Abstract
N-3-(Oxododecanoyl)-L-homoserine lactone (C12) is a small bacterial signaling molecule secreted by Pseudomonas aeruginosa (PA), which activates mammalian cells through TLR4-independent mechanisms. C12 acts as an immunosuppressant and it has been shown to modulate murine bone marrow-derived dendritic cell-mediated T-helper 2 (Th2) cell polarizations in vitro. In the present study, we initially examined the impact of C12 on the maturation of human monocyte-derived dendritic cells (Mo-DCs) and the induction of regulatory T-cells (iTregs) in culture. Our findings demonstrate that C12-treated Mo-DCs failed to undergo lipopolysaccharide (LPS)-induced maturation. At the molecular level, C12 blocked the upregulation of surface molecules, including CD11c, HLA-DR, CD40, and CD80, and it switched to an interleukin (IL)-10high, IL-12p70low phenotype. Moreover, C12 selectively inhibited the capacity of Mo-DCs to stimulate the proliferation of allogeneic CD4+ T-cells. Otherwise, the C12-treated Mo-DCs promoted the generation of CD4+CD25+Foxp3+-induced regulatory T-cells (iTregs) and enhanced their IL-10 and transforming growth factor (TGF)-β production associated with reduced interferon (IFN)-γ and IL-12p70 production. These findings provide new insights towards understanding the persistence of chronic inflammation in PA infection.
Keywords: Pseudomonas aeruginosa, dendritic cells, regulatory T-cells
Introduction
Pseudomonas aeruginosa (PA) is an opportunistic pathogen that often infects decubitus ulcers and burn wounds, thus delaying healing.1 This versatile bacterium is a major cause of pneumonia in immunocompromised and cystic fibrosis (CF) patients, in whom chronic PA infections are the main cause of morbidity and mortality.2 During the infectious process, PA expresses virulence factors in a cell density-dependent process termed “quorum sensing” (QS).3 In QS, N-3-(oxododecanoyl)-L-homoserine lactone (C12) is a small bacterial signaling molecule that modulates the gene expression of extracellular virulence factors, such as elastase, proteases, exotoxins, alkaline protease, and pyoverdine, which are capable of causing extensive tissue damage, invading the bloodstream, and consequently promoting systemic dissemination. Meanwhile, C12 interacts with eukaryotic cells and modulates immune responses.4,5 It has been reported C12 plays an important role in immune balance via TLR4-independent mechanisms,6 which have been shown to affect the function and activity of a range of mammalian cell types, such as lymphocytes,7 macrophages/monocytes,8 and neutrophils.9 There is evidence that C12 acts as an immunosuppressant, as is able to modulate murine bone marrow-derived dendritic cell (BM-DC)-mediated T-helper 2 (Th2) cell polarizations in vitro.10 However, the mechanism by which C12 promotes the induction of regulatory T-cells by human monocyte-derived dendritic cells (Mo-DCs) is still undefined.
DCs are professional antigen-presenting cells (APCs) that mediate the initial antigen presentation through the major histocompatibility complex (MHC) to naïve T-cells. They are responsible for the subsequent adaptive immune response.11 In addition to the polarization of conventional Th1 and Th2 cells, immunostimulatory DCs are able to generate adaptive iTregs, which are responsible for immune escape. A growing body of evidence indicates that adaptive iTregs can develop in the periphery from uncommitted naïve or memory T-cells triggered by immature or semi-mature DCs.12
To precisely determine the possible inhibitory mechanisms of C12, its effects on the maturation of human Mo-DCs and iTregs were examined. In the present study, we investigated whether C12 affects human Mo-DC maturation and induce the generation of CD4+CD25+Foxp3+ iTregs. Our findings could increase the understanding of the persistence of chronic inflammation in PA infections.
Materials and methods
Reagents
N-3-Oxododecanoyl-L-homoserine lactone (C12) and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO). RPMI 1640, fetal bovine serum (FBS), and carboxyfluorescein succinimidyl ester (CFSE) were purchased from Invitrogen (Carlsbad, CA). Ficoll/Isopaque Lymphoprep™ was purchased from Axis-shield (Oslo, Norway). Recombinant human GM-CSF and interleukin (IL)-4 were purchased from Protech (Rehovot, Israel). The Cell Counting Kit (CCK)-8 assay kits were purchased from DoJinDo Laboratories (Kumamoto, Japan). CD14+ and CD4+ microbeads were purchased from BD Biosciences (Franklin Lakes, NJ). Human regulatory T cell staining kit was purchased from eBioscience (San Diego, CA). Anti-CD14-FITC, anti-CD4-FITC, anti-CD80-FITC, anti-CD11c-APC, anti-CD40-PE, and anti-HLA-DR-PECy5 were purchased from eBioscience. Enzyme-linked immunosorbent assay (ELISA) kits for interferon (IFN)-γ, IL-12p70, IL-10, and transforming growth factor (TGF)-β were purchased from Dakewe Biotech Company (Guangzhou, China). Additionally, 24-well and 96-well culture plates were purchased from Corning Inc. (Corning, NY).
