Abstract
Platelets are key players in fundamental processes of vascular biology, such as angiogenesis, tissue regeneration, and tumor metastasis. However, the underlying mechanisms remain unclear. In this study, some tumor vascular endothelial cells were positively stained by antiplatelet antibodies. Further investigation revealed that platelets were taken up by endothelial cells in vitro and in vivo. Human umbilical vascular endothelial cells were rendered apoptotic under conditions of serum deprivation. However, endothelial apoptosis was suppressed and cell viability was enhanced when platelets were added to the cultures. Endothelial survival was paralleled by an upregulation of phosphorylated Akt and p70 S6K. In conclusion, this study demonstrated that platelets can be phagocytosed by endothelial cells, and the phagocytosed platelets could suppress endothelial apoptosis and promote cell viability level. The mechanism underlying this process involves the activation of Akt signaling.
Keywords: Phagocytosis, platelet, apoptosis
Introduction
Platelets play an important role in the initial stages of blood coagulation. In addition, increasing data indicate that platelets are also involved in fundamental processes of vascular biology, such as angiogenesis, tissue regeneration, and tumor metastasis.1–4 Platelets promote new blood vessel growth via numerous stimulators of angiogenesis. For example, vascular endothelial growth factor, fibroblast growth factor, and platelet-derived growth factor can induce endothelial cell proliferation and cell migration, stimulate chemotaxis, and increase tubule formation.5–8 In chronic wounds, platelet releasate can enhance endothelial cell proliferation and promote angiogenesis.9,10 Furthermore, platelet-derived microparticles have also been reported to play an essential role in neovascularization and angiogenesis.11,12 We have known that the platelet-contained biologically active molecules can be delivered to the endothelium upon platelet adhesion to endothelial cells. On the other hand, it was reported that platelets could be internalized by endothelial cells.13–15 However, the effects of the phagocytosed platelets on endothelial cells have been unclear until recently.
In this study, the effects of freshly isolated platelets on human umbilical vascular endothelial cell (HUVEC) viability and apoptosis were investigated in vitro. The significance of platelet and endothelial cell interactions in human pancreatic ductal adenocarcinoma and in mice was also assessed. Our data showed that endothelial viability was enhanced and apoptosis was markedly suppressed due to the phagocytosis of platelets. Furthermore, HUVEC survival was associated with the activation of Akt signaling. In addition, platelet staining was observed in some tumor vascular endothelial cells. Taken together, these findings indicate new mechanisms by which platelets promote angiogenesis in tumor tissues.
Materials and methods
Cell culture and platelet isolation
This study was performed according to the Code of Ethics of the World Medical Association. The ethics committee of Beijing Hospital and Beijing Institute of Geriatrics, Ministry of Health approved the protocol, and written informed consents were obtained from the donors.
HUVECs were isolated from the human umbilical vein as previously described by Baudin et al.16 Briefly, a segment of the umbilical vein was filled with 0.1% collagenase A (Sigma-Aldrich, Inc.) in phosphate-buffered saline (PBS) for 15 min. Endothelial cells were released by mechanical disruption and resuspended in endothelial culture medium (ECM, Sciencecell, Inc.) with 10% fetal bovine serum (FBS), 1% endothelial cell growth supplement, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate. The cells were seeded in gelatin-coated glass bottom well plates (1 × 105 cells/mL) and grown in a humidified chamber containing 5% CO2 at 37℃.
Platelets were prepared according to standard methods from 3 mL blood, which was obtained from informed healthy donors and anticoagulated with 0.01 M sodium citrate. Platelet-rich plasma was prepared by centrifugation at 500 × g at room temperature for 15 min, and the platelets were subsequently pelleted by centrifugation at 2000 × g for 6 min and washed in hydroxyethyl piperazine ethanesulfonic acid (HEPES) buffer at 37℃ (0.137 M NaCl, 2.68 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, and 0.1% glucose, pH 6.8). The concentration of the platelets was adjusted to 1 × 107–108/mL in the same buffer.
Co-incubation experiments
HUVECs were cultured until 80% confluence. Platelets were added to the medium at a HUVEC:platelet ratio of 1:40. After vigorous washing of the HUVEC/platelet co-cultures to remove non-adherent/non-phagocytosed platelets, the HUVECs were harvested for further analysis.
In vitro platelet phagocytosis assay
Phagocytosis of platelets was determined by specific membrane linking of platelets with a PKH26 red fluorescent cell linker kit (Sigma-Aldrich, Inc.) according to the manufacturer’s instructions and assessed using transmission electron microscopy (TEM) and fluorescence microscopy.
