Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal disease and considered as a cancer-like disease. The phosphatase and tensin homologue deleted on chromosome 10 (PTEN) tumor suppressor has drawn attention in the pathogenesis of IPF. However, the role of PTEN in phenotypic transformation of lung fibroblasts, particularly in the migratory and invasive phenotype, is still elusive. Our data showed that PTEN expression was markedly reduced in both fibroblasts and myofibroblasts from IPF patients. Furthermore, loss of PTEN led to the transformation of normal fibroblasts to myofibroblasts and increased proliferation, apoptosis resistance, and migration/invasion activities. PTEN deficiency upregulated hyaluronan synthase 2 expression and thereby enhanced the invasion ability of fibroblasts. Cross-talk between PTEN and the transforming growth factor β1 (TGF-β1) pathway and PTEN reduction by hypoxia were observed. These findings suggest that PTEN is implicated in multiple pathways and plays a crucial role in the pathogenesis of IPF.
Keywords: Fibroblasts, idiopathic pulmonary fibrosis, phenotype transformation, phosphatase and tensin homologue deleted on chromosome 10
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most severe chronic form of pulmonary fibrosis with unknown etiology and limited effective therapies. The median survival time of IPF patients is 2–3 years from diagnosis with a five-year mortality rate of 30–50%, similar to many cancers.1,2 The pathogenic mechanisms of IPF are still undefined. However, it is generally accepted that IPF histology arises from repetitive alveolar epithelial micro-injuries, resulting in aberrant wound healing and ultimately leading to formation of scar tissue and severe distortion of pulmonary architecture.3 Fibroblastic foci (FF) are characterized by the subepithelial accumulation of highly proliferative myofibroblasts with excessive deposition of fibrillar collagen, and accumulate at the sites of recent alveolar injury.4 FF are the histopathological hallmark of IPF, indicating current ongoing disease. The numbers of FF are significantly correlated with the mortality of IPF patients.4,5
Myofibroblasts, which express α-smooth muscle actin (α-SMA) and have contractile properties, are the main component cells in FF and the key effector cells in tissue remodeling and fibrosis.6,7 Previous studies have shown that lung resident fibroblasts comprise a major proportion of the myofibroblast population in FF.7 Fibroblasts isolated from IPF patients have distinct properties, including enhanced proliferative, apoptosis-resistant, migratory, and invasive capacities, analogous to malignant cancer cells.8–10 In particular, the ability of fibroblasts to invade extracellular matrix (ECM) promotes the progression of pulmonary fibrosis.11
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a tumor suppressor that is lost or mutated in several sporadic cancers and metabolic disorders.12 PTEN functions as a dual-specific lipid and protein phosphatase. As a lipid phosphatase, PTEN controls the phosphoinositide 3-kinase/Protein kinase B (PI3K/Akt) signal pathway through dephosphorylation of the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3).13,14 As a protein phosphatase, PTEN directly binds and dephosphorylates focal adhesion kinase (FAK).15 Loss of PTEN enhances the proliferation, migration, and invasion of cancer cells. Given the concept that IPF is a cancer-like disease,16 the role of PTEN in the pathogenesis of IPF has been the subject of attention. A previous study showed that PTEN expression was decreased in myofibroblasts within FF and that loss of PTEN led to transformation of fibroblasts to myofibroblasts.17 Additionally, Xia et al. demonstrated that decreased PTEN activity promoted fibroblast proliferation in polymerized collagen.18
Studies have shown that enhanced migration and invasion of fibroblasts are critical for progressive pathogenesis of IPF; however, the role of PTEN in the migratory/invasive phenotype of fibroblasts is still elusive. Therefore, in this study, we investigated the comprehensive effects of PTEN on fibroblast phenotype transformation and the pathways involved in this process.
Materials and methods
Subjects and cells
Thirteen IPF patients with usual interstitial pneumonia proven by surgical lung biopsy were included in this study. All patients were males, with a mean age of 51.08 ± 10.03 years. The diagnosis of IPF was made according to the standards accepted by ATS/ERS/JRS/ALAT.1 Three primary fibrotic fibroblast cell lines were isolated from fresh lung tissue of three IPF patients. Three primary normal fibroblast lines were isolated from histologically proven normal lung tissue from patients with primary spontaneous pneumothorax. One normal human fetal pulmonary fibroblast cell line (MRC-5) was purchased from American Type Culture Collection (ATCC, Manassas, VA). This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital of Capital Medical University, Beijing, China, and written informed consent was obtained according to institutional guidelines from all investigated subjects.
For normoxia conditions, MRC-5 cells were cultured in a humidified incubator (Thermo Scientific, Waltham, MA) at 37℃ in 95% air (21% O2) and 5% CO2. A Heracell incubator (Thermo Scientific) filled with nitrogen gas was used for hypoxia conditions (94% N2, 5% CO2 and 1% O2). Passages from five to eight of all cell lines were used in this study.
