Abstract
The number of individuals with gluten intolerance has increased dramatically over the last years. To date, the only therapy for gluten intolerance is the complete avoidance of dietary gluten. To sustain a strictly gluten-free diet, however, is very challenging. Therefore, there is need for a non-dietary therapy. Any such treatment must appreciate that the immunogenic part of gluten are gliadin peptides which are poorly degraded by the enzymes of the gastrointestinal tract. Probiotic therapy and oral enzyme therapy containing gluten-degrading bacteria (GDB) and their gliadin-digesting enzymes are possible new approaches for the treatment of gluten intolerance, however effectively isolating GDB for these treatments is problematic. The goal of this study was to develop an easy technique to isolate GDB rapidly and efficiently with the hope it might lead to newer ways of developing either probiotics or traditional medicines to treat gluten intolerance. Several researchers have already isolated successfully GDB by using gluten minimal or limited agar plates. Although these plates can be used to isolate bacteria which can tolerate gluten, further assays are needed to investigate if the same bacteria can also digest gluten. The agar plates we developed can detect bacteria which cannot only tolerate gluten but are able to digest it as well. Therefore, we were able to combine two steps into one step. Using such technologies, we were able to isolate five GDB from saliva and stool, and identified three bacterial reference strains with gluten-degrading activity. The technique we developed to isolate bacteria with gluten-degrading activity is fast, effective, and easy to use. The GDB isolated by our technology could have potential as part of a probiotic or enzymatic therapy for people with gluten intolerance.
Keywords: Gluten intolerance, gluten-digesting bacteria, probiotics, gluten
Introduction
In the last few years, the number of people known or suspected to suffer from Celiac Disease (CD) and non-celiac gluten intolerance (NCGI) has increased dramatically. In both cases, the main cause is an inability to digest gluten. NCGI patients have similar symptoms when digesting gluten as CD patients do; however, NCGI is milder than CD and does not induce the gastrointestinal damage seen in CD patients. CD is an autoimmune disease and is mediated by T-cell activation in the gastrointestinal mucosa.1,2 NCGI is neither an allergy nor an autoimmune diseases, it is a food intolerance.
Gluten is not only difficult to digest for patients with CD and NCGI, but it is also difficult to digest for individuals without gluten intolerance. The difficulties humans have in digesting gluten are attributed to the fact that gluten has a high content of the amino acids proline and glutamine which are largely resistant to cleavage by the major human gastrointestinal digestive enzymes.3 It has been hypothesized that bacterial enzymes have the potential to degrade gluten and may play an important role in preventing gluten intolerance. This was also confirmed by other studies which demonstrated that in CD gluten intolerance is caused by gluten peptides that escape intestinal degradation by the human body. Partially or undigested gluten in these patients can lead to a destructive immunological response in the proximal intestine of patients with CD or NCGI.4–6
The only cure for CD and NCGI at the present time is a gluten-free diet, which can be very difficult to adhere to. Although more widespread than in the past, gluten-free products are still less available than products containing gluten; additionally, gluten-free products are more expensive than their wheat-containing counterparts. Cross contamination of gluten-free products is very common and gluten-derived products are found in many food items that seem to contain no wheat. Because of the difficulties of staying on gluten-free diets alternative therapies have been investigated. The potential to develop probiotics-containing gluten-digesting bacteria has led to increased interest in finding bacteria that might be used in therapeutic products or could be a source of genetic material that could be safely transplanted into organism currently used in safe probiotics. Current therapy for gluten intolerance includes the use of bacterial enzymes for oral supplementation that degrade gluten proteins in food before they reach the small intestine.7 Another approach to benefit gluten intolerance is the supplementation of bacterial-derived enzymes to detoxify gluten during food processing and before administration to patients. Additionally it has been reported that probiotics, especially those found in the gastrointestinal tract (GI) have many benefits besides those related to gluten. These may have other health benefits for patients with other digestive diseases.8,9
There are many reports which have demonstrated that the GI may harbor many beneficial bacteria for patients.10 Most GI-colonizing microorganism live in symbiosis with the host. The importance of these symbiotic bacteria has been reported in the past.8,9
It has been demonstrated that certain probiotics contain elements that digest or alter gluten. Other studies have expanded on the potential anti-inflammatory effects of Bifidobacterium infantis on gluten intolerance and especially CDs. Ingestion of probiotics containing these bacteria reduces the damage caused by eating gluten-contaminated foods and may even accelerate mucosal healing after the initiation of a gluten-free diet.11
The problem with trying to identify GDB is that there are 20 billion oral microbes in the oral cavity alone. There are more than 700 bacterial species or phylotypes, of which, over 50% have not been identified.12 With such a high concentration of bacteria, it is difficult to find those bacteria which can digest gluten. Although the technology used by Fernandez-Feo et al.13 and Zamakhchari et al.14 was able to identify bacteria which can tolerate gluten, it could not determine if the same bacteria was also able to digest gluten. Since the number of gluten tolerant bacteria on minimal gluten agar plates can be high, it is very time consuming to pick the right colony. Additionally, they had to employ other techniques to test the gluten-degrading ability of the bacteria. We have developed a rapid and easy technique to isolate such bacteria by modifying the Kirby–Bauer agar diffusion test.15 Using our method we isolated five different bacterial species from saliva and feces capable of digesting gluten and believe we can easily isolate more.