In vivo experiment
To evaluate platelet phagocytosis by the endothelium in vivo, murine platelets were isolated from whole blood and labeled with PKH26. Next, PKH26-labeled platelets were injected (5 × 107 platelets) into mice via the tail vein. After 24 h, the mice were sacrificed and tissues including brain, liver, lung, and spleen were preserved at −150℃. The phagocytosed PKH26-labeled platelets were analyzed and quantified in serial sections by immunofluorescence and immunohistochemistry. The endothelial cells were confirmed by luminal staining for CD34 (GeneTex, Inc.).
All animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International approved facility, and animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Ethics Committee at the Beijing Institute of Geriatrics.
TEM
Typical morphological alterations indicative of platelet phagocytosis were evaluated using TEM. Briefly, the cells were washed off the culture plates, centrifuged, and fixed in ice-cold 1.0% glutaraldehyde in 0.1 M PBS. The cells were then postfixed in 1% osmium tetroxide, dehydrated in a graded alcohol series, embedded in Epon 812, sectioned using an ultramicrotome, and stained with uranyl acetate and lead citrate. The sections were then examined using a TEM (JEOL-1230, Japan).
Immunofluorescence
Briefly, platelets were resuspended in cell diluents and mixed with PKH26 dye in equal volumes at room temperature for 5 min. The staining reaction was blocked by the addition of 1 mL of 10% FBS. After centrifugation of platelets at 2000 × g for 10 min, the cells were extensively washed and resuspended in HEPES buffer. PKH26-labeled platelets were co-cultured with primary HUVECs for the indicated time. Next, the cells were washed three times with PBS, fixed with 4% paraformaldehyde–PBS solution and examined using fluorescence microscopy. The number of cells with internalized PKH26 platelets was quantified, and the phagocytosis index was defined as the number of platelets ingested per 100 cells. Data are expressed as the average of three independent experiments.
Immunohistochemistry
Immunohistochemistry was performed on thick sections. Slides were deparaffinized in xylene and rehydrated through graded alcohol solutions. The endogenous peroxidase activity was quenched by incubation in methanol containing 3% H2O2 for 10 min. After several washes in PBS, normal horse serum was applied for 30 min to block non-specific antibody binding, and sections were subsequently incubated with goat polyclonal CD42b (Santa Cruz Biotechnology, Inc.), mouse monoclonal platelet IIb/IIIa (Santa Cruz Biotechnology, Inc.), or rat monoclonal CD34 primary antibodies overnight at 4℃. For the secondary antibody, mouse anti-goat or goat anti-rat/mouse antibodies (Dako, Diagnostics (Shanghai) Co.) were incubated with the tissue for 30 min at room temperature. After several washes in PBS, the slides were developed in freshly prepared diaminobenzedine solution and then counterstained with hematoxylin, dehydrated, and mounted.
Tissue specimens
Ten specimens of pancreatic ductal adenocarcinomas coming from patients treated by a Whipple procedure at the Surgical Oncology Department of Beijing Hospital were selected. None of the patients had accepted radiotherapy before operation. Tumor tissues of all cases were fixed in 4% formaldehyde solution (pH 7.0) for about 24 h and then processed routinely for paraffin embedding. Four-µm-thick sections were cut and stained immunohistochemically.
Detection of cell viability and apoptosis
To estimate cell viability, cells were cultured in 96-well plates. Next, 15 µL of MTT 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide solution was added into each well and incubated for 4 h at 37℃. After removal of the medium, 100 µL of Dimethyl Sulphoxide (DMSO) was added to each well. After shaking, cell viability was determined by AD570 nm, and the results are expressed as the ratio of cell viability relative to the untreated control. The results were determined by three independent experiments.17
Cellular apoptosis was determined by Hoechst 33342 staining and immunoblotting of cleaved caspase3 (Cell signaling, Inc.). The detailed procedure was performed according to the manufacturer’s instructions.
Analysis of western blotting bands
The intensity of western blotting bands was analyzed by using software Image-Pro Plus 6.0.
Statistical analyses
Data are shown as the mean ± standard deviation. The non-parametric test was used to assess the differences of cell viability and western blotting. The differences among multiple groups were evaluated by Kruskal–Wallis test. Statistical analysis between two groups was performed using Mann–Whitney test. For the analysis of apoptotic differences in each group Chi-Square test was used. P < 0.05 was considered to be statistically significant. All data were analyzed with SPSS statistics software (Version 13.0, Chicago, IL, USA).
