Abstract
The development of new therapeutic strategies is necessary to reduce the worldwide social and economic impact of cardiovascular disease, which produces high rates of morbidity and mortality. A therapeutic option that has emerged in the last decade is cell therapy. The aim of this study was to compare the effect of transplanting human umbilical cord-derived stromal cells (UCSCs), human umbilical cord blood-derived endothelial cells (UCBECs) or a combination of these two cell types for the treatment of ischemic cardiomyopathy (IC) in a Wistar rat model. IC was induced by left coronary artery ligation, and baseline echocardiography was performed seven days later. Animals with a left ventricular ejection fraction (LVEF) of ≤40% were selected for the study. On the ninth day after IC was induced, the animals were randomized into the following experimental groups: UCSCs, UCBECs, UCSCs plus UCBECs, or vehicle (control). Thirty days after treatment, an echocardiographic analysis was performed, followed by euthanasia. The animals in all of the cell therapy groups, regardless of the cell type transplanted, had less collagen deposition in their heart tissue and demonstrated a significant improvement in myocardial function after IC. Furthermore, there was a trend of increasing numbers of blood vessels in the infarcted area. The median value of LVEF increased by 7.19% to 11.77%, whereas the control group decreased by 0.24%. These results suggest that UCSCs and UCBECs are promising cells for cellular cardiomyoplasty and can be an effective therapy for improving cardiac function following IC.
Keywords: Human umbilical cord-derived stromal cells, human umbilical cord blood-derived endothelial cells, cell therapy, ischemic cardiomyopathy
Introduction
Cardiovascular disease constitutes a major cause of morbidity and mortality worldwide,1–3 and it is important to develop new therapeutic strategies to reduce its immense social and economic impact. Ischemic cardiomyopathy (IC) involves changes in the cardiac structure, including thinning of the infarcted wall, cardiac dilatation, remodeling due to apoptosis and necrosis of cardiac myocytes, hypertrophy, fibrosis, and the infiltration of inflammatory cells.4,5
Cell therapy has shown promising results in several preclinical studies6–10 and clinical trials11–14 that have reported the safety and efficacy of cell transplantation for a wide range of therapeutic applications. Stem/progenitor cell-based therapies have been demonstrated to be effective for tissue engineering and have potential applicability for therapeutic neovascularization, vascular repair, and tissue engineering.15–18
Human umbilical cord blood (HUCB)19,20 and human umbilical cord (HUC)21,22 have been considered as potential cell sources for cell therapy, and they were recently studied as an option to treat/prevent cardiac diseases.23,24
The aim of this work was to evaluate the efficacy of umbilical cord-derived stromal cells (UCSCs), human umbilical cord blood-derived endothelial cells (UCBECs), and a combination of these two cell types to improve cardiac function in a rat model of IC. The transplantation of UCSCs and UCBECs was expected to not only improve cardiac function but also to facilitate the reduction of infarct size, attenuate the progression of left ventricular dysfunction in the ischemic tissue, and increase contractile function.
Materials and methods
Isolation and culture of UCSCs
Tissue collection for research was approved by the ethics committee of Pontifícia Universidade Católica do Paraná (number 2788/08). Under sterile conditions, the UC (n = 23) was washed with phosphate-buffered saline (PBS) three times to remove the blood. Then, the UC was cut open, and the Wharton’s jelly (WJ) tissue was excised and minced into fine pieces that were 0.5–1 mm2 in size. The WJ was immersed in an enzymatic solution, 1.49 µg/mL of collagenase type I (Invitrogen, Grand Island, NY, USA), incubated for 16 h at 37℃, and then washed with PBS and treated with 0.25% trypsin/EDTA (Invitrogen, Grand Island, NY, USA) for 30 min at 37℃. Finally, the cells were passed through a 40 µm nylon mesh (BD Falcon™, Franklin Lakes, NJ, USA), resuspended in Iscove’s modified Dulbecco’s medium (IMDM) (Invitrogen, Grand Island, NY, USA) supplemented with 1% penicillin/streptomycin (Invitrogen, Grand Island, NY, USA) and 15% fetal calf serum (FCS) (Invitrogen, Grand Island, NY, USA), and plated in 75 cm2 flasks for culturing at 37℃ with 5% CO2 in a humidified atmosphere.