Mo-DC culture
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood, which was obtained from healthy donors using Lymphoprep™ density separation, following the manufacturer’s instructions. Monocytes were isolated from the PBMCs by magnetic-activated cell sorting (MACS) using anti-CD14-coated microbeads and a magnetic cell separator (BD Biosciences) according to the manufacturer’s instructions. The recovered monocytes, which were >95% pure, as shown by flow cytometry with an anti-CD14 fluorescent antibody, were cultured at 1 × 106/mL in RPMI 1640 supplemented with 10% heat-inactivated FBS (complete medium) containing 50 ng/mL of GM-CSF and 1000 U/mL of IL-4 in 24-well culture plates at 37℃ and 5% CO2 for five days.
Mo-DC viability
The cytotoxic effects of C12 on Mo-DCs were evaluated by CCK-8 assays. On day 5 of culture, Mo-DCs were stimulated with 1 µg/mL of LPS in the absence and presence of C12 at concentrations of 5 µmol/L, 10 µmol/L, 15 µmol/L, 20 µmol/L, 25 µmol/L, 50 µmol/L, 100 µmol/L, and 200 µmol/L. All phosphate-buffered saline (PBS) control groups in this study were treated with 0.1% dimethylsulfoxide (DMSO) in PBS. Mo-DC viability was determined by the CCK-8 assay as follows: 10 µL of CCK-8 was added per well, 1 × 104 cells were incubated for an additional 4 h, and the absorbance at 450 nm was recorded using a 96-well plate reader. Cell viability was calculated as follows: % Cell viability = (the OD value of the treatment group/the OD value of the negative control group × 100%).
Immunophenotype analysis
On day 5 of the culture, generated Mo-DCs were stimulated with 1 µg/mL of LPS in the absence and presence of C12 at concentrations of 5 µmol/L, 10 µmol/L, 25 µmol/L, 50 µmol/L and 100 µmol/L at 37℃ for 48 h. Negative controls were prepared in triplicate wells at the same volume. To examine cell surface markers, stimulated cells were washed (cold PBS), fixed (PBS containing 1% formaldehyde), and then stained with anti-CD40-PE, anti-CD11c-APC, anti-CD80-FITC, and anti-HLA-DR-PEcy5 for 15 min at 4℃. Fluorescence-activated cell sorting (FACS) analysis was performed using a FACScan fluorocytometer (BD Biosciences). At least 10,000 cells were acquired for each sample and the surface marker expression on cells was analyzed using Cell Quest software.
Mixed leukocyte reactions
CD4+ T-cells that were used for the mixed lymphocyte reactions (MLRs) were purified (95% purity) from PBMCs obtained from different donors isolated using anti-CD4-coated microbeads and a magnetic cell separator, which was used according to the manufacturer’s instructions. Purified CD4+ T-cells were resuspended at a concentration of 1 × 106 cells/mL in complete medium and cocultured with fresh, allogeneic LPS-Mo-DCs or C12-LPS-Mo-DCs at a ratio of 10:1 for seven days. After this period of culture, T-cells stimulated with LPS-Mo-DCs and C12-LPS-Mo-DCs were stained with anti-CD4-FITC, anti-CD45-PE, and anti- Foxp3-PE. For the detection of Foxp3 cells, T-cells were fixed and permeabilized according to the manufacturer’s instructions and incubated with an anti-human Foxp3-PEcy5 mAb. All samples were analyzed using a FACScan fluorocytometer (Beckman Coulter, Brea, CA).