Results
Platelet–endothelial cell interactions are increased in tumor tissues
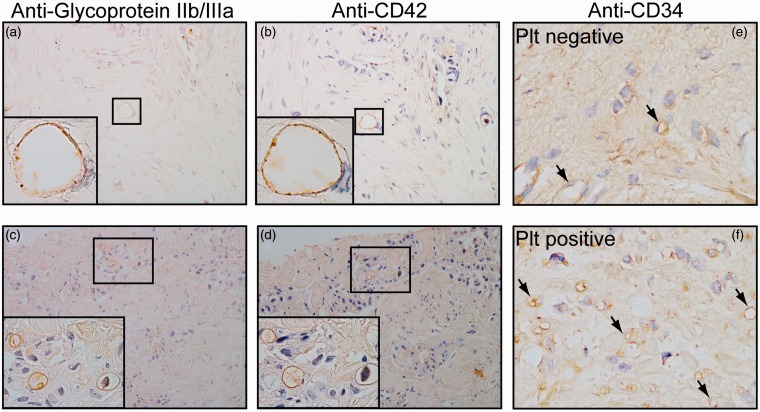
In four out of 10 pancreatic carcinoma specimens, the platelets were attached to some vascular endothelial cells in tumor tissues. To confirm that the positive staining was specific to platelets, two types of antiplatelet antibodies, antiglycoprotein IIb/IIIa and anti-CD42b, were used. The endothelium, which was stained by both antibodies, was considered positive for platelets. Platelet–endothelial cell interactions in both the middle and small vascular tissues are shown in Figure 1(a) to (d). In the remaining six cases, no platelets were observed to adhere to the vascular endothelium. The microvascular density of the 10 cases was also evaluated using the CD34 antibody. In Figure 1(e) and (f), tumors with high- and low-density microvasculature are shown as typical samples. The microvascular density was significantly higher in the tumors carrying platelet-positive vascular cells than in the tumors carrying platelet-negative vascular cells.
Figure 1.
Immunohistochemical staining for platelets and CD34 protein in pancreatic carcinoma. (a–d), Platelet–endothelial cell interactions in pancreatic carcinoma. Positive antiplatelet antibody glycoprotein IIb/IIIa staining (a, c) and antiplatelet antibody CD42b staining (b, d) were observed in intermediate (a, b) and small (c, d) vasculature (magnification × 200). (e, f) Microphotograph of pancreatic carcinoma stained using the anti-CD34 antibody. Arrows indicate microvascular. Note that the microvascular density is higher in tissues possessing platelet-positive vasculature (f) compared to tissues without platelet-positive vasculature (e) (magnification × 400). (A color version of this figure is available in the online journal.)
Platelets were phagocytosed by HUVECs
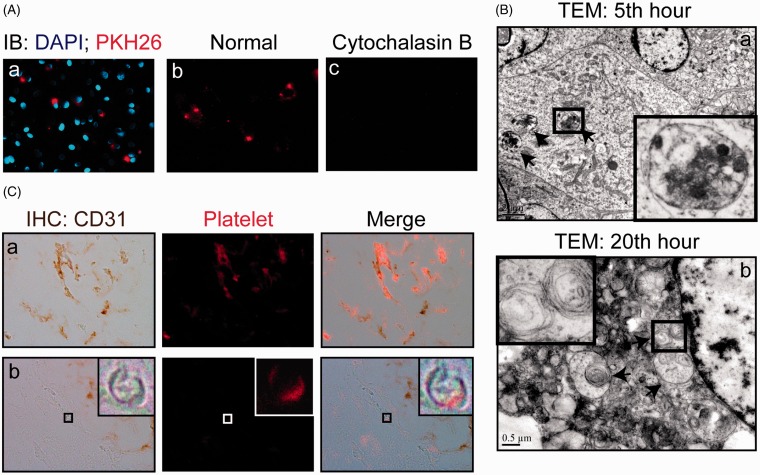
To examine the interaction between endothelial cells and platelets, platelets were prelabeled with PKH26 and cultured with HUVECs for 5 h. Fluorescence microscopy images obtained after washing showed that a significant proportion of platelets co-localized with endothelial cells (Figure 2(Aa)). When cytochalasin B (10 µg/mL), an inhibitor of phagocytosis,18 was mixed in the culture medium, the fluorescent signals dramatically decreased (Figure 2(Ac)), indicating that the number of endothelial cells with internalized platelets significantly decreased. Next, we used TEM to visualize platelet internalization. As expected, the engulfment of platelets by endothelial cells was observed. The phagocytosed platelets appeared round and were absent of membranous extensions (Figure 2(Ba)). Platelet internalization increased with incubation time. The platelet uptake level peaked at approximately the 20th hour (data not shown). TEM examination revealed myelin-like materials in the HUVECs at the 20th hour, which was indicative of platelet digestion by cells.19 (Figure 2(Bb)).
Figure 2.