Isolation of mononuclear cells from HUCB
The UCB (n = 23) was diluted 1:3 with IMDM and carefully loaded onto a density gradient of Ficoll-Histopaque (1.077 g/mL) (Sigma-Aldrich, Saint Louis, MO, USA) to isolate the mononuclear cells (MNCs). The MNCs were isolated by centrifugation (400 × g, 30 min, room temperature) and washed twice with IMDM. The MNCs were carefully removed and transferred to a new conical tube, washed twice with IMDM, and resuspended in IMDM supplemented with 1% penicillin/streptomycin and 15% FCS.
Immunomagnetic selection of CD133+ cells from UCB and culture of UCBECs
UCB MNCs expressing the CD133 antigen were immunomagnetically selected using the human CD133 MicroBeads and Magnetic Cell Sorting (MACS) system (Miltenyi Biotec®, Bergisch-Gladbach, Germany), as previously described by Senegaglia et al.25 Briefly, the MNCs were incubated with a blocking solution and anti-CD133 antibody (Miltenyi Biotech, Bergisch-Gladbach, Germany) linked to magnetic MicroBeads for 30 min at 4℃. The magnetically labeled CD133+ cells were retained in the column and were subsequently eluted as the positively selected cell fraction after removing the column from the magnetic field. The purity of the MACS-separated subpopulations was confirmed by flow cytometry with monoclonal antibodies (CD34, CD45, AC133). The isolated CD133+ cells were plated in 25 cm2 flasks in Endothelial Basal Medium 2 (EBM-2) medium supplemented with 10% FCS and Endothelial Cell Growth Medium 2 (EGM-2) MV Single Quots (Cambrex, Walkersville, MD, USA) at 37℃ with 5% CO2 in a humidified atmosphere.
Adipogenic, osteogenic, and chondrogenic differentiation
Adipogenic, osteogenic, and chondrogenic differentiation were conducted as previously described by Rebelatto et al.26 Briefly, the cells were seeded on glass coverslips in 24-well plates, and lineage-specific differentiation was induced by adipogenic or osteogenic medium (Invitrogen, Grand Island, NY, USA). For chondrogenic differentiation, 5 × 105 cells were centrifuged in a 15 mL conical tube at 400 × g for 10 min, and lineage-specific differentiation was induced by chondrogenic medium (Invitrogen, Grand Island, NY, USA). Adipogenesis was assessed by Oil Red O staining, osteogenesis was assessed by Alizarin Red S staining, and chondrogenesis was assessed by toluidine blue staining.
Flow cytometry
The UCSCs were incubated with the following monoclonal antibodies to determine their typical cell surface epitope profiles: anti-CD 14, anti-CD 19, anti-CD 29, anti-CD 31, anti-CD 34, anti-CD 44, anti-CD 45, anti-CD 73, anti-CD 90, anti-CD 117, HLA-DR (all from BD-Pharmingen™ San Jose, CA, USA), and anti-CD 105 (eBioscience Inc., San Diego, CA, USA). The UCBECs were incubated with the following monoclonal antibodies: anti-CD 29, anti-CD 31, anti-CD 34, anti-CD 45, anti-CD 106, anti-CD 117, anti-CD 144, anti-CD 146, anti-CD 166, anti-CD 309 (all from BD-Pharmingen™ San Jose, CA, USA), anti-CD 105 (eBioscience Inc., San Diego, CA, USA), and anti-CD 133 (Miltenyi Biotec®). Their viability was assessed by 7AAD (BD-Pharmingen™) staining. PE-, FITC-, APC-, and PerCP-conjugated anti-mouse IgG1 antibodies (all from BD-Pharmingen™ San Jose, CA, USA) were used as isotype controls. The data for cell staining were acquired using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Immunofluorescence of UCSCs and UCBECs
In brief, UCSCs and UCBECs were washed with PBS, fixed with 2% paraformaldehyde and permeabilized with 0.2% Triton X-100. After blocking with 1% Bovine serum albumin , the UCBECs were incubated for 3 h at room temperature with polyclonal mouse anti-von Willebrand factor (vWF) antibody (Dako, Glostrup, Denmark; diluted 1:200) followed by FITC labeled anti-rabbit IgG secondary antibody (Sigma-Aldrich, Saint Louis, MO, USA; diluted 1:80), as well as mouse anti-human CD 31 (BD-Pharmingen™ San Jose, CA, USA; diluted 1:100) followed by Texas red X labeled goat anti-mouse secondary antibody (Santa Cruz Biotechnology, CA, USA; diluted 1:1500). The UCSCs were stained with mouse anti-human CD 105 (BD-Pharmingen™; diluted 1:100) followed by Texas red X labeled goat anti-mouse secondary antibody (Santa Cruz Biotechnology; diluted 1:1500), as well as goat anti-vimentin (Santa Cruz Biotechnology; diluted 1:50) followed by Alexa Fluor 488 labeled rabbit anti-goat secondary antibody (Molecular Probes, Oregon, USA; diluted 1:1500). Nuclear staining was performed with 4′-6-diamidino-2-phenylindole (DAPI). The coverslips were mounted with mounting medium and observed by fluorescent microscopy (Leica).