FACS analysis of T-cell proliferation
Purified CD4+ T-cells were incubated with 1 µmol/L of CSFE at 1 × 106 cells/mL for 15 min at room temperature in the dark. CFSE was quenched for 3 min at room temperature with five volumes of ice-cold complete medium, and cells were washed twice. C12-LPS-Mo-DCs or LPS-Mo-DCs at 104 cells/200 µL per well in 96-well flat-bottom plates were cultured with 105 allogeneic T-cells (DCs:CD4+T = 1:10). After seven days of culture, cocultured cells were harvested, washed, and analyzed by a FACScan cytofluorometer (Beckman Coulter). Results are expressed as the percentage of positive cells.
Cytokine levels in supernatants of Mo-DC and MLR cultures
Cell-free supernatants from Mo-DCs and MLRs were harvested and frozen at −80℃ until measurement. IL-12p70 and IL-10 were detected in stimulated Mo-DC supernatants. Supernatants from MLRs were assessed for IFN-γ, IL-10, IL-12p70, and TGF-β expression using ELISA kits and measured at an extinction wavelength of 450 nm (MR5000 ELISA-reader and Bio-Linx Software; Dynatech, Chantilly, VA). TGF-β samples were acidified prior to the sample analysis. Results are expressed as the mean concentrations in triplicate culture supernatants.
Statistical analysis
Data are represented as mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA), and P < 0.05 was considered statistically significant.
Results
C12 influences Mo-DC viability
To determine whether C12 has a cytotoxic effect, we assessed the effect of C12 on cultured Mo-DCs using CCK-8 assays. Our findings showed that the viability of cells treated with C12 at concentrations of 5 µmol/L, 10 µmol/L, 15 µmol/L, 20 µmol/L, 25 µmol/L, and 50 µmol/L was >88.00%. However, at a concentration of 100 µmol/L and 200 µmol/L, C12 had harmful effects on Mo-DCs, as the viability of cells was reduced to 49.57% and 35.57%, respectively (Table 1).
Table 1.
Effect of C12 on the activity of Mo-DCs*
| Groups | Concentration | Activity of Mo-DCs (%) | P value |
|---|---|---|---|
| 3-O-C12-HSL | 5 µmol/L | 94.22 ± 6.58 | >0.05 |
| 10 µmol/L | 91.75 ± 6.49 | >0.05 | |
| 15 µmol/L | 91.58 ± 6.44 | >0.05 | |
| 20 µmol/L | 90.07 ± 6.23 | >0.05 | |
| 25 µmol/L | 89.90 ± 6.13 | >0.05 | |
| 50 µmol/L | 88.22 ± 6.02 | >0.05 | |
| 100 µmol/L | 49.57 ± 4.95 | <0.05 | |
| 200 µmol/L | 35.57 ± 4.01 | <0.05 | |
| PBS | PBS (0.1% DMSO) | 95.31 ± 6.36 | >0.05 |
| LPS | LPS(1 µg/mL) | 95.96 ± 6.45 | >0.05 |
Values are the mean ± SD of triplicate measurements. P values were measured using one-way ANOVA.
C12 influences the immune phenotype of Mo-DCs
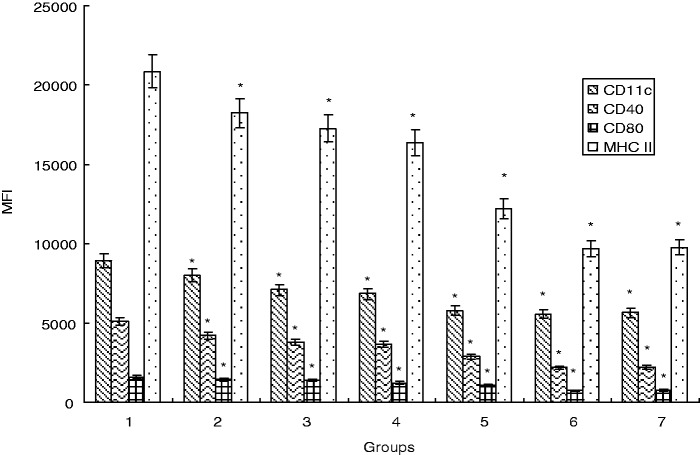
On day 5 of culture, in order to evaluate the effects of C12 on the immune phenotypic of Mo-DCs, Mo-DCs were harvested after 48 h of culture with LPS or in the presence of C12 at concentrations of 5 µmol/L, 10 µmol/L, 25 µmol/L, 50 µmol/L, and 100 µmol/L. In the LPS group, Mo-DCs were mature, with significant upregulation observed for CD11c, CD40, CD80, and HLA-DR. However, the upregulation pattern was partially attenuated in C12-treated Mo-DCs (Figure 1).
Figure 1.