In this study however, our goal was not to completely catalog all gluten-digesting bacteria but rather to develop a technology that would allow us to screen for bacteria with this ability. It is noteworthy, however, that using this method reported here, we identified two bacterial species previously reported by Fernandez-Feo et al.13 and Zamakhchari et al.14 to have gluten-digesting ability. Thus, we and the other authors identified Rothia species and Streptococcus species as GDB. However, we also identified six additional bacteria which have not been previously mentioned in the literature as gluten-digesting bacteria. Gluten-digesting bacteria isolated from the saliva or the feces could have great potentials in developing a probiotic therapy for CD and NCGI.
Material and methods
Collection of saliva samples
The collection of human saliva was approved by the Institutional Review Board at the University of California, Irvine. The saliva was collected from four healthy gluten tolerant individuals, two men and two women, using passive drool as described by Salimetrics (State College, PA, USA). Clients were asked to rinse their mouth with water 10 min prior sample collection. Subsequently, clients were asked to allow saliva to pool in the mouth and then to spit into a 50 mL tube. At least 2 mL saliva was collected per client. The saliva was immediately used and the rest was frozen at −20℃.
Collection of stool samples
Each subject collected stool specimens by passing feces into plastic wrap stretched loosely over the toilet bowl and transferred into container which was stored at −80℃ until use.
Determination of the optimal amount of gliadin to be dissolved in alcohol and added to Luria Broth (LB) agar plates
We used gliadin for our experiment since it has been reported that gliadin is the immunogenic part of gluten in individuals with gluten intolerance. Initial experiments determined the optimal amount of gliadin to add to LB agar plates. This was done by adding 1 g gliadin to various amounts of 60% ethanol. After determining the maximal amounts of gliadin that could be dissolved in ethanol, we added increasing increments to 20 mL of LB agar to produce an opaque appearance. The cloudiness of the plates was important for identification of GDB but too much gliadin dissolved in ethanol can inhibit bacteria growth on the plates so the optimization had to be done carefully.
Preparing gliadin plates to isolate gluten-degrading bacteria
One gram of gliadin was added to 22 mL of 60% ethanol and incubated overnight at room temperature on a shaker. This solution was centrifuged for 2 min at 300 r/min and the supernatant was removed. Eight hundred-fifty microliters of supernatant was then added to the 20 mL LB agar media. When the plates were solidified they were stored at 4℃.
Testing bacteria reference strains for gluten degradation activity
Reference strains were purchased from the American Type Culture Collection and included: Klebsiella pneumonia (K. pneumonia—ATCC 700603), Serratia marcescens (S. marcescens—ATCC 13477), Escherichia coli (E. coli—ATCC 25922), Enterobacter cloacae (E. cloacae—ATCC 10699), and Staphylococcus aureus (S. aureus—ATCC 29213). A strain of Pseudomonas aeruginosa (P. aeruginosa) was obtained from the clinical laboratories at UCI Medical Center and typed by Vitek Method. Bacteria were inoculated on gliadin plates and inoculated at 37℃ for two days aerobically and anaerobically. After two days the plates were observed for a clear zone around the bacterial colony.