Double positive staining with acetylated low-density lipoprotein and lectin
To confirm their EC phenotype, the UCBECs were examined for the ability to bind acetylated low-density lipoprotein (AcLDL) (Biomedical Technologies Inc., Stoughton, MA, USA) and lectin (Sigma). The cells were incubated with 2 mg/mL of AcLDL for 1 h at 37℃, fixed in 4% paraformaldehyde and counterstained with 50 mg/mL of lectin for 1 h at 37℃ in the dark. Nuclear staining was performed with DAPI. The cells displaying double-positive fluorescence for AcLDL and lectin were considered to be ECs. After staining, the samples were viewed with an inverted fluorescent microscope (Leica, Solms, Germany).
Animal model
The animal studies were approved by the Ethics Committee in Research of the Pontifícia Universidade Católica do Paraná (no. 401). Male adult Wistar rats (Rattus norvegicus) were used for the study. The animals were housed in groups of four rats/cage at room temperature (18–21℃) in a humidity-controlled (55–65% relative humidity) environment with a 12-h light-dark cycle and ad libitum access to standard rodent chow and water.
Induction of IC
IC was produced as previously described.27 Briefly, the rats received intramuscular injections of 5 mg/kg of meperidine with 0.04 mg/kg atropine. After 10 min, they were anesthetized with ≈4% halothane in an anesthesia chamber. A left thoracotomy was performed between the 4th and the 5th intercostal spaces. The thorax was opened, the left anterior descending coronary artery was occluded at 2 mm from its origin by ligating the artery between the pulmonary artery and the left atrial auricle with 4-0 silk thread. Then, the heart was rapidly returned to its normal position in the thorax, and the surgical incision was closed. The rat was placed in a recovery cage with a supply of oxygen for approximately 30 min. Analgesia (morphine 1 mg/kg/SC; flunixin meglumine 2.5 mg/kg) and antibiotic therapy (enrofloxacin 10 mg/kg/IM) were scheduled for up to 72 h.
Echocardiographic analysis
Baseline echocardiographs were performed seven days after IC induction using an echocardiographic system equipped with a 7.5-MHz phased-array transducer (Hewlett-Packard, Andover, MA, USA). The animals were anesthetized with intramuscular injections of ketamine chlorhydrate (50 mg/kg) and xylazine (5 mg/kg). All of the measurements were averaged from three consecutive cardiac cycles and were analyzed by one independent observer who was blinded to the treatment status of the animals. Animals with a LVEF of ≤40% were selected for the study.
Cell transplantation
The rats were first premedicated by intraperitoneal injections of 1.25 mg/kg diazepam and 12.5 mg/kg ketamine, as well as an intramuscular injection of 5 mg/kg of meperidine. Anesthesia was induced by ≈4% halothane in 100% oxygen in a glass induction chamber. Each rat was then endotracheally intubated, and anesthesia was maintained by ≈2% halothane vaporized in 100% oxygen (≈150 mL/min) in a semi-closed breathing circuit. Each rat was mechanically ventilated using a ventilator (Harvard Apparatus, South Natick, MA, USA), which was set to 70–80 breaths/min and 175–200 mL/min. The heart was exposed through a thoracotomy of the breastbone. The cells in IMDM or medium alone were administrated intramyocardially in three separated equivolumetric injections in the infarct border zone, totalizing 200 µL. The recovery and postsurgical care were identical to the procedures after surgical induction of IC.