Phagocytosis of platelets by HUVECs. (A) Phagocytosis of platelets by HUVECs in vitro. PKH26-labeled platelets (red) were co-cultured with 80% confluent HUVECs in ECM supplemented with 10% FBS for 20 h. Cells were fixed and stained with DAPI (a) or without DAPI (b, c) and observed using fluorescence microscopy (magnification, × 200). (B) Transmission electron microscopy (TEM) reveals the complete ingestion of platelets. HUVECs were co-incubated with platelets for 5 h (a) and 20 h (b) (40 platelets per endothelial cell) and then examined using TEM. Platelets (a) and myelin-like structure (b) were found inside of endothelial cells at 5 and 20 h (magnification, × 7100), as indicated by the arrow. (C) Accumulation of PKH26-labeled platelets in endothelial cells in vivo. Mouse platelets were labeled with PKH26 and administered intravenously via the tail vein. The mice were sacrificed 20 h later. The fluorescence-labeled platelets were visualized in endothelial cells in the liver (a) and brain (b). The endothelium was identified using CD34 immunostaining. (A color version of this figure is available in the online journal.)
Interaction of endothelial cells and platelets in vivo
Phagocytosis of platelets by endothelial cells was also examined in vivo. Fluorescence microscopy demonstrated that PKH26-labeled platelets punctuated the vascular wall of the liver and brain (Figure 2(C)). Furthermore, sections were analyzed using immunohistochemical staining. The results showed that PKH26-labeled platelets co-localized with CD34-positive endothelial cells in the liver and brain. Compared with the larger vasculature, the endothelium in smaller vasculature preferentially phagocytosed PKH26-labeled platelets (Figure 2(C)).
Phagocytosis of platelets by HUVECs enhances HUVEC viability
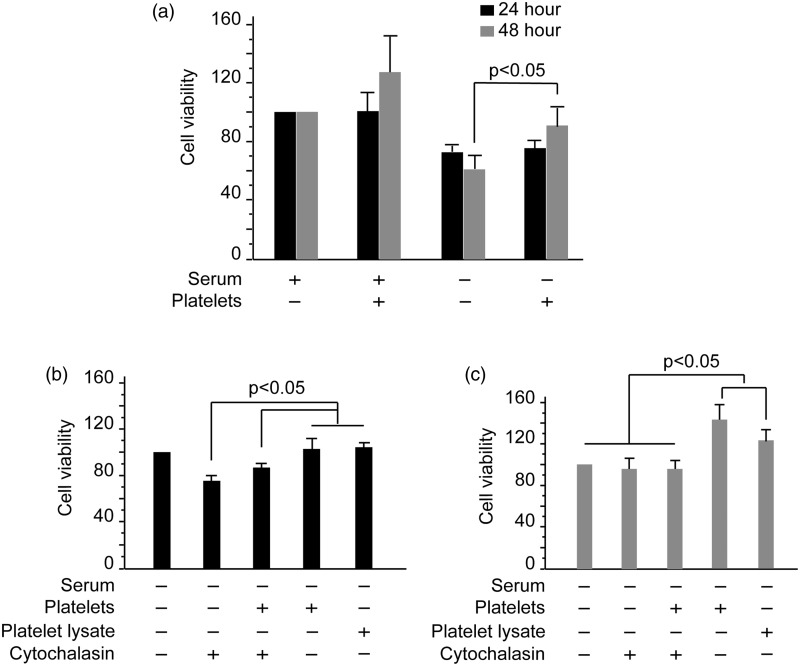
HUVECs were co-cultured with platelets in normal (containing 10% FBS) or serum-deprived ECM, and cell viability was assessed. Cell viability in the serum (+) and platelet (–) group was considered as 100%. As shown in Figure 3(a), after platelets were added to the culture, the viability of HUVECs significantly increased at 24 and 48 h, especially in the platelet (–) group. Platelets promoted the viability level of HUVECs by 25% when cultured in normal ECM, and the viability was improved by 50% in serum-deprived groups (both P < 0.05). This finding was consistent with the result that engulfment of platelets by endothelial cells was dramatically enhanced in serum-deprived medium (data not shown). In general, 5% of HUVECs were fluorescent after incubation with PKH26-labeled platelets, whereas HUVECs demonstrated a 19.8% increase in fluorescence under conditions of serum deprivation (supplemental Figure 1(a) and (b)). The effect of the platelets on endothelial viability was platelet specific, and red blood cells did not alter the viability level (data not shown). In the serum-deprived groups, cell viability induced by platelet incubation was significantly inhibited at 24 (Figure 3(b)) and 48 h in the presence of cytochalasin B (Figure 3(c)) (P < 0.05). When platelet lysate was added to the medium, the viability of HUVECs increased by 20% at 48 h. Furthermore, when cells were co-cultured with platelets, HUVEC viability increased by 50% (Figure 3(c)).