C12 hampers the maturation of Mo-DCs. Monocytes were positively selected from PBMC by anti-CD14 magnetic beads and examined by flow cytometry. The results shown are representative of at least three independent experiments with mean fluorescence intensity (MFI). Data are given as the mean ± SD of triplicate measurements. *P < 0.05 compared with the LPS group. (Note: 1: LPS group; 2: 3-O-C12-HSL group with a final concentration of 5 µmol/L with 1 µg/mL of LPS; 3: 3-O-C12-HSL group with a final concentration of 10 µmol/L with 1 µg/mL of LPS; 4: 3-O-C12-HSL group with a final concentration of 25 µmol/L with 1 µg/mL of LPS; 5: 3-O-C12-HSL group with a final concentration of 50 µmol/L with 1 µg/mL of LPS; 6: 3-O-C12-HSL group with a final concentration of 100 µmol/L with 1 µg/mL of LPS; 7: PBS group.)
C12 induces functional human CD4+ Treg cells in vitro
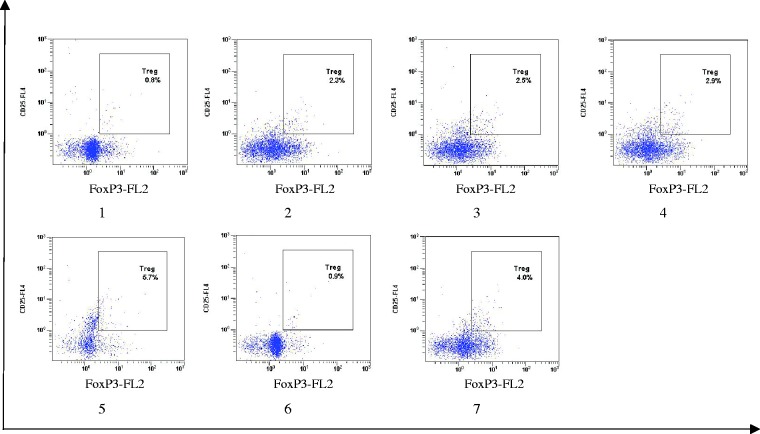
Several markers, such as CD25 and Foxp3, have been previously reported to be expressed by iTregs. We, therefore, investigated the expression of these markers after MLRs and found more positive cells expressing CD25 and Foxp3 in CD4+ T-cells in the C12-treated groups (Figure 2). These results indicate that C12 treatment increased the number of CD4+ CD25+Foxp3+ iTregs.
Figure 2.
C12-treated Mo-DCs promote the generation of CD4+CD25+Foxp3+ Tregs. CD4+ T-cells were cocultured with Mo-DCs at ratio of 10:1 for seven days. Mo-DCs were prestimulated with LPS in the presence or absence of C12 for 48 h. Cocultured cells were stained with anti-CD4, CD25, then fixed and probed for Foxp3 protein expression. The gated CD4+CD25+ population was further analyzed for their expression of Foxp3. The value represents the percentage of cells expressing Foxp3. *P < 0.05 compared with the LPS group. (Note: 1: LPS group; 2: 3-O-C12-HSL group with a final concentration of 5 µmol/L with 1 µg/mL of LPS; 3: 3-O-C12-HSL group with a final concentration of 10 µmol/L with 1 µg/mL of LPS; 4: 3-O-C12-HSL group with a final concentration of 25 µmol/L with 1 µg/mL of LPS; 5: 3-O-C12-HSL group with a final concentration of 50 µmol/L with 1 µg/mL of LPS; 6: 3-O-C12-HSL group with a final concentration of 100 µmol/L with 1 µg/mL of LPS; 7: PBS group.) (A color version of this figure is available in the online journal.)