Histology
The hearts were sectioned from the apex to the base into four transverse sections. Histological sections from formalin-fixed and paraffin-embedded tissues were cut at 4 mm thickness and stained with Masson trichrome. For each section, 10 randomly selected fields of view were captured using a microscope coupled to a video camera (Leica, Solms, Germany), which sent digital images to a computer, and were analyzed using Image Pro-plus 6.0 image analysis software (Media Cybernetics®, Silver Spring, MD, USA). To identify the effects of cells on the myocardial capillary density, the heart sections were stained with a monoclonal anti-laminin antibody (Dako, Glostrup, Denmark). For quantification, microscopic fields of view were selected from the infarct region, and the positively stained capillaries were counted. The capillary density was assessed by counting the number of capillaries in fields of view from tissue sections, and the data are expressed as the number of capillaries/field.
Statistical analysis
The results obtained from the study are expressed as the mean ± SD, the median, and the minimum and maximum values. A one-way analysis of variance (ANOVA) was used to compare the groups with respect to the quantitative variables that were assessed pretransplantation. An analysis of co-variance (ANCOVA) was used to compare the groups in relation to the posttransplant evaluations as well as to compare the differences between the pre- and post-transplant values, and the baseline values were used as the covariate. The nonparametric Kruskal-Wallis test was used to compare the groups in terms of the percentage of collagen and the number of capillaries. Student’s t-test was used to compare the pre- and post-transplant values for paired samples. P values <0.05 were considered to be statistically significant. All statistical analyses were performed using SPSS v.20.0 software.
Results
Isolation of UCSCs, MNCs and purification of HUCB-derived CD133+ cells
A total of 5.26 × 106 ± 6.32 cells were isolated from HUC. The mean volume of HUCB was 94.4 ± 42.30 mL, and the number of MNCs after isolation was 26.74 × 106 ± 19. Flow cytometry analysis demonstrated that 68.15% (±8.17) of the MNCs were CD45+, 1.66% (±0.43) were CD133+, and 3.93% (±5.19) were CD34+. After MACS-separation 17.26 × 105 ± 19.67 cells were isolated. Flow cytometry analysis demonstrated that 10.9% (±9.57) of the MACS-separated cells were CD45+, 84.4% (±10.24) were CD133+, and 68.6% (±21.44) were CD34+.
Characterization of UCSCs and UCBECs by morphology and flow cytometry assays
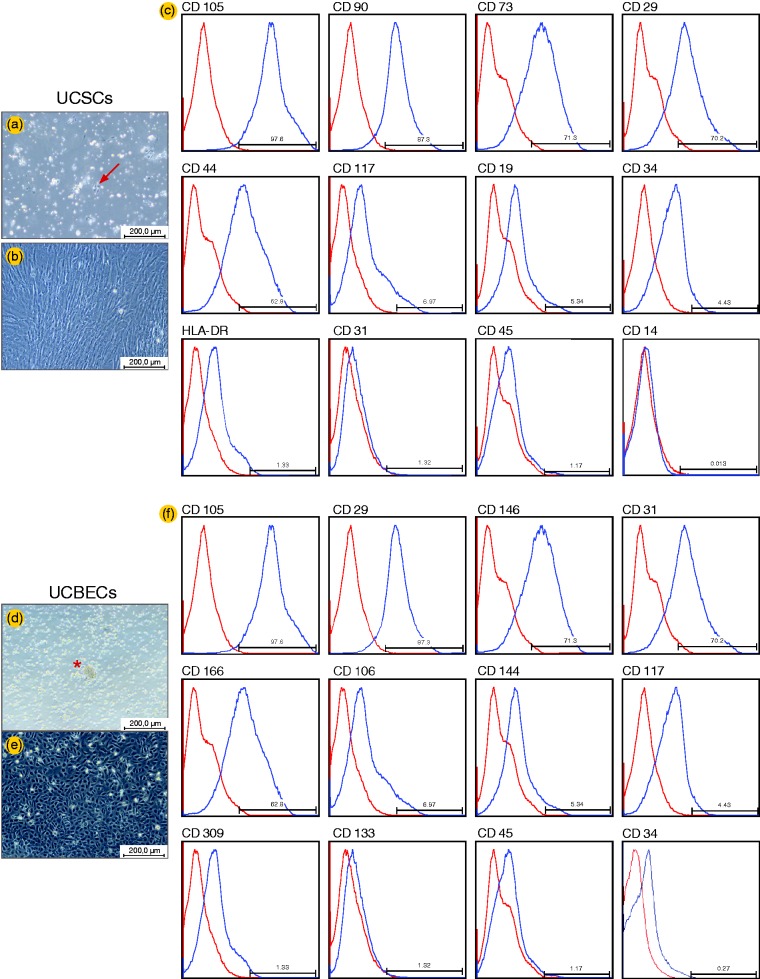
The cell cultures were examined daily under phase contrast microscopy, and a nearly homogeneous population of UCSCs with a fibroblast-like spindle-shaped morphology was observed. The UCBEC cultures contained multiple thin, flat cells that emanated from central clusters of rounded cells. After 15 days in culture, the UCBECs displayed a typical endothelial-like cobblestone morphology (Figure 1). Flow cytometry analysis was performed to characterize the UCSCs and UCBECs. The staining profiles of the cells for specific markers are provided in Figure 1.
