MCP-1/CCL2, CXCL2, MMP-9).30,31 Importantly, Nielsen et al.32 found that IL-6, but not TNF-α, infusions in humans result in elevated plasma CHI3L1 levels, suggesting that IL-6 plays a regulatory role of CHI3L1 production in individuals with elevated BMI following exercise-mediated acute inflammation. However, increased CHI3L1 may provide a negative feedback mechanism to downregulate pro-inflammatory cytokine expression, including IL-6. Specifically, Görgens et al.33 have recently shown that in human skeletal muscle cell culture CHI3L1 decreases IL-6 production via the inhibition of NF-κB activation. While exercise training has been shown to provide physiological benefits and can serve as an adjuvant therapy in various inflammatory diseases,4 the present study clearly indicated that acute aerobic exercise elicits a mixture of pro- and anti-inflammatory responses, which magnitudes, however, seem to be distinct between normal and obese individuals.
In this regard, a provocative finding is that acute exercise increases circulating levels of ITLN-1 in both obese and normal-weight subjects, although the magnitude is greater in normal-weight than obese subjects. Yamawaki et al.34 have demonstrated that ITLN-1 inhibits TNF-α-mediated inflammation (e.g. COX-2) in human endothelial cells. While the relationship between ITLN-1 and IL-6 was not observed in this study, the administration of ITLN-1 in rats decreases IL-6 mRNA intra-thoracic pericardial adipose tissue.35 Additionally, Saremi et al.36 showed increased circulating levels of ITLN-1 in obese subjects following 12 weeks of aerobic training; this elevated ITLN-1 is also associated with improved insulin resistance. Taken together, exercise-induced changes in ITLN-1 may play an important anti-inflammatory role in attenuation of cardiovascular complications and insulin resistance in obesity. Finally, although this study is consistent with previous research showing no gender difference in CHI3L137 and ITLN-1,38 future study is warranted to elucidate whether the levels of these lectins would differ between phases of the menstrual cycle.
In summary, our study demonstrates that acute aerobic exercise not only immediately increases plasma ITLN-1, CHI3L1, and IL-6 in normal-weight subjects, but also obese subjects. While ITLN-1 could be a predictor of outcome of exercise interventions, further investigation should include a larger sample size to examine whether the upregulation of circulating ITLN-1 following exercise would potentially be an obligatory step toward improving exercise-mediated inflammation in obesity.
ACKNOWLEDGEMENTS
Funding for this study was provided by the Departments of Exercise Science and Health Promotion and Biomedical Science at Florida Atlantic University. The University had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Authors' contributions
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. Conception and design: C-JH, YS. Data collection: C-JH, ALS, MW, AM, YS. Data analysis and interpretation: C-JH, MW, YS. Manuscript writing: C-JH, ALS, MW, MW, YS. Final approval of manuscript: C-JH, ALS, MW, AM, YS.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 2006; 26: 968–76. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd CM, Saglani S. Eosinophils in the spotlight: finding the link between obesity and asthma. Nat Med 2013; 19: 976–7. [DOI] [PubMed] [Google Scholar]
- 3.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol 2005; 100: 93–9. [DOI] [PubMed] [Google Scholar]
- 4.Lira F, Rosa Neto J, Antunes B, Fernandes R. The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr Diabetes Rev 2014; 10: 391–6. [DOI] [PubMed] [Google Scholar]
- 5.Kyrgios I, Galli-Tsinopoulou A, Stylianou C, Papakonstantinou E, Arvanitidou M, Haidich A-B. Elevated circulating levels of the serum acute-phase protein YKL-40 (chitinase 3-like protein 1) are a marker of obesity and insulin resistance in prepubertal children. Metabolism 2012; 61: 562–8. [DOI] [PubMed] [Google Scholar]
- 6.El-Mesallamy HO, Mostafa AM, Amin AI, El Demerdash E. The interplay of YKL-40 and leptin in type 2 diabetic obese patients. Diabetes Res Clin Pract 2011; 93: e113–e6. [DOI] [PubMed] [Google Scholar]
- 7.Huang C-J, Beasley KN, Acevedo EO, Franco RL, Jones TL, Mari DC, Shibata Y. Chitin enhances obese inflammation. ex vivo. Hum Immunol 2014; 75: 41–6. [DOI] [PubMed] [Google Scholar]
- 8.Andersen OA, Dixon MJ, Eggleston IM, van Aalten DM. Natura product family 18 chitinase inhibitors. Nat Prod Rep 2005; 22: 563–79. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Lee H-W, Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer's disease. J Neurol 2011; 258: 2181–5. [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi E. Chitinase 3–like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006; 130: 398–411. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen AR, Erikstrup C, Johansen JS, Fischer CP, Plomgaard P, Krogh-Madsen R, Taudorf S, Lindegaard B, Pedersen BK. Plasma YKL-40 A BMI-independent marker of type 2 diabetes. Diabetes 2008; 57: 3078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vind I, Johansen J, Price P, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol 2003; 38: 599–605. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbrüchel DA, Friis T, Bindslev L, Haack-Sorensen M, Jorgensen E, Kastrup J. YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J 2008; 42: 295–302. [DOI] [PubMed] [Google Scholar]