C12-treated Mo-DCs inhibit CD4+ T-cell proliferation
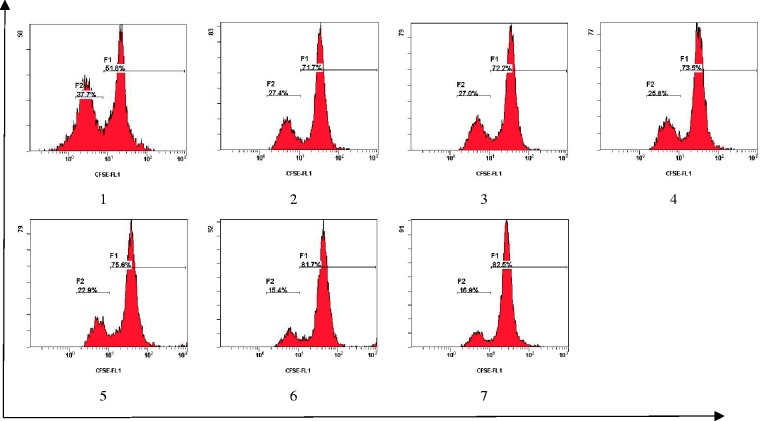
To evaluate the ability of Mo-DCs treated with LPS (with and without the presence of C12) to inhibit allogeneic CD4+ T-cell proliferation, allogeneic CD4+ T-cells labeled with CSFE were cocultured with C12- and/or LPS-treated Mo-DCs for seven days. Mo-DCs treated with both LPS and C12 consistently inhibited the proliferation of CD4+ T-cells compared with Mo-DCs treated with LPS alone (Figure 3). These results suggest that C12 inhibits CD4+ T-cell proliferation stimulated by Mo-DCs.
Figure 3.
C12-treated Mo-DCs exhibit a decreased stimulatory effect on CD4+ T-cell proliferation. Monocytes were cultured for six days with GM-CSF and IL-4; Mo-DCs were prestimulated with LPS in the presence or absence of C12 for 48 h. The stimulatory effect on T-cell proliferation was evaluated by coculturing Mo-DCs with 2 × 105 CFSE-stained allogeneic CD4+ T-cells at a ratio of 1:10 for seven days. The proliferation of T-cells was determined by flow cytometry. *P < 0.05 compared with the LPS group. (Note: 1: LPS group; 2: 3-O-C12-HSL group with a final concentration of 5 µmol/L with 1 µg/mL of LPS; 3: 3-O-C12-HSL group with a final concentration of 10 µmol/L with 1 µg/mL of LPS; 4: 3-O-C12-HSL group with a final concentration of 25 µmol/L with 1 µg/mL of LPS; 5: 3-O-C12-HSL group with a final concentration of 50 µmol/L with 1 µg/mL of LPS; 6: 3-O-C12-HSL group with a final concentration of 100 µmol/L with 1 µg/mL of LPS; 7: PBS group.) (A color version of this figure is available in the online journal.)
Altered IL-10 and IL-12p70 production by activated Mo-DCs
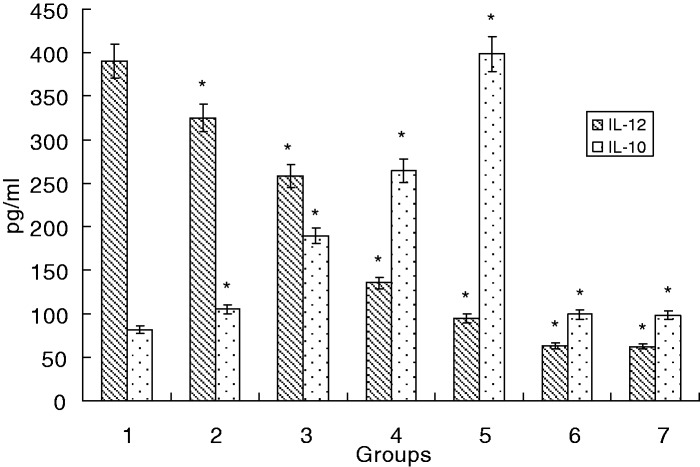
IL-10 has been shown to play an important role in promoting the differentiation of Th2 and Tregs and in inhibiting Th1 responses. In contrast, the level of IL-12p70 production by Mo-DCs during the activation of T-cells is a major factor driving the development of Th1 cells. Therefore, we measured IL-10 and IL-12p70 production in immature Mo-DCs stimulated with LPS in the presence or absence of C12. Upon C12 stimulation, Mo-DCs secreted higher amounts of IL-10 and less IL-12p70 (P < 0.05). These findings provide strong evidence that C12 affects T-cell-mediated responses by provoking Mo-DCs to release iTreg- and Th2-promoting cytokines (Figure 4).
Figure 4.