Small interfering RNA (siRNA) transfection
MRC-5 cells were seeded in six-well plates and incubated overnight. PTEN siRNA (5 µmol/L) and negative control (NC) siRNA (5 µmol/L) were separately mixed with RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. siRNA–RNAiMAX complexes were added to MRC-5 cells following two washes with serum-free medium. After 24 h, the transfection medium was replaced by culture medium for another 24 h. PTEN siRNA #1(s325), PTEN siRNA #2 (s326), and NC siRNA were all purchased from Invitrogen Silence® Select (Invitrogen). Both PTEN siRNA (PTENsi) #1 and PTEN siRNA (PTENsi) #2 had good efficiency at 24 h after siRNA transfection (Supplementary Figure 1).
RNA isolation and real-time RT-PCR analysis
RNA isolation, reverse transcription and real-time reverse transcription polymerase chain reaction (RT-PCR) analysis were performed as previously described.19 The primers were PTEN-F 5′-CGGCAGCAAATGTTTCAG-3′ and PTEN-R 5′-AACTGGCAGGTAGAAGGCAACTC-3′. β-Actin was used for normalization (β-actin-F 5′-TGCTATCCAGGCTGTGCTAT-3′ and β-actin-R AGTCCATCACGATGCCAGT-3′). The fold-change of the target genes was calculated using the 2−ΔΔCT method. Detailed information is provided in the online Supplement.
Western blot analysis
The expression of PTEN and other proteins in cell lysates was determined by Western blot assay. The primary antibodies used were human PTEN (Abcam, Cambridge, MA), Akt (Cell Signaling Technology, CST, Boston, MA), p-Akt (s473) (CST), FAK (CST), p-FAK (CST), hyaluronan synthase 2 (HAS2) (Abcam), matrix metalloproteinase (MMP)9 (Abcam), and β-actin (California Bioscience, Coachella, CA). Detailed information is provided in the online Supplement.
Immunofluorescent staining
MRC-5 cells seeded on glass slides were fixed in 90% cold ethanol, permeabilized by 0.1% Triton X100 in phosphate-buffered saline (PBS), blocked with 5% BSA, and incubated with anti-α-SMA (R&D, Minneapolis, MN) and anti-type I collagen (Abcam) overnight at 4℃. Cells were washed and incubated with Alexa Fluor® 594-conjugated goat anti-mouse/488-conjugated goat anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 50 min in the dark. Cells were washed three times, mounted with 4′,6-diamidina-2-phenylindole (DAPI)-containing mounting media (ZSGB-BIO, Beijing, China), and visualized using a confocal laser scanning microscope (Leica Microsystems, Wetzlar, Hesse, Germany).
Immunohistochemistry
Immunohistochemistry analysis was performed using formalin-fixed, paraffin-embedded lung specimens. All slides were scanned using the Aperio ScanScope®AT (Leica, Nussloch, Germany). Staining of proteins was analyzed using Aperio software.20 Detailed information is provided in the online Supplement.
Bromodeoxyuridine (BrdU) incorporation assay
MRC-5 cells were seeded on glass slides in 24-well plates and transfected with PTEN siRNA or NC siRNA for 24 h without fetal bovine serum (FBS), followed by incubation with minimum essential medium-α (MEM-α) containing 10% FBS and antibodies for another 24 h. BrdU incorporation was measured using a BrdU labeling and detection kit (Roche, Mannheim, Germany) in accordance with the manufacturer’s protocol. Detailed information is provided in the online Supplement.
H2O2 treatment and apoptosis assay
MRC-5 cells were seeded in six-well plates and transfected with PTEN siRNA or NC siRNA for 24 h without FBS, and then exposed to 100 µmol/L H2O2 for 4 h. At 24 h after H2O2 exposure,21 cells were harvested using trypsin-EDTA solution and assessed using an Annexin V/fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences, San Diego, CA) as instructed. Detailed information is provided in the online Supplement.
Migration/invasion assay
Migration assays were performed using 8 -µm pore Transwell chambers (Millipore, Billerica, MA). In invasion assays, Transwell chambers with 8 -µm pores were coated with 30 µL of Matrigel (BD Biosciences) gel mixture (1:5 in MEM-α containing 0.1% FBS), and allowed to solidify for 1 h at 37℃. Cells were transfected with PTEN siRNA or NC siRNA for 24 h prior to detachment with 0.125% trypsin (HyClone, Logan, UT). A total of 2 × 104 cells were resuspended in MEM-α media containing 0.1% FBS and added to the upper chamber. MEM-α media containing 10% FBS was added to the lower chamber. Cell viability was assessed by staining with crystal violet and microscope evaluation.
Microarray assay
At 48 h after transfection, total RNA from PTEN siRNA- and NC siRNA-transfected human lung fibroblasts was isolated with TRIzol reagent (Invitrogen) and purified using the Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. cDNA was hybridized to HTA 2.0 (GeneChip® Human Transcriptome Array 2.0, Affymetrix, Santa Clara, CA) according to the User Manual. Data analysis was performed with Affymetrix® Expression Console Software (version 1.2.1).