Culturing of oral microorganisms from saliva
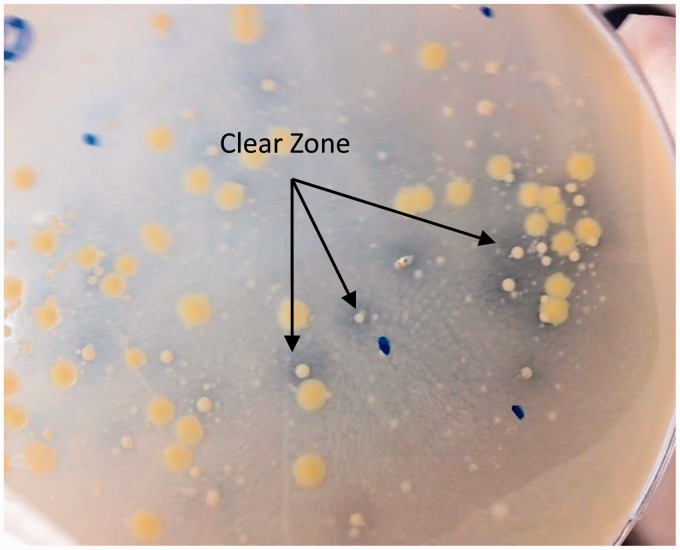
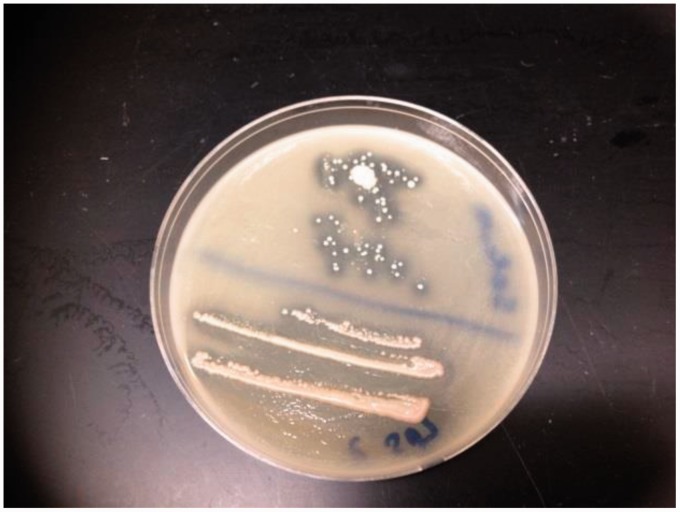
Saliva samples were diluted either 100-fold or 1000-fold and 20 µL of each dilution was plated on LB plates containing gliadin, respectively. Subsequently, the plates were incubated for a period of two days aerobically or anaerobically and checked periodically for the development of a clear zone around the colonies (Figure 1). Each colony found to have a surrounding clear zone was subcultured onto a gluten-containing plate and incubated at 37℃ for one day to verify their ability to digest gluten (Figure 2). Bacteria which demonstrated gluten-digesting activity after subculture were kept at −80℃ in a glycerol/LB broth mixture (20/80% v/v).
Figure 1.
Bacteria isolated from saliva on gliadin plates. The clear zone around the bacteria demonstrated gliadin-digesting activity from the bacteria. (A color version of this figure is available in the online journal.)
Figure 2.
Subcultured bacteria on gliadin plates. Bacterial colonies surrounded by a clear zone were subcultured onto another gliadin plate. (A color version of this figure is available in the online journal.)
Culturing of microorganism from the stool
One gram of stool was dissolved in 10 mL of saline and diluted 100-fold, 1000-fold, and 10,000-fold. Twenty microliters of each dilution was spread on gliadin plates and incubated aerobically or anaerobically at 37℃ for 1–3 days maximum. Each day the plates were inspected for gluten-digesting bacteria, determined by a clear zone around the bacteria colony. Each colony was subcultured on a new gliadin plate and incubated at 37℃ overnight and observed again in the development of a clear zone around the colony to ensure their ability to digest gliadin.
Bacterial identification by 16 S rDNA
Bacterial colonies with gluten-degrading activity were identified by 16 S rDNA analysis. DNA extraction was performed using the Zymo DNA extraction kit (Zymo, Irvine, CA), following the manufacturer’s instructions for the isolation of genomic DNA. Briefly, 2 µL of the isolated bacterial DNA was used to amplify the 16 s rRNA using 25 µL Zymotaq PCR premix, 2 µL primer Forward 27 F (AGA GTT TGA TGC TGG CTC AG) (20 pmol), 2 µL primer Reverse 735 R (TAT ATC CTG TTC GCT ACC) (20 pmol), 2 µL dNTP (dATP/dCTP/dGTP/dTTP), and 19 µL H2O, having a final volume of 50 µL. The samples were amplified in the MJ thermal cycler using 35 cycles with the initial denaturation step of 95℃ for 10 min, followed by 35 cycles using 95℃ for 30 s, an annealing temperature of 55℃ for 40 s, and an extension step of 72℃ for 1 min. After 35 cycles, a 72℃ extension step for 7 min was applied. To analyze the PCR product the samples were loaded onto a 1.5% agarose gel containing ethidium bromide. The amplified DNA fragment had a size of 708 bp and was visualized under UV light. The samples were sequenced by Retrogen. The sequences of the individual bacteria were identified by using BLAST search at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
Degradation of gluten by bacteria or the bacteria free supernatant
The supernatant from media in which gluten-digesting bacteria were grown was tested for the ability to digest gluten. Each individual bacterial strain was incubated overnight in LB media at 37℃. The next day the OD was measured for each culture and diluted to an OD of 1.2. One hundred microliters of a bacterial suspension with an OD of 1.2 was cultured in LB media containing 6.5% gliadin or no gliadin. Each strain was incubated alone or in combination with one, two, or three other strains for two days at 37℃. After two days, the bacteria were centrifuged at 3500 g for 5 min and the supernatant was transferred into another tube and filtered through a 0.22 µm nitrocellulose filter. Seventy-five microliters of the filtered supernatant or the bacteria was added to each well within the diffusion assay agar plates which contain LB media and 0.2% gliadin. After 24 and 48 h the plates were investigated for a clear zone around the bacterial colonies.