Figure 3.
Platelets promote HUVEC viability. (a) HUVECs were plated at 5 × l04 cells/mL in 96-well plates in ECM supplemented with 10% FBS and incubated for 24 h. Next, the medium was replaced with serum-deprived or 10% FBS-containing ECM and platelets were added. Cells were cultured continuously for 24 and 48 h. Cell viability was determined using the MTT assay. Error bars represent the SD value. (b, c) HUVECs were cultured in serum-deprived ECM in combination with platelets, cytochalasin B, or platelet lysates for 24 (b) and 48 h (c). Cell viability was determined using the MTT assay. Data are expressed as the mean and SD values of quadruplicate wells of three independent experiments
Phagocytosis of platelets by endothelial cells inhibited endothelial cell apoptosis
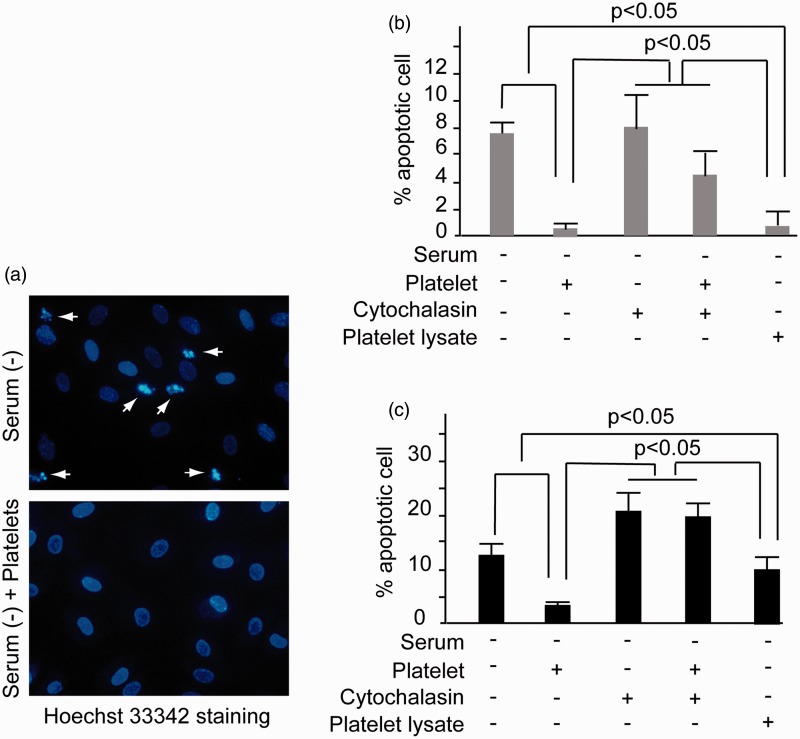
Consistent with TEM findings (data not shown) and Hoechst 33342 staining, apoptosis was induced when HUVECs were cultured in serum-deprived medium for 48 h. The ratio of apoptotic cells was quantified using Hoechst 33342 staining, which exhibited a clear apoptotic body (Figure 4(a)). When platelets or platelet lysate was added, the apoptosis levels of HUVECs at 48 h decreased by 7 and 6%, respectively (Figure 4(b)). When the co-incubation time was extended to 72 h, both platelets and platelet lysate were sufficient to induce this antiapoptosis effect (Figure 4(c)). Compared to platelet lysates, platelets induced more significant apoptotic inhibition. Platelets and platelet lysates reduced the HUVEC apoptotic level by 76 and 23%, respectively (P < 0.05) (Figure 4(c)). Moreover, administration of cytochalasin B inhibited platelet-induced apoptosis suppression at both 48 and 72 h (Figure 4(b) and (c)). To further confirm the apoptotic ratio, the level of cleaved caspase 3 was investigated by western blotting. As was shown in supplemental Figure 1(c) and (d), platelet could reduce the level of cleaved caspase3.
Figure 4.
Inhibition of HUVEC apoptosis by platelet phagocytosis. (a) HUVECs were cultured in the serum-deprived ECM in the presence or without platelet for 48 h. Then Hoechst33342 staining analysis was performed. The dense nuclear condensation and shrinkage marked by arrows indicated apoptotic cells. Magnification × 400. (b) HUVECs were cultured in serum-deprived ECM together with platelets, platelet lysate, or cytochalasin B, respectively, for 48 h. Then the apoptotic ratio of HUVECs was examined by Hoechst33342 staining analysis. (c) HUVECs were cultured in serum-deprived ECM in the presence of platelets, platelet lysate, or cytochalasin B, respectively, for 72 h. Then the apoptotic ratio of HUVECs was examined by Hoechst33342 staining analysis. Significant inhibition of apoptosis was noted in the platelet and platelet lysate incubation groups (p < 0.05). All of the experiments were performed thrice. (A color version of this figure is available in the online journal.)