ELISA assay
Equal numbers of MRC-5 cells were seeded on glass slides in 24-well plates and transfected with PTEN siRNA or NC siRNA for 48 h without FBS. The levels of TGF-β1 in the cellular supernatant were measured with a commercial human enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen) following the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) where applicable. We assessed the differences in measured variables using the unpaired/paired two-sided Student’s t-test or Mann–Whitney test for nonparametric data. P < 0.05 was considered statistically significant. SPSS 16.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5.0 (Graphpad Software, La Jolla, CA) were used for statistical analysis and graph creation.
Results
Loss of PTEN expression results in multiple phenotype changes of fibroblasts in IPF
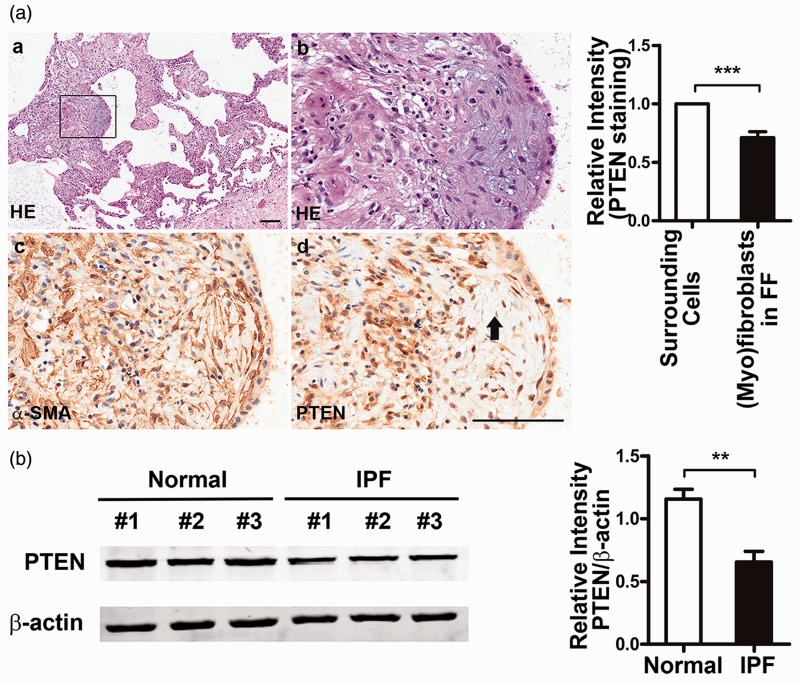
To evaluate PTEN expression in IPF, we first performed immunohistochemistry of the surgical lung biopsy specimens from 13 IPF patients with pathologic diagnosis of usual interstitial pneumonia. In 11 out of 13 IPF patients, PTEN staining was diminished or absent in myofibroblasts within FF compared with the overlying epithelial cells (Figure 1a). We then examined PTEN expression in the primary human lung fibroblasts isolated from lung tissues of three IPF patients and three normal controls. As shown in Figure 1 (b), much less PTEN expression was seen in the primary IPF lung fibroblasts compared with normal fibroblasts. These data demonstrated that PTEN was downregulated in lung FF and fibroblasts of IPF patients.
Figure 1.
Decreased phosphatase and tensin homolog deleted on chromosome 10 (PTEN) expression in idiopathic pulmonary fibrosis (IPF) lung tissues and primary lung fibroblast cell lines. (a) PTEN expression was decreased in myofibroblasts within fibroblastic foci. Representative serial lung sections from one patient with IPF were stained for (a, b) hematoxylin and eosin (HE), (c) α-smooth muscle actin (SMA), and (d) PTEN. Myofibroblasts were identified by strong α-SMA staining. Fibrotic focus is indicated with a black arrow. Scale bar, 100 µm. Relative intensity of cytoplasmic PTEN in myofibroblasts within fibroblastic focus compared with the surrounding cells. Quantitative analysis was performed by ScanScope®AT. (b) The expression of PTEN was decreased in primary fibroblast cell lines of IPF patients. Protein levels were assessed by Western blot. Representative patients (n = 3) and controls (n = 3) are shown. Relative intensity analysis of PTEN expression was measured by Prism 5. β-Actin was used as a loading control. Relative differences were assessed by fold-change of target protein/β-actin compared with normal control #1. Data are representative of at least three independent experiments. Error bars indicate mean ± SEM. **P < 0.01, ***P < 0.001