Results
Optimization for gliadin plates
The optimal concentration of gliadin was 1 g in 22 mL of 60% ethanol. It was further determined that 0.85 mL of this solution when added to 20 mL of the LB agar was optimal for the production of the plates used to test for gliadin-digesting bacteria. Plates produced in this manner were opaque and used to differentiate between GDB and non-GDB without affecting the growth of the bacteria. The cloudiness of the plates reflects the concentration of gliadin within the agar. Plates prepared according to this protocol were stored at 4℃ until use.
Testing of bacterial reference strains for gliadin degradation activity
Gluten-degrading activity could be visualized through the clear zone produced on the gliadin-containing agar plates. This clear zone around the bacteria or the bacterial supernatant indicates the ability of the bacteria/supernatant to remove the gliadin from the plates and thus demonstrates the ability to digest gliadin, the immunogenic part of gluten.
Of the six reference strains tested, S. marcescens and P. aeruginosa demonstrated significant ability to digest gliadin under aerobic and anaerobic conditions. The supernatant of P. aeruginosa, devoid of bacteria, was also found to degrade bacteria. S. aureus demonstrated weak gluten-degrading activity compared to our other stains. All bacteria were able to grow on gliadin plates (Table 1).
Table 1.
Different bacterial reference strains and their ability to digest gliadin
| Reference strain | Gliadin degradation activity |
|---|---|
| Klebsiella pneumonia | No |
| Serratia marcescens | Yes |
| Pseudomonas aeruginosa | Yes |
| Escherichia coli | No |
| Staphylococcus aureus | Yes ± very weak |
| Enterobacter cloacae | No |
Isolation of bacteria from the saliva and the stool
Eleven bacterial strains were isolated from the saliva of four individuals (two men and two women) and three bacteria were isolated from the stool of one individual (Tables 2 and 3). All were able to digest gluten as demonstrated by the appearance of a clear zone around colonies when cultured on plates as described earlier. Each colony which could digest gluten was subcultured to verify gluten-digesting activity. Only one of the 14 strains failed to digest gluten after subcultured.
Table 2.
Bacteria species isolated from four different individuals
| Bacterial strains | Individual 1 (men) | Individual 2 (men) | Individual 3 (women) | Individual 4 (women) |
|---|---|---|---|---|
| Streptococcus salivarius | + | – | – | – |
| Bacillus pumilus | – | – | + | – |
| Rothia dentocariosa | – | + | + | + |
| Bacillus subtilis | – | – | + | – |
| Staphylococcus epidermis | – | + | – | + |
Table 3.
All gliadin-digesting bacteria were isolated from the saliva, except B. subtilis, which was only isolated from stool samples. B. pumilus was isolated from the saliva and stool
| Bacterial strains | Isolated from saliva | Isolated from stool |
|---|---|---|
| Streptococcus salivarius | + | – |
| Bacillus pumilus | + | + |
| Rothia dentocariosa | + | – |
| Bacillus subtilis | – | + |
| Staphylococcus epidermis | + | – |
Identification of the isolated bacteria
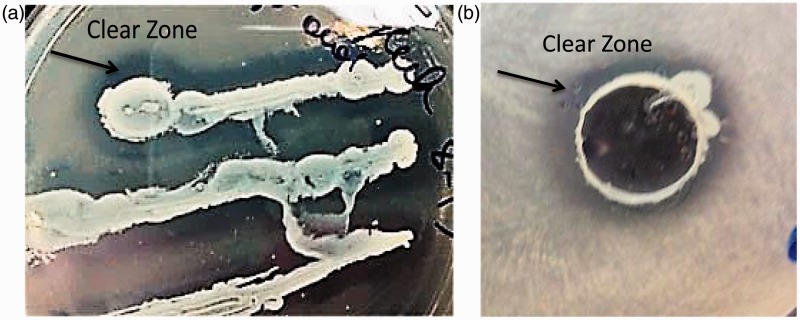
To determine the species of the bacteria, the 16sRNA was amplified and subsequently sequenced. All 13 bacteria strains were sequenced. The sequence of the individual bacteria was identified using BLAST methods searched on the NCBI website (http://www.ncbi.nlm.nih.gov). The 13 isolated strains were identified as follows: Streptococcus salivarius (S. salivarius—one strain), Bacillus pumilus (B. pumilus—four strains), Rothia dentocariosa (R. dentocariosa—five strains), Bacillus subtilis (B. subtilis—one strain), and Staphylococcus epidermis (S. epidermidis—two strains). B. pumilus was isolated from both the stool and also from the saliva. The other bacterial strains could only be isolated from the saliva (Table 4). Rothia species and Streptococcus species were previously described in the literature as gluten-digesting bacteria and thus help validate our technology as a useful tool for the isolation of GDB (Figure 3).
Table 4.
We were able to isolate several gliadin-digesting bacteria species multiple times from the saliva or feces. R. dentocariosa was isolated five times and thus the most frequent isolated bacteria strain followed by B. pumilus.
| Bacterial strains | Number of isolation |
|---|---|
| Streptococcus salivarius | 1 |
| Bacillus pumilus | 4 |
| Rothia dentocariosa | 5 |
| Bacillus subtilis | 1 |
| Staphylococcus epidermis | 2 |
Figure 3.