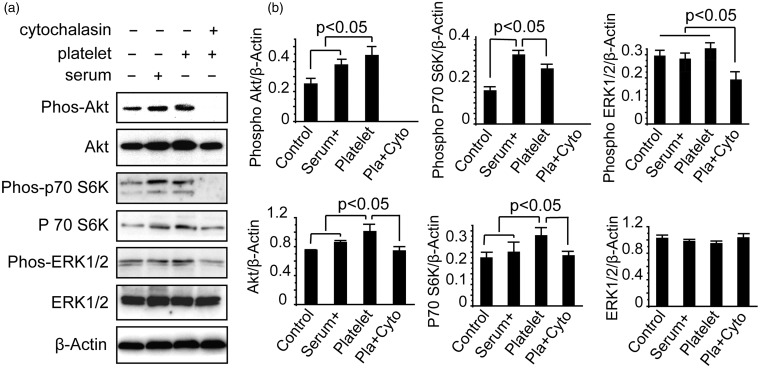
Increased levels of phosphorylated Akt and p70 S6K protect against apoptosis by platelets
Akt and ERK1/2 are essential mediators of several cellular processes, including cell proliferation and programmed cell death. Thus, we examined the expression of active Akt, the Akt substrate p70 S6K, and ERK1/2 using western blotting analyses with respect to platelet-dependent downregulation of endothelial cell apoptosis. HUVECs were treated with serum deprivation, incubation with platelets, or a mixture of cytochalasin B for 10 h. Next, the cells were harvested and analyzed using western blotting.
Inhibition of Akt signaling by serum deprivation was characterized by a reduction of activated phosphorylated Akt and phosphorylated p70 S6K, which were induced by the addition of serum or platelets (Figure 5(a) and (b)). Phosphorylation of Akt and p70 S6K increased after the addition of serum or platelets. Furthermore, activation of Akt signaling induced by platelets was completely suppressed by cytochalasin B. Our immunoblotting analysis on the proximal regulation of ERK1/2 demonstrated that ERK1/2 was not affected by serum deprivation. Moreover, phosphorylation of ERK1/2 was slightly elevated after the addition of platelets. In addition, ERK1/2 activation induced by the platelets was inhibited by cytochalasin B (Figure 5).
Figure 5.
Platelet phagocytosis enhances activation of Akt signaling. (a) Whole-cell lysates from endothelial cells were immunoblotted using antibodies that specifically recognize phosphorylated Akt, Akt, phosphorylated p70 S6K, p70 S6K, phosphorylated ERK1/2, ERK1/2, or β-actin. (b) Densitometric analysis (intensity ratio) for phosphorylated Akt, Akt, phosphorylated p70 S6K, p70 S6K, phosphorylated ERK1/2 and ERK1/2 protein (intensity compared to β-actin). Band intensity of each protein was analyzed using Pro-Plus Image 6.0 quantitative digital imaging system. The experiment was performed thrice
Discussion
Five years ago, Kuckleburg et al. found that activated bovine platelets are engulfed by bovine endothelial cells.13 In the following years, several studies have confirmed that platelets or platelet-derived vesicles are internalized by the liver or brain endothelium.14,15,20 Endothelial cells possess a strong ability to engulf a variety of cell types.21–23 Thus, it is reasonable that endothelial cells are considered “non-professional phagocytes.”23 Similar to these reports, we found that endothelial cells derived from humans can engulf human platelets. The internalization of platelets can be inhibited by cytochalasin B and intact platelets are localized in HUVECs. We propose that platelets are internalized by endothelial cells via phagocytosis.
Under physiological conditions, platelets flowing in the bloodstream show minimal interactions with endothelial cells. In contrast, an intravital microscopic study reported that platelets could roll or firmly adhere to postischemic microvascular endothelial cells during ischemia–reperfusion injury.24 In this study, we demonstrated that HUVECs cultured in serum-deprived medium phagocytose more platelets, and thus, we propose that a shortage of nutrition will result in a promotion of platelet internalization. In addition, platelet–endothelium interaction exists in some pancreatic carcinomas. Perhaps the platelet engulfment by endothelial cells was enhanced since shortage of nutrition condition is often found in tumor tissues. Further analysis is needed to confirm this hypothesis.