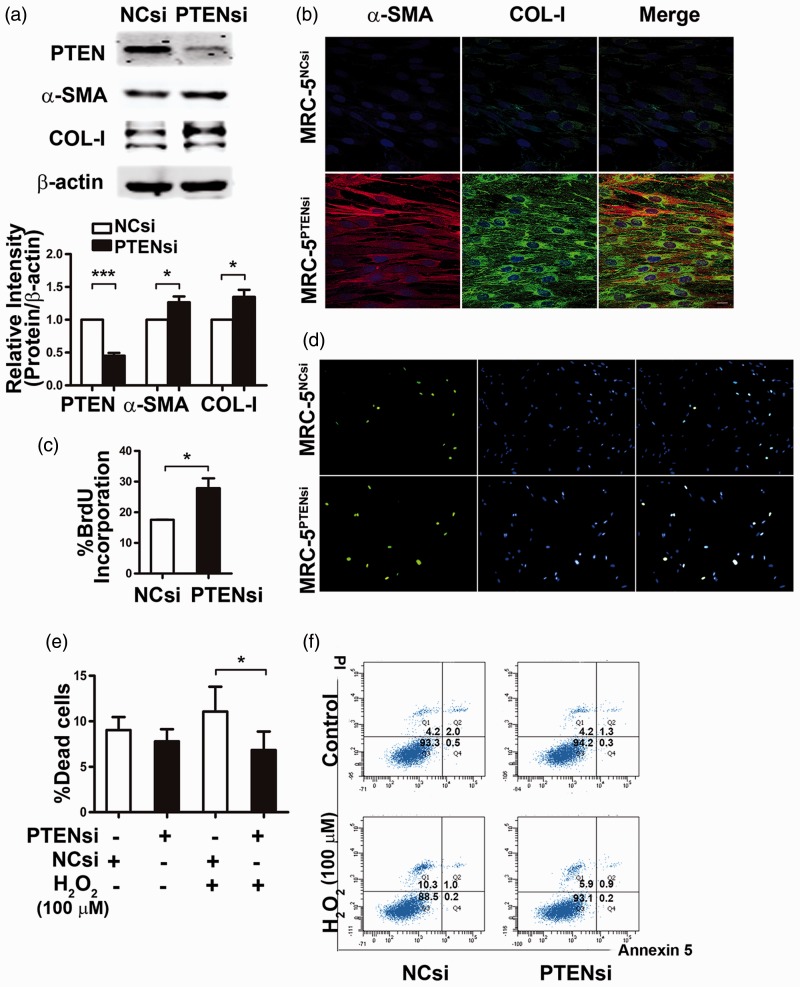
Next, we knocked down PTEN expression in normal lung fibroblasts (MRC-5 cells) using siRNA interference. We used two different siRNAs against PTEN and confirmed their ability to knock down PTEN expression (Supplementary Figure 1). We then evaluated the effects of diminished PTEN expression on myofibroblast transformation. MRC-5 cells were transfected with PTEN siRNA (MRC-5PTENsi cells) or NC siRNA (MRC-5NCsi cells) for 48 h. As shown in Figure 2(a), α-SMA expression was markedly higher in MRC-5PTENsi cells than that in MRC-5NCsi cells. MRC-5PTENsi cells also produced more type I collagen. The confocal immunofluorescence staining results (shown in Figure 2b) were consistent with these data.
Figure 2.
Loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) expression results in phenotype transformation of fibroblasts. Compared with negative control (NCsi), knock down of PTEN by siRNA (PTENsi) led to fibroblast transformation to myofibroblasts with enhanced α-smooth muscle actin (α-SMA) and type I collagen (COL-I) expression by (a) Western blot and (b) immunofluorescence staining. α-SMA (red) and type I collagen (green) were evaluated as indicated. Nuclei were visualized by 4′, 6-diamidina-2-phenylindole (DAPI) (blue) staining. (c, d) PTEN siRNA-transfected MRC-5 cells (MRC-5PTENsi) were more proliferative than negative control (NC) siRNA-transfected cells (MRC-5NCsi), as assessed by 5-bromo-2′-deoxyuridine (BrdU) incorporation assay. (e, f) MRC-5PTENsi showed enhanced apoptosis resistance compared with MRC-5NCsi. Percentage of the dead cells (Q1 + Q2 + Q4) from three different experiments is presented. Error bars indicate mean ± SEM. *P < 0.05, ***P < 0.001
We then examined the influence of PTEN deficiency on the proliferative and apoptosis-resistant capacities of fibroblasts. BrdU incorporation assay was used to measure fibroblast proliferation. As shown in Figure 2(c) and (d), MRC-5PTENsi cells displayed an increased proliferative capacity compared with MRC-5NCsi cells. To examine the effects of PTEN on the apoptosis response of fibroblasts, we treated MRC-5 cells with PTEN siRNA or NC siRNA for 24 h without FBS, followed by 100 µmol/L H2O2 for 4 h. PTEN deficiency led to fibroblasts with significant apoptosis resistance and decreased cell death rate (Figure 2e and f).