R. dentocariosa and S. salivarius cultured on gliadin plates. (a) S. salivarius was inoculated on gliadin plates. The clear zone around S. salivarius demonstrates gluten-degrading activity. (b) R. dentocariosa activity to degrade gliadin was determined by using the diffusion gliadin agar plate assay. The clear zone around the R. dentocariosa demonstrates gluten-degrading activity. (A color version of this figure is available in the online journal.)
Degradation of gliadin by bacteria or bacteria free supernatant
The bacteria and the bacteria free supernatant from all bacteria described earlier were used to determine if the supernatant from the bacteria culture or only the bacteria could degrade gliadin. All bacteria were able to degrade gluten. The supernatants of B. subtilis, B. pumilus, and P. aeruginosa from a two-day culture were also able to degrade the gluten without the presence of the living bacteria (Figure 4). S. salivarius, R. dentocariosa, S. marcescens, S. aureus, and S. epidermis were only able to degrade gluten in the presence of the bacteria, and their supernatants demonstrated no gluten-degrading activity (Table 5).
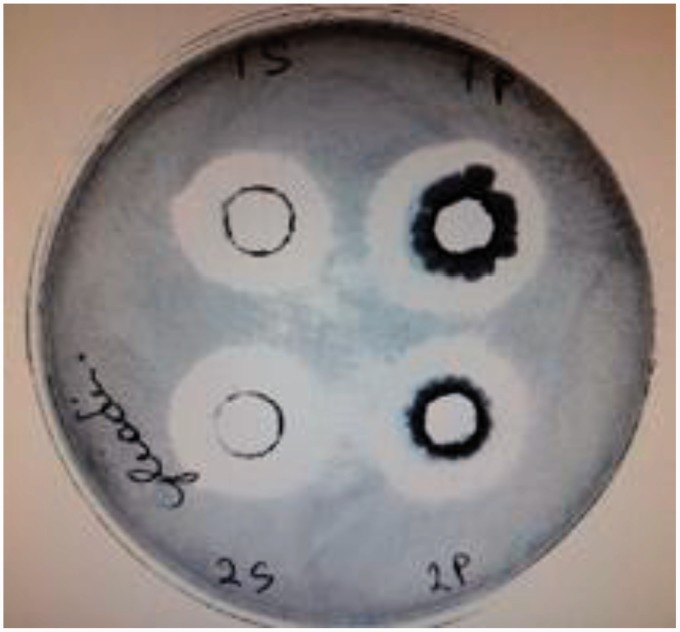
Figure 4.
A diffusion gliadin agar plate assay was used to determine if the isolated bacteria and the supernatant of the media in which they were grown have the ability to digest gliadin. B. subtilis and B. pumilus (2 P) and their supernatant (2 S) exhibited the ability to digest gluten. (A color version of this figure is available in the online journal.)
Table 5.
Bacterial strains and the supernatants of the media in which they were grown were tested for gliadin-digesting activity. The supernatant of only three strains was capable of digesting gliadin without the presence of bacteria
| Bacterial strains | Supernatant | Bacteria alone |
|---|---|---|
| Bacillus subtilis | + | + |
| Bacillus pumilus | + | + |
| Pseudomonas aeruginosa | + | + |
| Streptococcus salivarius | – | + |
| Rothia dentocariosa | – | + |
| Serratia marcescens | – | + |
| Staphylococcus aureus | – | + |
| Staphylococcus epidermis | – | + |
Discussion
We developed a rapid and easy technique to isolate bacteria with gluten-degrading activity from various parts of the GI tract. While agar plates are a common technology to isolate bacteria, there are no agar plates which can identify GDB. There are manufactures who have developed gluten-containing agar plates to identify bacteria which can tolerate gluten.13,14 The disadvantage of these plates is that further assays are needed to determine if the gluten tolerant bacteria can also digest gluten. However, we modified the Kirby–Bauer agar diffusion assay15 and developed an agar plate that could not only isolate bacteria which can tolerate gluten but which could also identify bacteria which can digest gluten. Using our developed technique we were able to successfully identify five bacterial strains from human saliva and feces capable of degrading gluten. In addition, six bacterial reference strains were tested for gluten degradation activity. Three of the six strains were able to degrade gluten under both aerobic and anaerobic conditions. This easy technique demonstrates how gluten-degrading bacteria can be easily isolated from the feces and saliva. While we used this method to isolate bacteria from humans, we believe this same method can be used to isolate bacteria that degrade gluten from other environmental sources as well.