Figure 1.
Characterization of UCSCs and UCBECs by morphology and flow cytometry assays. UCSCs displayed a typical spindle-shaped morphology (a and b), and UCBECs demonstrated a typical endothelial cobblestone morphology (d and e), as observed by phase microscopy. Flow cytometry histograms of UCSCs (c) and UCBECs (f). The blue line indicates the positively staining cells, whereas the red line indicates the isotype-matched monoclonal antibody control. The values are representative of three independent experiments
UCSC: umbilical cord-derived stromal cells; UCBEC: umbilical cord blood-derived endothelial cells. (A color version of this figure is available in the online journal.)
Characterization of UCSCs and UCBECs by immunofluorescence and differentiation assays
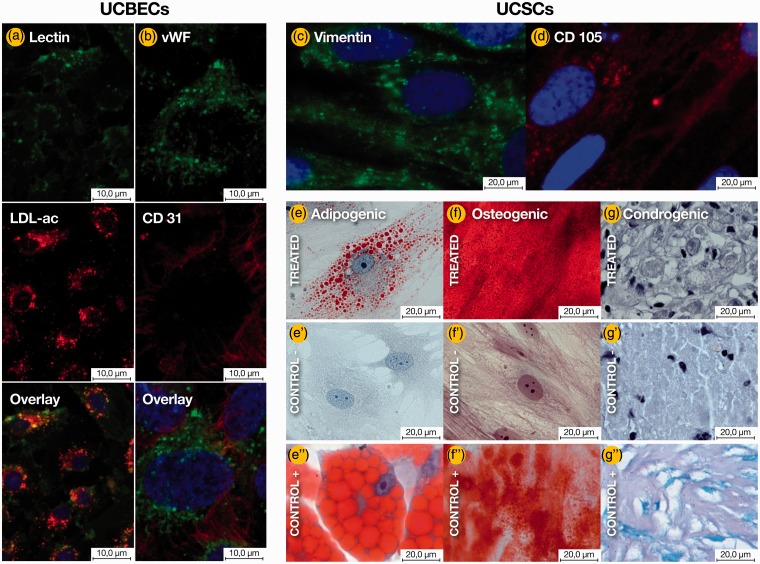
The cultured UCBECs stained positive for CD31, vWF, and vascular endothelial growth factor, indicating an appropriately enriched culture of ECs. The UCSCs stained positive for vimentin and CD105. The negative control did not produce a positive signal. UCBECs in the third passage were positive for DiI-AcLDL uptake and lectin binding. The UCSCs differentiated into osteoblasts, adipocytes, and chondrocyte-like cells, demonstrating their multipotency (Figure 2 and Figure S1).
Figure 2.
Detection of endothelial and stromal cell marker expression by immunofluorescence analysis. The UCBECs were shown to simultaneously bind fluorescein isothiocyanate UEA-1 (lectin) (a) and engulf DiI-acLDL (a’). (a”) Overlay of lectin+DiI-acLDL+DAPI. The UCBECs were labeled with vWF/FITC (b) and CD31/TexasRed (b’). (b”) Overlay of vWF+CD31+DAPI. The UCSCs were labeled with vimentin/FITC (c) and endoglin (CD105)/TexasRed (d). The nuclei were stained with DAPI (a”, b”, c and d). Characterization of MSC-like differentiation potential toward the osteogenic, chondrogenic, and adipogenic lineages. After three weeks in the respective induction media, the UCSCs stained positively for lipid vacuoles with Oil Red O (e), demonstrated the formation of mineralized matrix, as assessed by alizarin staining (f), and were positive for intracellular matrix mucopolysaccharides by toluidine blue staining (g). Cells cultured in growth medium without inductive factors served as negative controls (e’, f’, g’). BM–derived MSCs were utilized as a positive control (e”, f”, g”). Scale bars are specified in each image.