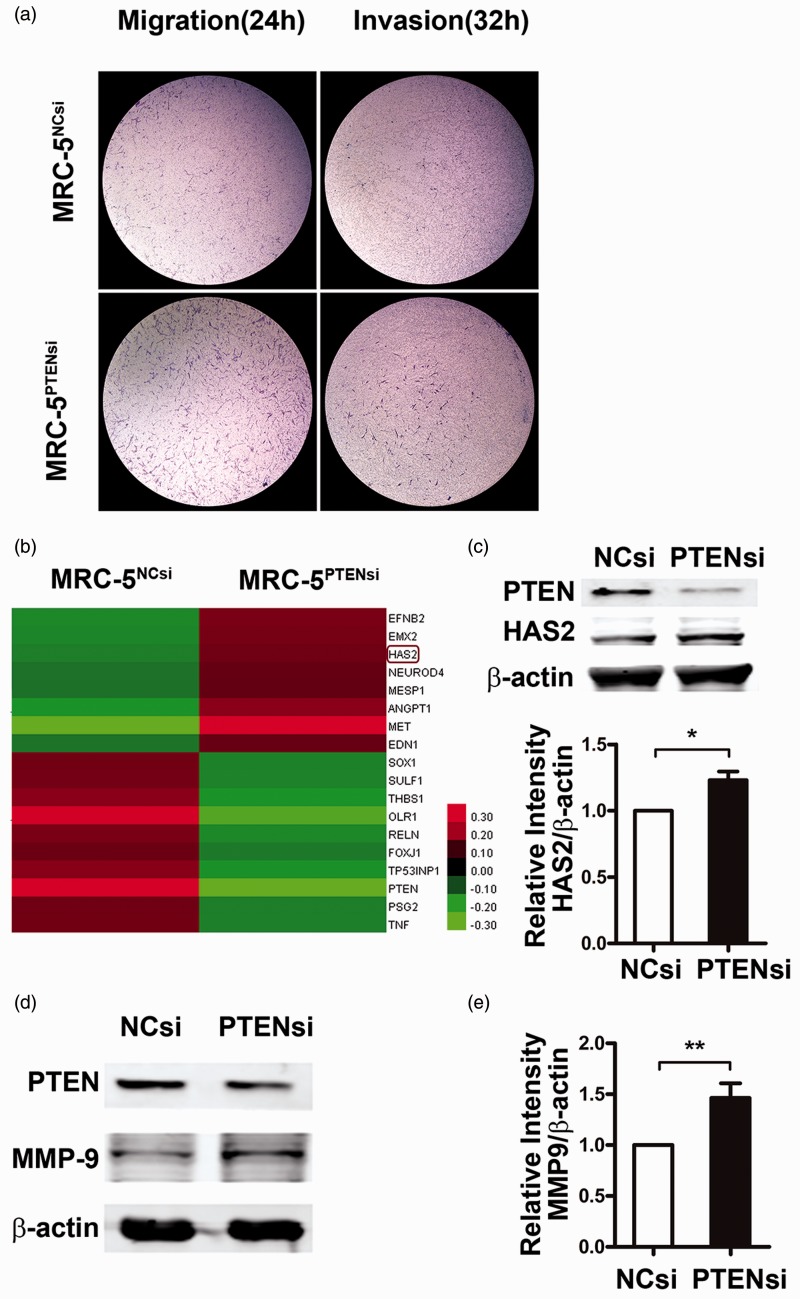
We further assessed whether loss of PTEN expression led to a migratory and invasive phenotype in fibroblasts. After transfection with PTEN siRNA or NC siRNA for 24 h, equal numbers of fibroblasts were loaded into a transwell chamber with or without Matrigel. As shown in Figure 4(a), in PTEN siRNA-transfected cells, the number of cells that migrated through the filter with or without Matrigel was significantly higher than the NC siRNA group.
Figure 4.
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) deficiency enhances invasion of fibroblasts via upregulation of hyaluronan synthase 2 (HAS2) expression. (a) Twenty-four hours after transfection with either PTEN siRNA or NC siRNA, equal numbers of fibroblasts were loaded into transwell chambers without/with Matrigel. Migratory fibroblasts (left panel) and invasive fibroblasts (right panel) were examined by crystal violet staining assay at indicated time points. Data are representative of at least three independent experiments. (b) RNA was extracted from MRC-5PTENsi cells and MRC-5NCsi cells, and migration- and invasion-related gene expression profiling are shown. (c, d) Inhibition of PTEN increased the expression of HAS2 and matrix metalloproteinase (MMP)9. MRC-5 cells were transfected with PTEN siRNA or negative control (NC) siRNA for 48 h, and the protein expression of HAS2 and MMP9 were measured by Western blot analysis and densitometric quantification. β-Actin was used as a loading control. Data are representative of at least three independent experiments. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01
PTEN depletion leads to fibroblast phenotypic changes independent of FAK phosphorylation
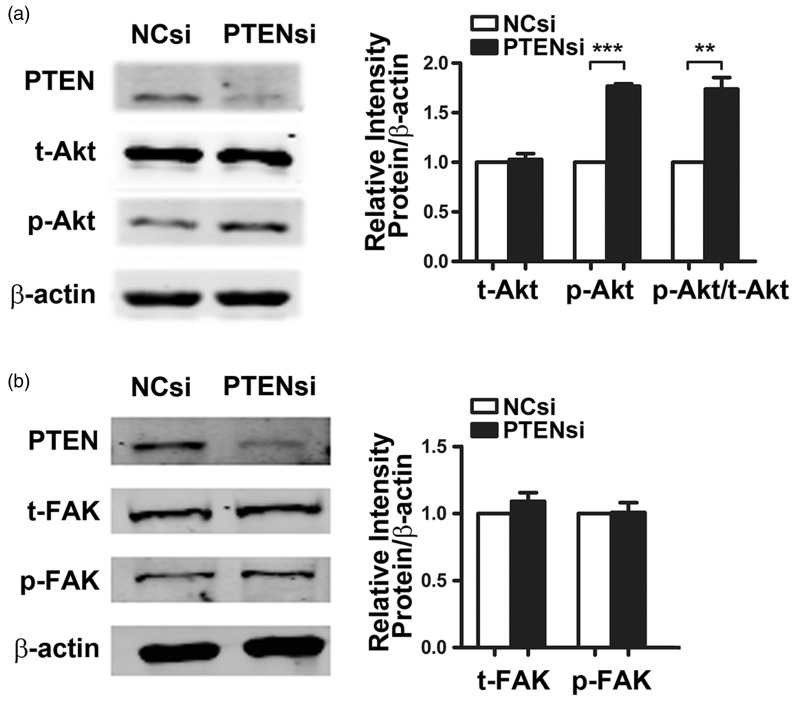
To investigate the aberrant regulation induced by PTEN deficiency and responsible for fibroblast phenotypic changes, we first assessed the phosphorylation status of FAK, a known target of PTEN and a key molecule implicated in integrin-mediated cell proliferation, survival, and motility. As shown in Figure 3(a), knock down of PTEN had a negligible effect on the phosphorylation of tyrosine residue 397 (p-FAKTyr397) in MRC-5 cells. We then testified the phosphorylation status of Akt, another well-known target of PTEN responsible for cell proliferation, apoptosis, and growth factor-mediated cell migration. As shown in Figure 3(b), inhibition of PTEN by siRNA transfection led to a notable increased phosphorylation of serine 473 in Akt (p-AktSer473) and a dramatically elevated ratio of p-AktSer473/Akt. These data suggest that the activated PI3K/Akt signaling pathway may account for the increased proliferation and apoptosis resistance in PTEN-deficient fibroblasts.