We focused on isolating bacteria with gluten-degrading activity since it has been hypothesized that inadequate digestion of gliadin is responsible for CD and NCGI. Gliadin is a part of gluten that can be found in wheat, rye, and barley. Gluten is classically divided into two groups: the monomeric gliadins and the polymeric glutenins. There are at least 50 gliadin epitopes that exert immunomodulatory, cytotoxic, and gut-permeating activities.16 The most important gluten peptide, regarded as one of the most CD-immunodominant gluten peptides, is 33-mer α-gliadin which is resistant to the action of proteases.17,18 However, there are other gluten peptides also in the ϒ-gliadin group which have immunomodulatory activities.18–22
Although a gluten-free diet is the cure for gluten sensitivity, life on a gluten-free diet can be difficult due to several reasons—expense, contamination of gluten-free products with gluten, products with gluten-derived ingredients, availability, inadequate food labeling, and insufficient knowledge about gluten. Complications of untreated CDs or gluten intolerance can be severe. Gluten sensitivity is not just a disease of the gut, it is a multiorgan, multisymptom disease. It has been reported that CD can lead to malnutrition,23 osteoporosis,24 neurological25–27 and psychiatric28 complications and cancer such as lymphoma.29,30 Additionally, individuals with CD have a significant increase of other autoimmune disorders.31 NCGI has overlapping similarities with CD but the effects of gluten are generally less severe. NCGI has been detected in numerous individuals with irritable bowel syndrome. In the large study performed by Carroccio et al.,32 30% of subjects with IBS-like symptoms suffered from wheat sensitivity or multiple food hypersensitivities. Leffler et al.33 demonstrated that the sensitivity to gluten exposure varies greatly between individuals with gluten intolerance. In some cases, a tiny amount of gluten is enough to induce disease. Since some of these reactions are silent, they may not be recognized but may still result in damage to the body.
Other treatment options besides a gluten-free diet for people with CD or NCGI have been suggested. One of these treatments is the development of an oral probiotic and enzyme therapy. The eight different bacteria strains isolated in this experiment, three reference strains and five strains isolated from saliva or stool were able to degrade gliadin and could possibly be part of such treatment. B. subtilis, B. pumilus, and P. aeruginosa did not have to be present to permit gluten digestion. At the present time we are not sure what substances these three bacteria secrete which have extracellular gliadin-degrading activity. We did not observe this in the rest of the bacteria. However, it has been demonstrated that certain bacteria contain glutenase, a proline-specific and glutamine-specific endopeptidases which can modulate the toxic effect of gliadin. Glutenase has been isolated from various organisms such as fungi, barley, and also bacteria.34,35 To achieve a more complete digestion of gluten and specifically its immunogenic domains, the proline- and glutamine-rich regions which are highly resistant to degradation by gastric and pancreatic proteases36–38 need to be digested before they can be absorbed and used by the body. Therefore, treatments which can accomplish digestion of gluten could be useful.
Glutenases are currently intended as oral enzyme supplements which could be used in conjunction with a gluten-free diet to diminish the toxicity of accidental gluten exposure. The bacteria we isolated from the saliva have all gliadin degradation activity. Since four of these bacteria have been shown to colonize the intestine and also the oral cavity they would be good candidates for a gluten digestive probiotic treatment. B. pumilus and B. subtilis have also been reported to be of great value as probiotics.39 B. subtilis can also enhance viability of other probiotics such as Lactobacillus reuteri,40 and in combination with lactulose is an effective and safe therapeutic method for elders with functional constipation.41 S. salivarius has already been tested as an oral probiotic and is in use for the prevention of oral diseases.42 R. dentocariosa is very common in the oral cavity and is described in many papers.43 The other identified strains such as S. marcescens and S. aureus which have gliadin degradation activity are unlikely to be considered for probiotic therapy since they can be human pathogens. However, since all three have a strong gliadin-degrading activity, their enzymes and/or substance responsible for this activity could be used for treating CD and NCGI. This might be especially true for the substance derived from P. aeruginosa, since the supernatant of this bacteria culture could digest gliadin without the presence of the bacteria. The most important enzyme reported for the use of an oral enzyme or probiotic therapy is glutenase. Since a number of microbes exhibit glutenase activity, we hypothesize that the gliadin degradation activity of our bacteria is also glutenase based. However, since at the present moment we were only interested in developing a rapid and easy technique to identify bacteria with gliadin degradation activity, we did not define the gliadin-degrading substance any further; however, two of our bacteria, R. dentocariosa and S. salivarius, belong to a group of bacterial species which have already been reported in the literature to have gliadin-degrading activity and especially glutenase activity.13,14,44 That we were able to identify two bacterial species which have been well described in the literature as bacteria with gluten-digesting abilities13,14 helps validate the usefulness of our technology. The clear zone around these two bacteria, R. dentocarios and S. salivarius (Figure 3), which both belong to a group of bacterial species which are well characterized as gluten-digesting bacteria, confirms that GDB produce a clear zone around their colonies demonstrating their ability to digest the gluten in the agar plate. Using this technology the isolation and identification of gluten-digesting bacteria can be done within two days, which is also an improvement over other techniques used to isolate such bacteria. Further studies are underway to isolate and investigate more gluten-digesting bacteria in the oral cavity.