Cytokine levels in Mo-DC culture supernatants. Immature Mo-DCs were differentiated from monocytes with GM-CSF and IL-4. Mo-DCs were then stimulated with LPS in the presence or absence of C12 for five days. The cytokine concentrations in the supernatants of the C12 groups and control groups were determined by ELISA and expressed as pg/mL. The illustrated data represent the mean ± SD of the results from three separate experiments; *P < 0.05 compared with the LPS group. (Note: 1: LPS group; 2: 3-O-C12-HSL group with a final concentration of 5 µmol/L with 1 µg/mL of LPS; 3: 3-O-C12-HSL group with a final concentration of 10 µmol/L with 1 µg/mL of LPS; 4: 3-O-C12-HSL group with a final concentration of 25 µmol/L with 1 µg/mL of LPS; 5: 3-O-C12-HSL group with a final concentration of 50 µmol/L with 1 µg/mL of LPS; 6: 3-O-C12-HSL group with a final concentration of 100 µmol/L with 1 µg/mL of LPS; 7: PBS group.)
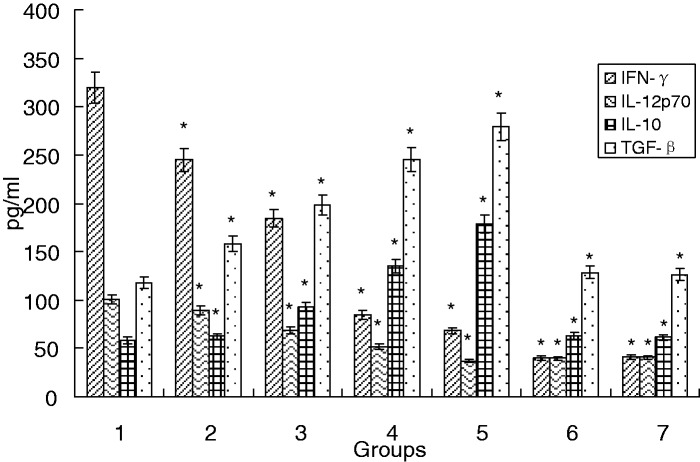
Treg and Th2 cytokine production is enhanced by C12
Supernatants from the MLRs were analyzed for their cytokine profiles. C12 induced the production of TGF-β and IL-10 (Treg and Th2 proinflammatory cytokines) at 5 µmol/L, 10 µmol/L, 25 µmol/L, and 50 µmol/L (P < 0.05). Production of IL-12p70 and IFN-γ (Th1 proinflammatory cytokines) was decreased. These results suggest that C12-LPS-Mo-DCs induced CD4+ T-cells toward the Treg and Th2 types (Figure 5).
Figure 5.
Cytokine levels in MLR supernatants. MLRs were performed with Mo-DCs:CD4+ T-cells at a ratio of 1:10. Mo-DCs were prestimulated with LPS in the presence or absence of C12 for 48 h, then cocultured for seven days and analyzed for IFN-γ, IL-10, IL-12p70, and TGF-β by ELISA. Data are given as the mean ± SD of three separate experiments. *P < 0.05 compared with the LPS group. (Note: 1: LPS group; 2: 3-O-C12-HSL group with a final concentration of 5 µmol/L with 1 µg/mL of LPS; 3: 3-O-C12-HSL group with a final concentration of 10 µmol/L with 1 µg/mL of LPS; 4: 3-O-C12-HSL group with a final concentration of 25 µmol/L with 1 µg/mL of LPS; 5: 3-O-C12-HSL group with a final concentration of 50 µmol/L with 1 µg/mL of LPS; 6: 3-O-C12-HSL group with a final concentration of 100 µmol/L with 1 µg/mL of LPS; 7: PBS group.)
Discussion
The Gram-negative bacterium PA produces a member of the N-acylhomoserine lactone (AHL) family including C12. C12 possesses the ability to establish a persistent state of infection, particularly in the lungs of patients suffering from CF and other chronic obstructive pulmonary diseases.2,3 The establishment of chronic PA infections is correlated with the disturbance of the host immune system. It has been shown that C12 modulates murine BM-DCs in terms of cytokine production, maturation marker expression, and Th2 cell polarization.10 These results are consistent with the hypothesis that C12 contributes to the establishment and maintenance of chronic infection with PA through its effects on mammalian cells.
The physiological relevance of the immunomodulatory effects of C12 on host cells is obvious and these effects are typically observed in vitro at 50 µmol/L. Previous studies revealed that concentrations of C12 in vivo vary to a large extent depending on the growth status of PA. whether the bacteria are growing as planktonic culture (1–5 µmol/L)13,14 or as a part of biofilms (100–600 µmol/L).15 The physiological concentration of C12 is clinically relevant because biofilms are formed in vivo (e.g. in the lungs of CF patients).16,17 Thus, it is most likely that the host cells that are exposed to these high C12 concentrations are also simultaneously stimulated by the bacterial LPS and, therefore, are downregulated by C12 rather than stimulated by it.