UCSC: umbilical cord-derived stromal cells; UCBEC: umbilical cord blood-derived endothelial cells; LDL: low-density lipoprotein; vWF: von Willebrand factor. (A color version of this figure is available in the online journal.)
Effect of UCSC, UCBEC, and UCSC + UCBEC transplantation on cardiac function
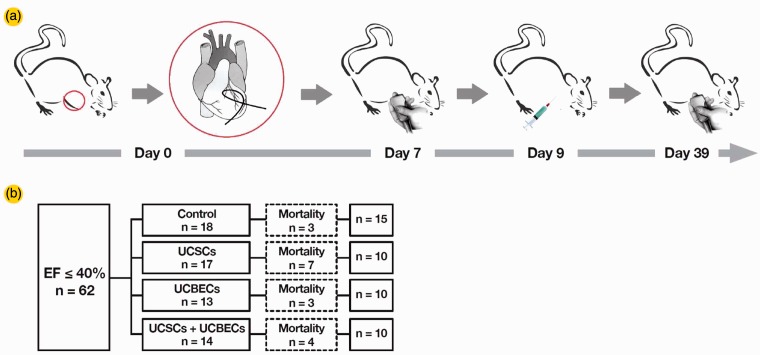
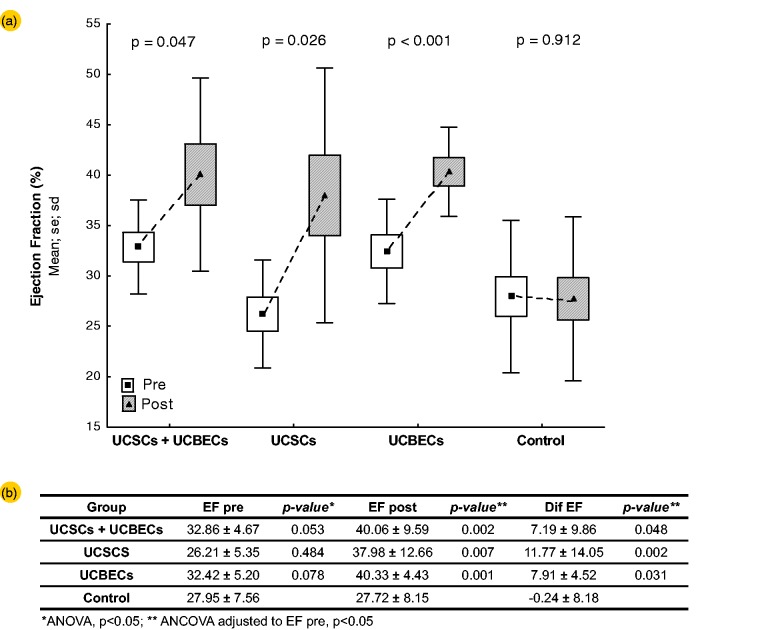
A total of 62 male Wistar rats with a left ventricular ejection fraction (LVEF) of less than or equal to 40% were randomized into four groups: Control (n = 18), UCSC (n = 17), UCBEC (n = 13) and UCSC + UCBEC (n = 14; Figure 3). After transplantation, a total of 17 animals died remaining 10 animals in each of the cell transplantation groups and 15 in the control group. The results from one month posttransplantation showed that there were significant differences between the pre- and post-transplant LVEF in the treatment and control groups (P = 0.006). However, the differences among the cell transplant groups were not significant (P = 0.980). LVEF improved by 7.19 ± 9.86% from the pretransplant value in the UCSC+UCBEC group, 7.91 ± 4.52% in the UCBEC group, and11.77 ± 14.05% in the UCSC group. In contrast, LVEF decreased by 0.24 ± 8.18% in the control group (Figure 4).
Figure 3.
(a) Flow chart of the study design. Day 0: Exteriorization of the heart for coronary artery ligation. Day 7: The animals were subjected to echocardiography. Day 9: Animals with an LVEF of less than 40% were randomized and received a transplant. Day 39: A second ECHO was performed on the 30th day post-transplantation. (b) Mortality/survival rate for each group. Dashed boxes indicate the number of animals dead after the treatment (cell transplantation or medium injection)
UCSC: umbilical cord-derived stromal cells; UCBEC: umbilical cord blood-derived endothelial cells. (A color version of this figure is available in the online journal)
Figure 4.