We believe that the method developed here to isolate GDB could be an important tool for the detection of gluten-digesting bacteria in our environment and the human flora. This technique could have great potential for the development of an oral probiotic or enzyme therapy that could be helpful for people with CD and NCGI.
ACKNOWLEDGEMENTS
We would like to thank the Institute of Clinical and Translational Science at the University of California Irvine for valuable technical advice, helpful discussion, and for granting us access to some of their equipment. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through GrantΜL1 TR000153. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors’ Contributions
All authors participated in the interpretation of the studies and analysis of the data and review of the manuscript “Rapid Isolation of gluten-digesting bacteria from human stool and saliva by using gliadin-containing plates”; MB and TC designed the study and wrote the manuscript. MB, DO, CS, and JS conducted the experiments. All individuals who made contributions to this study are included either as authors or are acknowledged.
References
- 1.Marsh MN. Studies of intestinal lymphoid tissue: the cytology and electron microscopy of gluten-sensitive enteropathy, with particular reference to its immunopathology. Scand J Gastroenterol Suppl 1981; 70: 87–106. [PubMed] [Google Scholar]
- 2.Ferguson A, Ziegler K, Strobel S. Gluten intolerance (coeliac disease). Ann Allergy 1984; 53: 637–42. [PubMed] [Google Scholar]
- 3.Siegel M, Bethune M, Gass J, Ehren J, Xia J, Johannsen A, Stuge TB, Gray GM, Lee PP, Khosla C. Rational design of combination enzyme therapy for celiac sprue. Chem Biol 2006; 13: 649–58. [DOI] [PubMed] [Google Scholar]
- 4.Koning F, Gilissen L, Wijmenga C. Gluten: a two-edged sword. Immunopathogenesis Celiac Dis 2005; 27: 217–32. [DOI] [PubMed] [Google Scholar]
- 5.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol 2006; 3: 516–25. [DOI] [PubMed] [Google Scholar]
- 6.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009; 137: 1912–33. [DOI] [PubMed] [Google Scholar]
- 7.Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implication for coeliac diseases. Gut 2008; 57: 25–32. [DOI] [PubMed] [Google Scholar]
- 8.Petschow B, Doré J, Hibberd P, Dinan T, Reid G, Blaser M, Cani PD, Degnan FH, Foster J, Gibson G, Hutton J, Klaenhammer TR, Ley R, Nieuwdorp M, Pot B, Relman D, Serazin A, Sanders ME. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serban DE. Gastrointestinal cancers: influence of gut microbiota, probiotics and prebiotics. Cancer Lett 2013;345:258–70. [DOI] [PubMed]
- 10.Dunne C. Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm Bowel Dis 2001; 7: 136–45. [DOI] [PubMed] [Google Scholar]
- 11.Lindfors K, Blomqvist T, Juuti-Uusitalo K, Stenman S, Venäläinen J, Mäki M, Kaukinen K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin Exp Immunol 2008; 152: 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol 2005; 13: 589–95. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Feo M, Wei G, Blumenkranz G, Dewhirst FE, Schuppan D, Oppenheim FG, Helmerhorst EJ. The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clin Microbiol Infect 2013; 19: e386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamakhchari M, Wei G, Dewhirst F, Lee J, Schuppan D, Oppenheim FG, Helmerhorst EJ. Identification of Rothia bacteria as gluten-degrading natural colonizers of the upper gastro-intestinal tract. PLoS One 2011;6:e24455–65. [DOI] [PMC free article] [PubMed]
- 15.Bauer A, Perry DM, Kirby WM. Single-disk antibiotic-sensitivity testing of staphylococci; an analysis of technique and results. AMA Arch Intern Med 1959; 104: 208–16. [DOI] [PubMed] [Google Scholar]
- 16.Nijeboer P, Bontkes HJ, Mulder CJ, Bouma G. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J Gastrointest Liver Dis 2013; 22: 435–40. [PubMed] [Google Scholar]
- 17.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science 2002; 297: 2275–9. [DOI] [PubMed] [Google Scholar]
- 18.Qiao SW, Bergseng E, Molberg Ø, Xia J, Fleckenstein B, Khosla C, Sollid LM. Antigen presentation to celiac lesion-derived T-cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol 2004; 173: 1757–62. [DOI] [PubMed] [Google Scholar]
- 19.Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Ráki M, Kwok WW, Jung G, Lundin KE, Sollid LM. HLA-DQ2 and -DQ8- signatures of gluten T cell epitopes in celiac disease. J Clin Invest 2006; 116: 2226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salentijn EM, Mitea DC, Goryunova SV, van der Meer IM, Padioleau I, Gilissen LJ, Koning F, Smulders MJ. Celiac disease T-cell epitopes from gamma-gliadins: immunoreactivity depends on the genome of origin, transcript frequency, and flanking protein variation. BMC Genomics 2012;13:277–89. [DOI] [PMC free article] [PubMed]