In the present study, we demonstrated that C12 inhibits the maturation of human Mo-DCs, and that C12-treated Mo-DCs selectively promote the generation of CD4+CD25+ Foxp3+ iTreg cells. Specifically, we observed that the incubation of immature Mo-DCs with C12 partially prevented IL-12p70 production and decreased the expression of HLA-DR, CD11c, CD80, and CD40. Furthermore, we analyzed the influence of C12-LPS-Mo-DCs on CD4+ T-cells and demonstrated that C12-LPS-Mo-DCs induced IL-10-producing CD4+ iTregs with a low proliferative capacity. These results were similar to those using DCs treated with different biological and pharmacological agents, which are known to be able to induce Tregs.18,19 Although the precise mechanisms remain unknown, several possibilities account for the generation of Tregs by C12-treated Mo-DCs. The activation of naïve CD4+ T-lymphocytes requires two signals delivered by DCs: one mediated through an antigen/HLA-DR-TCR interaction (signal 1) and another mediated by the interaction of costimulatory molecules such as CD80/CD86-CD28 and CD40-CD40L (signal 2). CD40 appears to be a key determinant as to whether tolerance or immunity is established. The characteristic phenotype of C12-treated Mo-DCs showed low expression of HLA-DR and costimulatory molecules, which would deliver the stimulatory but not the costimulatory signal, this is in agreement with their tolerance-inducing ability. In addition, the observation that C12-treated Mo-DCs secrete IL-10 may be linked to the stability of their tolerogenic-like phenotype. IL-10 has been shown to inhibit the expression of costimulatory molecules on APCs and to induce CD4+CD25+ Tregs in the periphery.20 In addition to IL-10, TGF-β seems to be the other major driver of peripherally induced Foxp3+ Tregs, regardless of whether it is available in its bound or secreted form.21 Many studies have demonstrated that TGF-β could function directly to mediate the Treg suppression of T-cell activation, differentiation, and proliferation.22,23 Read et al.24 first demonstrated that the immunosuppressive effect of Tregs could be abrogated by anti-TGF-β in a mouse model of colitis. In addition, Nakamura et al.25 showed that Treg suppression was cell contact-dependent and mediated by TGF-β using in vitro experiments.
Tregs are a component of the immune system that plays an important role in immune tolerance, autoimmune diseases, graft rejection, and tumor development. Currently, various subsets of regulatory T-cell populations have been identified and are subdivided based on their expression of cell surface markers, the production of cytokines, and their mechanisms of action.25 In addition to their role in maintaining immune homeostasis, evidence is now emerging that Tregs can be induced by infectious pathogens, either as an evasion strategy to subvert protective Th1 responses or as a protective mechanism of the host to limit pathogen-induced immunopathology. In this study, we found that the coculture of C12-treated Mo-DCs with autologous T-cells leads to an increase in the number of CD4+CD25+ Foxp3+ iTregs, which is in agreement with Banerjee et al.,26 who reported that human Mo-DCs effectively expand CD4+CD25+Foxp3+ T-cells in vitro and in vivo. Immunosuppression in chronic infection is a major obstacle in eradicating pathogens. The data presented in this study suggest that when using human cells, C12 was able to generate iTregs by inhibiting human Mo-DC maturation induced by LPS in vitro. However, the C12-binding receptor on Mo-DCs was not investigated in the present study. Further studies are necessary to determine how to regulate the interactions between the pathogen and the host.
Conclusion
C12-treated dendritic cells promote the generation of CD4+ CD25+Foxp3+ iTregs in vitro. These findings provide a new perspective toward understanding the persistence of the chronic inflammation that accompanies PA infection.
Acknowledgments
This work was supported by the foundation for excellent youth of Guangzhou University of traditional Chinese medicine of China (No: 2013KT1478), Medical Scientific Research Foundation of Guangdong Province, China (No: B2014182), Science and Technology Planning Project of Guangdong Province, China (No: 2013B021800241), Guangdong Natural Science Foundation (No: S2013010012970) and National Nature Science Foundation of China (No: 81071397).
Author contributions
CC and HZ designed the experiments. YL carried out immunophenotype analysis and written the article, YZ carried out T-cell proliferation analysis. BH and PQ carried out mixed leukocyte reactions. JZ and ES carried out cell culture and viability analysis. XZ carried out ELISA, JL carried out the statistical analysis.