The effects of platelet phagocytosis on endothelial cells have been unclear until recently. It was reported that platelets and platelet microparticles could support the integrity of the endothelium.25–27 In this study, when platelets were added to cultured HUVECs, endothelium viability was enhanced and cell apoptosis was highly suppressed. Our data support the promotion of cell survival via platelet internalization. Previously, Lou et al.28 reported that platelet–endothelial adhesion and fusion resulted in an enhanced endothelium sensitivity to TNF-induced injury. Thus, further investigation is needed to clarify the effects of platelet internalization on endothelial cells.
Interestingly, platelets exhibit a more significant tendency to promote cell viability at 48 h and suppress apoptosis at 72 h compared to platelet lysates. Similar data were reported by Lang et al.,27 where they found that platelet supernatants and the contents of platelet granules were ineffective in mimicking the cytoprotective capacity of platelets. This raises the question, what components provide this extra driving force? It has been well established that platelets are a transferring module for various factors, including platelet receptors, chemokines, bioactive lipids, and messenger RNAs.29–31 These factors are essential for the phenotype of the recipient cells. The enhanced cell survival is potentially induced by some of these platelet components. Further investigations are needed to clarify these questions.
We also examined the mechanisms of HUVEC viability induced by platelets. Western blotting analysis demonstrated that platelets induced a profound activation of Akt, as well as its substrate p70 S6K. This finding was consistent with a previous report in which the phosphorylation of Akt was markedly enhanced when platelet microparticles were incorporated into endothelial cells.25 Because activation of Akt contributes to cell proliferation and survival, we proposed that Akt signaling is involved in platelet phagocytosis to promote endothelial survival and proliferation. In this study, platelet incubation induced a moderate activation of ERK1/2 at 72 h. In contrast, Ranzato et al. reported that endothelial proliferation was predominantly under the control of ERK1/2 signaling when the cells were stimulated by platelet lysates.32 Taken together, these data suggested that different components of platelets potentially promoted different signaling pathways.
In summary, our study demonstrated that HUVECs have the ability to phagocytose platelets, and platelet phagocytosis was enhanced under conditions of serum deprivation. We demonstrated that phagocytosis played an essential role in enhancing endothelial viability and attenuating cell apoptosis. Furthermore, activation of the Akt signaling pathway in the endothelium is one of the underlying mechanisms mediating endothelial viability and cell apoptosis. Moreover, the enhancement of endothelium viability triggered by platelets may be involved in tumor angiogenesis. Our data provide insights into a novel concept by which platelet internalization via phagocytosis might directly result in endothelial proliferation beyond an indirect involvement of angiogenic factors.
Ping Jiang1, Ya-Li Ren2, Yong Lan3, Jia-Liang Li4, Jun Luo1, Jian Li1 and Jian-Ping Cai1
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31371160), China Postdoctoral Science Foundation special funded project (201104043), and Scientific Research Foundation of the Education Ministry for returned Chinese scholars (jws1433).
Authors’ contribution
PJ and Y-LR equally contributed to this work. PJ and JL performed the cellular studies. PJ and Y-LR drafted the manuscript. YL and J-LL drew blood from healthy volunteers. Y-LR performed the electronic microscopic analyses. JL and J-PC conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.de Groot PG, Urbanus RT, Roest M. Platelet interaction with the vessel wall. Handb Exp Pharmacol 2012; 210: 87–110. [DOI] [PubMed] [Google Scholar]
- 2.Sopova K, Tatsidou P, Stellos K. Platelets and platelet interaction with progenitor cells in vascular homeostasis and inflammation. Curr Vasc Pharmacol 2012; 10: 555–62. [DOI] [PubMed] [Google Scholar]
- 3.Everts PA, Hoogbergen MM, Weber TA, Devilee RJ, van Monftort G, de Hingh IH. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol 2012; 13: 1163–72. [DOI] [PubMed] [Google Scholar]
- 4.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011; 9: 237–49. [DOI] [PubMed] [Google Scholar]
- 5.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. Epub ahead of print 2013. DOI: 10.1002/jcp.24539. [DOI] [PubMed]
- 6.Radziwon-Balicka A, Moncada de la Rosa C, Jurasz P. Platelet-associated angiogenesis regulating factors: a pharmacological perspective. Can J Physiol Pharmacol 2012; 90: 679–88. [DOI] [PubMed] [Google Scholar]
- 7.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22: 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). Thromb Haemost 2002; 88: 834–42. [PubMed] [Google Scholar]