- 21.Dorum S, Qiao SW, Sollid LM, Fleckenstein B. A quantitative analysis of transglutaminase 2-mediated deamidation of gluten peptides: implications for the T-cell response in celiac disease. J Proteome Res 2009; 8: 1748–55. [DOI] [PubMed] [Google Scholar]
- 22.Dorum S, Arntzen MØ, Qiao SW, Holm A, Koehler CJ, Thiede B, Sollid LM, Fleckenstein B. The preferred substrates for transglutaminase 2 in a complex wheat gluten digest are peptide fragments harboring celiac disease T-cell epitopes. PLoS One 2010;5:e14056–66. [DOI] [PMC free article] [PubMed]
- 23.Waqar Rabbani M, Imran Khan W, Bilal Afzal A, Rabbani W. Causes of short stature identified in children presenting at a tertiary care hospital in Multan Pakistan. Pak J Med Sci 2013; 29: 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Stefano M, Mengoli C, Bergonzi M, Corazza GR. Bone mass and mineral metabolism alterations in adult celiac disease: pathophysiology and clinical approach. Nutrients 2013; 5: 4786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currie S, Hadjivassiliou M, Clark MJ, Sanders DS, Wilkinson ID, Griffiths PD, Hoggard N. Should we be ‘nervous’ about coeliac disease? Brain abnormalities in patients with coeliac disease referred for neurological opinion. J Neurol Neurosurg Psychiatry 2012; 83: 1216–21. [DOI] [PubMed] [Google Scholar]
- 26.Ghazal FA, Singh S, Yaghi S, Keyrouz SG. Gluten ataxia: an important treatable etiology of sporadic ataxia. Int J Neurosci 2012; 122: 545–6. [DOI] [PubMed] [Google Scholar]
- 27.Licchetta L, Bisulli F, Di Vito L, La Morgia C, Naldi I, Volta U, Tinuper P. Epilepsy in coeliac disease: not just a matter of calcifications. Neurol Sci 2011; 32: 1069–74. [DOI] [PubMed] [Google Scholar]
- 28.Smith DF, Gerdes LU. Meta-analysis on anxiety and depression in adult celiac disease. ACTA Psychiatr Scand 2012; 125: 189–93. [DOI] [PubMed] [Google Scholar]
- 29.Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, Green PH, Ludvigsson JF. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med 2013; 159: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Lebwhol B, Kampe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid 2013; 23: 971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013:127589–06. [DOI] [PMC free article] [PubMed]
- 32.Carroccio A, Brusca I, Mansueto P, D’alcamo A, Barrale M, Soresi M, Seidita A, La Chiusa SM, Iacono G, Sprini D. A comparison between two different in vitro basophil activation tests for gluten- and cow’s milk protein sensitivity in irritable bowel syndrome (IBS)-like patients. Clin Chem Lab Med 2013; 51: 1257–63. [DOI] [PubMed] [Google Scholar]
- 33.Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, Dennis M, Kelly CP. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013; 62: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoven S, Murray J, Marietta E. Celiac disease: advances in treatment via gluten modification. Clin Gastroenterol Hepatol 2012; 10: 859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bethune M, Khosla C. Oral enzyme therapy for celiac sprue. Methods Enzymol 2012; 502: 241–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan L, Qiao SW, Arentz-Hansen H, Molberg Ø, Gray GM, Sollid LM, Khosla C. Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue. J Proteome Res 2005; 4: 1732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornell HJ, Rivett DE. In vitro mucosal digestion of synthetic gliadin-derived peptides in celiac disease. J Protein Chem 1995; 14: 335–9. [DOI] [PubMed] [Google Scholar]
- 38.Piper JL, Gray GM, Khosla C. Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J Pharmacol Exp Ther 2004; 311: 213–9. [DOI] [PubMed] [Google Scholar]
- 39.Duc LH, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 2004; 70: 2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Xiong H, Guo X. Enhanced viability of Lactobacillus reuteri for probiotics production in mixed solid-state fermentation in the presence of Bacillus subtilis. Folia Microbiol 2014; 59: 31–6. [DOI] [PubMed] [Google Scholar]
- 41.Liu YP, Liu X, Dong L. Lactulose plus live binary Bacillus subtilis in the treatment of elders with functional constipation Zhonghua Yi Xue Za Zhi 2012;92:2961–4. [DOI] [PubMed]
- 42.Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, Wescombe PA. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol 2013; 62: 875–84. [DOI] [PubMed] [Google Scholar]
- 43.Lexner MO, Blomgvist S, Dahlén G, Twetman S. Microbiological profiles in saliva and supragingival plaque from caries-active adolescents before and after a short-term daily intake of milk supplemented with probiotic bacteria – a pilot study. Oral Health Prev Dent 2010; 8: 383–8. [PubMed] [Google Scholar]
- 44.Helmerhorst EJ, Zamakhchari M, Schuppan D, Oppenheim FG. Discovery of a novel and rich source of gluten-degrading microbial enzymes in the oral cavity. PLoS One 2010; 5: 13264–13264. [DOI] [PMC free article] [PubMed] [Google Scholar]