Figure 3.
Depletion of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) increases protein kinase B (Akt) phosphorylation but has no effects on focal adhesion kinase (FAK) phosphorylation in fibroblasts. MRC-5 cells were transfected with PTEN siRNA or negative control (NC) siRNA for 48 h, and (a) total Akt (t-Akt), Ser473 phosphorylated Akt (p-Akt), (b) total FAK (t-FAK), and Tyr397 phosphorylated FAK (p-FAK) were measured by Western blot. β-Actin was used as a loading control. Data are representative of at least three independent experiments. Error bars indicate mean ± SEM. **P < 0.01, ***P < 0.001
PTEN deficiency augments HAS2 and enhances invasion of fibroblasts
Considering that the migratory/invasive property of fibroblasts is essential for progressive pathogenesis of IPF, we examined whether loss of PTEN expression resulted in a more aggressive migratory/invasive phenotype than normal controls (Figure 4a). To investigate the genes implicated in this process caused by PTEN loss, we compared DNA expression profiles on migration/invasion between MRC-5PTENsi cells and MRC-5NCsi cells and identified 18 differently expressed genes (P < 0.05) (Figure 4b). In addition to genes involved in PI3K/Akt pathways, such as MET and ANGPT1, we also identified HAS2. HAS promotes fibroblast invasion through the enhanced production of MMP.11 To further confirm our findings, we performed Western blot analysis and found that PTEN reduction by siRNA transfection led to an obvious increase in the expression levels of HAS2 and MMP9 (Figure 4c).
PTEN inhibition promotes TGF-β1 production and secretion
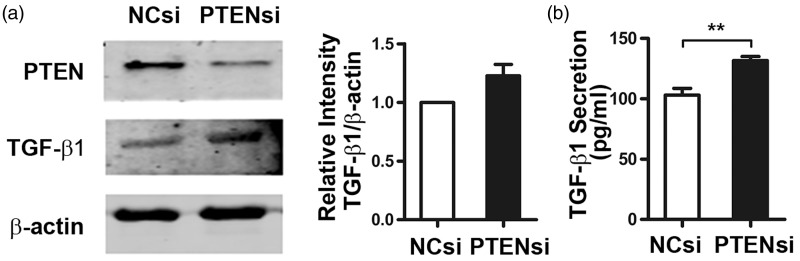
TGF-β1 is a central mediator of fibrotic response, and therefore we examined whether knock down of PTEN affected TGF-β1 production and secretion. MRC-5 cells were transfected with PTEN siRNA or NC siRNA and cultured in medium without FBS for 48 h. TGF-β1 protein expression and TGF-β1 concentration in the cellular culture supernatant were assessed (Figure 5a and b). Western blot and ELISA results showed that both TGF-β1 expression and secretion were increased by PTEN inhibition.
Figure 5.