- 9.Kakudo N, Morimoto N, Kushida S, Suzuki K, Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 2014; 47: 83–89. [DOI] [PubMed] [Google Scholar]
- 10.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011; 118: 1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayon Y, Dashevsky O, Shai E, Brill A, Varon D, Leker RR. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res 2012; 9: 185–92. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka M, Sasaki K, Ueno T, Seki R, Nakayoshi T, Koiwaya H, Toyama Y, Yokoyama S, Mitsutake Y, Chibana H, Itaya N, Okamura T, Imaizumi T. Platelet-derived microparticles augment the adhesion and neovascularization capacities of circulating angiogenic cells obtained from atherosclerotic patients. Atherosclerosis 2013; 227: 275–82. [DOI] [PubMed] [Google Scholar]
- 13.Kuckleburg CJ, McClenahan DJ, Czuprynski CJ. Platelet activation by Histophilus somni and its lipooligosaccharide induces endothelial cell proinflammatory responses and platelet internalization. Shock 2008; 29: 189–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Q, Yeh H, Wei L, Enjyoj K, Machaidze Z, Csizmad E, Schuetz C, Lee KM, Deng S, Robson SC, Markmann J, Buhler L. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS One 2012; 7: e47273–e47273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paris LL, Chihara RK, Sidner RA, Tector AJ, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation 2012; 19: 31–39. [DOI] [PubMed] [Google Scholar]
- 16.Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc 2007; 2: 481–5. [DOI] [PubMed] [Google Scholar]
- 17.Mao JZ, Jiang P, Cui SP, Ren YL, Zhao J, Yin XH, Enomoto A, Liu HJ, Hou L, Takahashi M, Zhang B. Girdin locates in centrosome and midbody and plays an important role in cell division. Cancer Sci 2012; 103: 1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grønlien HK, Berg T, Løvlie AM. In the polymorphic ciliate Tetrahymena vorax, the non-selective phagocytosis seen in microstomes changes to a highly selective process in macrostomes. J Exp Biol 2002; 205: 2089–97. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara T, Akizuki S, Yokota T, Takahashi M, Uchino F, Matsumoto N. Foamy cells associated with platelet phagocytosis. Am J Pathol 1984; 114: 104–11. [PMC free article] [PubMed] [Google Scholar]
- 20.Faille D, El-Assaad F, Mitchell AJ, Alessi MC, Chimini G, Fusai T, Grau GE, Combes V. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J Cell Mol Med 2012; 16: 1731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fens MH, van Wijk R, Andringa G, van Rooijen KL, Dijstelbloem HM, Rasmussen JT, de Vooght KM, Schiffelers RM, Gaillard CA, van Solinge WW. A role for activated endothelial cells in red blood cell clearance: implications for vasopathology. Haematologica 2012; 97: 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao C, Xie R, Li W, Zhou J, Liu S, Cao F, Liu Y, Ma R, Si Y, Liu Y, Bi Y, Gilbert GE, Shi J. Endothelial cell phagocytosis of senescent neutrophils decreases procoagulant activity. Thromb Haemost 2013; 109: 1079–90. [DOI] [PubMed] [Google Scholar]
- 23.Peng B, Koga K, Cardenas I, Aldo P, Mor G. Phagocytosis of apoptotic trophoblast cells by human endometrial endothelial cells induces proinflammatory cytokine production. Am J Reprod Immunol 2010; 64: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 1998; 92: 507–15. [PubMed] [Google Scholar]
- 25.Johnson SA, Balboa RS, Dessel BH, Monto RW, Siegesmund KA, Greenwalt TJ. The mechanism of the endothelial supporting function of intact platelets. Exp Mol Pathol 1964; 3: 115–27. [DOI] [PubMed] [Google Scholar]
- 26.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Müller-Newen G, Soehnlein O, Weber C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 2010; 122: 495–506. [DOI] [PubMed] [Google Scholar]
- 27.Lang D, Dohle F, Terstesse M, Bangen P, August C, Pauels HG, Heidenreich S. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol 2002; 168: 6152–8. [DOI] [PubMed] [Google Scholar]
- 28.Lou J, Donati YR, Juillard P, Giroud C, Vesin C, Mili N, Grau GE. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol 1997; 151: 1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 29.Passaretti F, Tia M, D’Esposito V, Pascale MD, Corso MD, Sepulveres R, Liguoro D, Valentino R, Beguinot F, Formisano P, Sammartino G. Growth-promoting action and growth factor release by different platelet derivatives. Platelets 2014; 25: 252–6. [DOI] [PubMed] [Google Scholar]
- 30.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013; 24: 173–82. [DOI] [PubMed] [Google Scholar]
- 31.Svensson Holm AC, Bengtsson T, Grenegård M, Lindström EG. Platelet membranes induce airway smooth muscle cell proliferation. Platelets 2011; 22: 45–55. [DOI] [PubMed] [Google Scholar]
- 32.Ranzato E, Boccafoschi F, Mazzucco L, Patrone M, Burlando B. Role of ERK1/2 in platelet lysates-driven endothelial cell repair. J Cell Biochem 2010; 110: 783–93. [DOI] [PubMed] [Google Scholar]