References
- 1.Higaki S, Kitagawa T, Kagoura M, Morohashi M, Yamagishi T. Characteristics of Pseudomonas aeruginosa isolated from skin infections. Drugs Exp Clin Res 2001; 27: 121–6. [PubMed] [Google Scholar]
- 2.El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K. Persistent infection with Pseudomonas aeruginosa in ventilator associated pneumonia. Respir Crit Care Med 2008; 178: 513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkett A, Vandemheen KL, Giesbrecht-Lewis T, Ramotar K, Ferris W, Chan F, Doucette S, Fergusson D, Aaron SD. Persistency of Pseudomonas aeruginosa in sputum cultures and clinical outcomes in adult patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2012; 31: 1603–10. [DOI] [PubMed] [Google Scholar]
- 4.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. N-(3-Oxo-acyl) homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem 2006; 281: 28822–30. [DOI] [PubMed] [Google Scholar]
- 5.Bjarnsholt T, Givskov M. The role of quorum sensing in the pathogenicity of the cunningaggressor Pseudomonas aeruginosa. Anal Bioanal Chem 2007; 387: 409–414. [DOI] [PubMed] [Google Scholar]
- 6.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 1994; 91: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie AJ, Jansson A, Stallberg J, Nilsson P, Lysaght P, Cooley MA. The Pseudomonas aeruginosa quorum-sensing molecule N-3-(oxododecanoyl)-L-homoserine lactone inhibits T-cell differentiation and cytokine production by a mechanism involving an early step in T-cell activation. Infect Immun 2005; 73: 1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 2003; 71: 5785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner C, Zimmermann S, Brenner-Weiss G, Hug F, Prior B, Obst U, Hänsch GM. The quorum-sensing molecule N-3-oxododecanoyl homoserine lactone (3-O-C12-HSL) enhances the host defence by activating human polymorphonuclear neutrophils (PMN). Anal Bioanal Chem 2007; 387: 481–7. [DOI] [PubMed] [Google Scholar]
- 10.Skindersoe ME, Zeuthen LH, Brix S, Fink LN, Lazenby J, Whittall C, Williams P, Diggle SP, Froekiaer H, Cooley M, Givskov M. Pseudomonas aeruginosa quorum sensing signal molecules interfere with dendritic cell induced T cell proliferation. FEMS Immunol Med Microbiol 2009; 55: 335–45. [DOI] [PubMed] [Google Scholar]
- 11.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327: 656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z, Garg SK, BanerjeeR Regulatory T cells interfere with glutathione metabolism in dendritic cells and T Cells. J Biol Chem 2010; 285: 41525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson DL, Endersby R, Kirkham A, Stuber K, Vollman DD, Rabin HR, Mitchell I, Storey DG. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect Immun 2002; 70: 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyairi S, Tateda K, Fuse ET, Ueda C, Saito H, Takabatake T, Ishii Y, Horikawa M, Ishiguro M, Standiford TJ, Yamaguchi K. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol 2006; 55: 1381–7. [DOI] [PubMed] [Google Scholar]
- 15.Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2000; 2: 530–41. [DOI] [PubMed] [Google Scholar]
- 16.Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr 2007; 19: 83–8. [DOI] [PubMed] [Google Scholar]
- 17.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000; 407: 762–4. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Giamberardino C, Thomas JM, Gowdy K, Pastva AM, Rae Wright J. Surfactant protein A modulates induction of regulatory T cells via TGF-β. Immunology 2012; 188: 4376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Li S-P, Minc J, Zheng L. Hepatoma cells inhibit the differentiation and maturation of dendritic cells and increase the production of regulatory T cells. Immunol Lett 2007; 114: 38–45. [DOI] [PubMed] [Google Scholar]
- 20.Bonacci B, Edwards B, Jia S, Williams CB, Hessner MJ, Gauld SB, Verbsky JW. Requirements for growth and IL-10 expression of highly purified human T regulatory cells. J Clin Immunol 2012; 32: 1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+IL-10+Foxp3+ effector T cells. Nat Immunol 2009; 9: 1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3+ regulatory T cells. J Mol Cell Biol 2012; 4: 29–37. [DOI] [PubMed] [Google Scholar]
- 23.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 2007; 82: 1365–74. [DOI] [PubMed] [Google Scholar]
- 24.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med 2000; 192: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001; 194: 629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 2006; 108: 2655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]