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) reduction increases the production and secretion of transforming growth factor β1 (TGF-β1). MRC-5 cells were transfected with PTEN siRNA or negative control (NC) siRNA for 48 h in serum-free medium. (a) Western blot analysis was used to detect alterations in TGF-β1 protein levels, and (b) the concentration of TGF-β1 in the MRC-5 culture supernatant was evaluated by ELISA. Error bars indicate mean ± SEM. **P < 0.01
Hypoxia downregulates PTEN expression in fibroblast cells
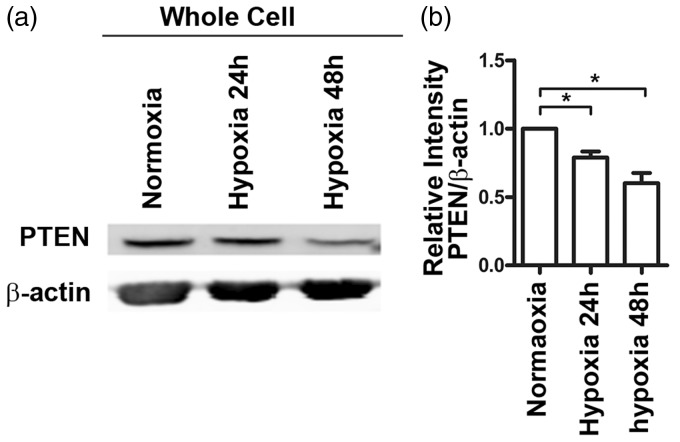
Hypoxia has been recognized as one of the significant microenvironmental factors in tissue injury, normal wound healing, and fibrosis.22 To explore the relationship between hypoxia and decreased PTEN expression in IPF pathogenesis, we cultured MRC-5 cells in normoxic (21% O2) or hypoxic (1% O2) conditions for 48 h. As shown in Figure 6, PTEN expression was markedly reduced under hypoxia conditions.
Figure 6.
Hypoxia induces an obvious downregulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN). MRC-5 cells were exposed to normoxic (21% O2) or hypoxic (1% O2) conditions for 24 h and 48 h. Western blot analysis showed that PTEN protein levels were markedly reduced in MRC-5 cells under hypoxic conditions for 48 h. Data are representative of at least three independent experiments. Error bars indicate mean ± SEM. *P < 0.05
Discussion
IPF is characterized by fibroblast accumulation and activation with unremitting matrix deposition in the distal airspaces of the lung. Phenotype transformation of fibroblasts is a crucial event in the pathogenesis of IPF.9 Consistent with previous reports, our data showed that PTEN expression was decreased in FF of lung tissues from 11 IPF patients and 3 primary IPF fibroblast lines. Knock down of PTEN led to fibroblasts transformation to myofibroblasts and enhanced proliferative, apoptosis-resistant, and migratory/invasive properties, which are all characteristics of IPF fibroblasts. These data suggest that PTEN, as a crucial regulator of fibroblast phenotype transformation, is involved in multiple pathways in the pathogenesis of IPF.
PTEN directly interacts with and dephosphorylates FAK. FAK functions as an integrin-associated signaling modulator that is upstream of PI3K/Akt signaling and regulates various cellular processes including adhesion, migration, proliferation, and cytoskeletal organization.23 Upregulation of FAK activity protected fibroblasts from contraction-induced apoptosis by activating the PI3K/Akt pathway.24 Interestingly, we found that PTEN deficiency resulted in the activation of Akt, while FAK dephosphorylation at Try397 in fibroblasts was not affected, suggesting that activation of PI3K/Akt signaling pathway is not dependent on FAK phosphorylation in PTEN-deficient fibroblasts.
Our data demonstrated that in the absence of PTEN, fibroblasts displayed an invasive phenotype. Liliental et al. reported that the migration of PTEN−/− cells was promoted and enhanced motility was due to increased cortical actin polymerization.25 Moreover, our data demonstrate that PTEN deficiency significantly augmented HAS2 expression and upregulated the expression of MMP9, and thereby enhanced invasive capacity.
Upregulation of TGF-β gene expression in human lung fibroblasts is an important determinant of phenotype transformation during the repair process.26 White et al. showed inhibition of PTEN was necessary for TGF-β induced α-SMA expression.17 Additionally, a previous study showed that PTEN was rapidly repressed by TGF-β1.27 Our data showed that loss of PTEN expression potentiates the generation and secretion of TGF-β1 from fibroblasts. Taken together, these data suggest a positive feedback loop between TGF-β1 upregulation and PTEN downregulation during the phenotype transformation of fibroblasts in IPF. Together, increased TGF-β1 production and decreased PTEN expression contribute to excess ECM production and fibrogenic cytokine secretion by fibroblasts/myofibroblasts, promoting fibroblast proliferation and migration, and thereby aggravating and perpetuating the fibrotic response.
Hypoxia is being recognized as a prominent microenvironment factor in various lung pathological processes, including pulmonary fibrosis.28 Previous studies showed that under hypoxic stimulus, fibroblasts undergo a phenotype switch to myofibroblasts and show enhanced proliferation.29,30 Our study shows that hypoxia induced an obvious decrease in the expression of PTEN, implying that PTEN may be implicated in the mechanisms of hypoxia-induced phenotype transformation of fibroblasts.
In conclusion, our study illustrates that loss of PTEN leads to fibroblast transformation to an aggressive phenotype that shows increased proliferation, migration/invasion, and apoptosis-resistance activities, and ultimately transform to myofibroblasts. In this complex process, PTEN interacts with multiple pathways, which strongly supports that downregulation of PTEN in fibroblasts is a key event in the pathogenesis of IPF.