- 14.Hempen M, Kopp H-P, Elhenicky M, Höbaus C, Brix J-M, Koppensteiner R, Schernthaner G, Schernthaner GH. YKL-40 is elevated in morbidly obese patients and declines after weight loss. Obes Surg 2009; 19: 1557–63. [DOI] [PubMed] [Google Scholar]
- 15.Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Increased circulating and visceral adipose tissue expression levels of YKL-40 in obesity-associated type 2 diabetes are related to inflammation: impact of conventional weight loss and gastric bypass. J Clin Endocrinol Metab 2011; 96: 200–9. [DOI] [PubMed] [Google Scholar]
- 16.de Souza Batista CM, Yang R-Z, Lee M-J, Glynn NM, Yu D-Z, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007; 56: 1655–61. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji S, Yamashita M, Hoffman DR, Nishiyama A, Shinohara T, Ohtsu T, Shibata Y. Capture of heat-killed Mycobacterium bovis bacillus Calmette-Guérin by intelectin-1 deposited on cell surfaces. Glycobiology 2009; 19: 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voehringer D, Stanley SA, Cox JS, Completo GC, Lowary TL, Locksley RM. Nippostrongylus brasiliensis: identification of intelectin−1 and −2 as Stat6-dependent genes expressed in lung and intestine during infection. Exp Parasitol 2007; 116: 458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-a-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol 2012; 686: 116–23. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Navarrete JM, Catalán V, Ortega F, Gómez-Ambrosi J, Ricart W, Frühbeck G, Fernandez-Real JM. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamer M, Steptoe A. Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosom Med 2007; 69: 660–6. [DOI] [PubMed] [Google Scholar]
- 22.Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L. InCHIANTI Investigators. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2005; 60: 760–7. [DOI] [PubMed] [Google Scholar]
- 23.McAnulty LS, Nieman DC, Dumke CL, Shooter LA, Henson DA, Utter AC, Milne G, McAnulty SR. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab 2011; 36: 976–84. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen T, Bruun JM, Paulsen SK, Ølholm J, Overgaard K, Pedersen SB, Richelsen B. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur J Appl Physiol 2013; 113: 1635–42. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab 2014; 25: 348–55. [DOI] [PubMed] [Google Scholar]
- 26.Yang R-Z, Lee M-J, Hu H, Pray J, Wu H-B, Hansen BC, Shuldiner AR, Friend SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 2006; 290: E1253–E61. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans, 2008. Available at: http://health.gov/paguidelines/pdf/paguide.pdf.
- 28.Mor G, Cardenas I. Review Article: the immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010; 63: 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9. [DOI] [PubMed] [Google Scholar]
- 30.Libreros S, Garcia-Areas R, Shibata Y, Carrio R, Torroella-Kouri M, Iragavarapu-Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer 2012; 131: 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Létuvé S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol 2008; 181: 5167–73. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen AR, Plomgaard P, Krabbe KS, Johansen JS, Pedersen BK. IL-6, but not TNF-α, increases plasma YKL-40 in human subjects. Cytokine 2011; 55: 152–5. [DOI] [PubMed] [Google Scholar]
- 33.Görgens S, Eckardt K, Elsen M, Tennagels N, Eckel J. Chitinase-3-like protein 1 protects skeletal muscle from TNFalpha-induced inflammation and insulin resistance. Biochem J 2014; 459: 479–88. [DOI] [PubMed] [Google Scholar]
- 34.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun 2011; 408: 339–43. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti L, Leone S, Orlando G, Ferrante C, Recinella L, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A, Vacca M. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol Rep 2014; 66: 991–5. [DOI] [PubMed] [Google Scholar]
- 36.Saremi A, Asghari M, Ghorbani A. Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci 2010; 28: 993–8. [DOI] [PubMed] [Google Scholar]
- 37.Johansen JS, Lottenburger T, Nielsen HJ, Jensen JEB, Svendsen MN, Kollerup G, Christensen IJ. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev 2008; 17: 2603–8. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L, Weng M, Qi M, Qi T, Tong L, Hou X, Tong Q. Aberrant expression of intelectin-1 in gastric cancer: its relationship with clinicopathological features and prognosis. J Cancer Res Clin Oncol 2012; 138: 163–72. [DOI] [PubMed] [Google Scholar]