Evaluation of cardiac function. (a) The left ventricular ejection fractions measured by echocardiography on the seventh day post-AMI (open boxes) and the 30th day post-transplantation (solid boxes) were significantly different for all transplantation groups (i.e. UCSCs + UCBECs, UCSCs, and UCBECs; P values are shown by each pair of boxes). The control group did not show a significant difference before and after transplantation. (b) LVEF pre transplant, post transplant, and the difference between pre-and post-transplant, for each cell transplantation group compared to the control group. The data are presented as the means. The P value was calculated by a ANOVA for the pre-transplant values and an ANCOVA for the post-transplant values and the comparisons between the cell groups and the control group (pre-transplant as a covariate; P < 0.05 was considered statistically significant). EF = ejection fraction. The boxes are the means ± standard error, and the bars are the means ± SD.
UCSC: umbilical cord-derived stromal cells; UCBEC: umbilical cord blood-derived endothelial cells; ANOVA: one-way analysis of variance; ANCOVA: analysis of co-variance. (A color version of this figure is available in the online journal.)
Reduction of myocardial fibrosis and increase in capillary density by UCSC, UCBEC, and UCSC + UCBEC transplantation
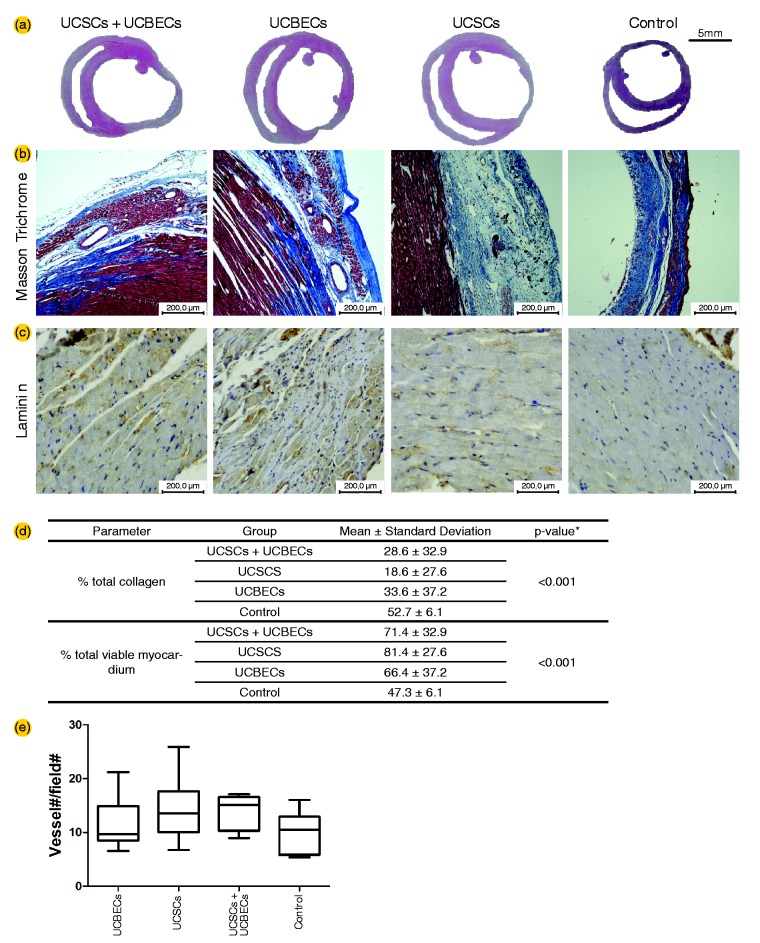
The transplantation of UCSCs, UCBECs, and UCSCs + UCBECs significantly attenuated the development of myocardial fibrosis. In addition, a trend of increased capillary density was observed in all the cell transplantation groups. However, the differences were not significant. Masson trichrome staining revealed that the areas containing collagen deposits were significantly smaller in the treated groups than in the control group. To evaluate the effects of the transplanted cells on cardiac fibrosis, we quantified the fibrotic area and the area of viable myocardium in the myocardial sections using image analysis software (Image-pro plus 6.0). The number of capillaries, which was counted using the technique described by Zhao et al.,28 with modifications, showed a trend of increased numbers of blood vessel in the infarct area in the UCSC and UCSC + UCBEC groups (Figure 5).
Figure 5.