ACKNOWLEDGMENT
We would like to thank Dr Jinbai Miao and Dr Qirui Chen, Department of Thoracic Surgery, Beijing Chao-Yang Hospital for their kind supply of the lung tissue specimens from informed patients; Dr Mulan Jin and Dr Ping Wei for their helpful comments on the pathologic diagnosis of IPF; and Weiping Tang and Hanqian Xu for excellent technical assistance.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the General Program of National Natural Science Foundation of China (No.81270123), the Key Program of National Natural Science Foundation of China (No. 81430001), and the Key Program of Beijing Natural Science Foundation of China (No. 7131008).
Authors’ contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript; JG designed and performed the experiments, analyzed and interpreted the data, and wrote the manuscript; YL and XX contributed to the design of the study and revision of the article; SL, DYJ, and ZL participated in the experiments; and HD contributed to the conception and design of the study, the revision of the manuscript and the final approval of the version to be published.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai M, Zhu M, Ban C, Su J, Ye Q, Liu Y, Zhao W, Wang C, Dai H. Clinical features and outcomes of 210 patients with idiopathic pulmonary fibrosis. Chin Med J (Engl) 2014; 127: 1868–73. [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A, American Thoracic S, European Respiratory S, American College of Chest P. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001; 134: 136–51. [DOI] [PubMed] [Google Scholar]
- 4.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001; 345: 517–25. [DOI] [PubMed] [Google Scholar]
- 5.Tiitto L, Bloigu R, Heiskanen U, Paakko P, Kinnula VL, Kaarteenaho-Wiik R. Relationship between histopathological features and the course of idiopathic pulmonary fibrosis/usual interstitial pneumonia. Thorax 2006; 61: 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol 2013; 8: 241–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet 2012; 380: 680–8. [DOI] [PubMed] [Google Scholar]
- 8.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 2001; 24: 591–8. [DOI] [PubMed] [Google Scholar]
- 9.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 2003; 201: 343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suganuma H, Sato A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 1995; 50: 984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 2011; 208: 1459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 2012; 13: 283–96. [DOI] [PubMed] [Google Scholar]
- 13.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–8. [DOI] [PubMed] [Google Scholar]
- 14.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of pkb/akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998; 95: 29–39. [DOI] [PubMed] [Google Scholar]
- 15.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 1998; 280: 1614–17. [DOI] [PubMed] [Google Scholar]
- 16.Vancheri C. Idiopathic pulmonary fibrosis: an altered fibroblast proliferation linked to cancer biology. Proc Am Thorac Soc 2012; 9: 153–7. [DOI] [PubMed] [Google Scholar]
- 17.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, Toews GB. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10). Am J Respir Crit Care Med 2006; 173: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 2008; 205: 1659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Wan X, Geng J, Li F, Yang T, Dai H. Rapamycin regulates connective tissue growth factor expression of lung epithelial cells via phosphoinositide 3-kinase. Exp Biol Med (Maywood) 2013; 238: 1082–94. [DOI] [PubMed] [Google Scholar]
- 20.Staniszewski W. Virtual microscopy, data management and image analysis in aperio scanscope system. Folia Histochem Cytobiol 2009; 47: 699–701. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto S, Tomita T, Matsui H, Ohga E, Matsuse T, Ouchi Y. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: protective roles of glutathione. Jpn J Pharmacol 1999; 79: 33–40. [DOI] [PubMed] [Google Scholar]
- 22.Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells 2014; 37: 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/akt cell survival pathway. J Biol Chem 1999; 274: 20693–703. [DOI] [PubMed] [Google Scholar]
- 24.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/akt in regulating fibroblast survival in response to contraction of type i collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 2004; 279: 33024–34. [DOI] [PubMed] [Google Scholar]
- 25.Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y, Sun H, Wu H. Genetic deletion of the PTEN tumor suppressor gene promotes cell motility by activation of RAC1 and CDC42 GTPases. Curr Biol 2000; 10: 401–4. [DOI] [PubMed] [Google Scholar]
- 26.Kelley J, Shull S, Walsh JJ, Cutroneo KR, Absher M. Auto-induction of transforming growth factor-beta in human lung fibroblasts. Am J Respir Cell Mol Biol 1993; 8: 417–24. [DOI] [PubMed] [Google Scholar]
- 27.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997; 57: 2124–9. [PubMed] [Google Scholar]
- 28.Araneda OF, Tuesta M. Lung oxidative damage by hypoxia. Oxid Med Cell Longev 2012;2012:856918. [DOI] [PMC free article] [PubMed]
- 29.Mizuno S, Bogaard HJ, Voelkel NF, Umeda Y, Kadowaki M, Ameshima S, Miyamori I, Ishizaki T. Hypoxia regulates human lung fibroblast proliferation via p53-dependent and -independent pathways. Respir Res 2009; 10: 17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol 2004; 286: C416–25. [DOI] [PubMed] [Google Scholar]