Histological analysis of the total collagen content, evaluated using Masson trichrome staining to detect myocardial fibrosis (collagen stained blue, viable myocardium stained red) (a), and capillary density analysis using an anti-laminin antibody (b). Quantification of the percentage of total collagen and the total viable myocardium. The total area of necrosis was significantly reduced in all three treatment groups compared to the control group (c). Quantification of the total capillary density in the myocardium. A trend of increased capillary density was observed in all the cell transplantation groups. However, the differences were not significant (d). UCSC: umbilical cord-derived stromal cells; UCBEC: umbilical cord blood-derived endothelial cells. (A color version of this figure is available in the online journal.)
Discussion
In the present study, we evaluated the effects of UCSCs, UCBECs, and a combination of these two cell types for the treatment of IC in a rat model of permanent ligation of the left coronary artery. Studies have shown that the transplantation of stem/progenitor cells improves cardiac function in experimental models of ischemic heart disease.29–33 However, studies comparing the regenerative capacity of UCSCs and UCBECs are scarce, if not absent, for IC. Our study demonstrates, for the first time, the effects of UCSCs and UCBECs in animals with LVEF less than or equal to 40%, which corresponds to significant cardiac dysfunction. In humans, the effect of bone marrow-derived stem cells on LVEF was also greater and more statistically significant when participants had LVEF ≤40% of baseline compared to participants with LVEF >40% of baseline.34
The major findings of our study demonstrated that in an animal model of IC induced by ligation of the left coronary artery, the transplantation of 2 × 106 UCSCs, UCBECs, or UCSCs + UCBECs significantly improved heart function compared to the control group. However, there was not a significant difference among the cell therapy groups in global LVEF 30 days after the procedure, as revealed by ECHO analysis. The improved global heart function and decreased cardiac fibrosis in rats with IC imply the potential benefits of the direct transplantation of UCSCs, UCBECs, or UCSCs + UCBECs to the damaged area. Henning et al.35 reported that directly injecting HUCB-derived cells into the myocardium was effective in reducing infarct size and improving ventricular function in non-immunosuppressed rats. The heterogeneity between the findings of clinical trials and experimental research can be attributed to differences in the disease context, patient population, cell isolation protocols, cell dose/type, timing of cell infusion, and the route of delivery.34,36
Another important result of this study was that UCSCs, UCBECs, and UCSCs + UCBECs significantly attenuated collagen deposition, which indicates that they inhibited the formation of a collagenous scar. This result suggests that the primary benefit may be reducing the number of apoptotic/necrotic cells in the border zone or favorably modulating the matricellular environment. We conducted a direct comparison of different cell types for the stimulation of angiogenesis in the same IC Wistar rat model. Injections of UCSCs, UCBECs, and UCSCs + UCBECs into infarcted rat hearts do not induced angiogenesis significantly, although a tendency was observed when treated with UCSCs and with both cell types together. This observation is at least partially discordant with the increased capillary density reported in other studies.37–39 Nonetheless, differences in the procedures to generate heart ischemia, in the pathways of cell injection, cell number, cell sources/isolation, and even in the assays to quantify vessels might account for the conflicting results.
The mechanisms by which UCB and UC cells protect the heart and improve cardiac function appear to be complex and multifactorial.40 Some studies suggest that the cells induce neovascularization in the necrotic area,37–39 modulate the inflammatory reaction induced by ischemic cascades41 and secrete growth factors42,43 in animal models of IC.
In summary, these results demonstrate that the transplantation of ex vivo expanded UCSCs, UCBECs, and UCSCs + UCBECs promotes cardiac repair in IC animal models and could be used to improve the recovery of cardiac function after IC. Thus, these cells are promising tools for cardiovascular regenerative medicine, confirming their value for banking purposes and future therapeutic use.
Acknowledgements
We thank Monica Hansen for her assistance with the figures.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; PHS, LGAC, FB, LM, DJ, LF, AVS, ACS and CLKR conducted the experiments, MO performed statistical analysis, PHS and AC wrote the manuscript and PRSB review of the manuscript.
Funding
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (CT-Saúde/MS/SCTIE/DECIT/MCT/CNPq N° 17/2008) and Fundação Araucária (Convênio no. 416/2009).
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367: 1747–57. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115: e169–71. [DOI] [PubMed] [Google Scholar]
- 3.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 2008; 118: 2057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anversa P, Kajstura J, Olivetti G. Myocyte death in heart failure. Curr Opin Cardiol 1996; 11: 245–51. [DOI] [PubMed] [Google Scholar]
- 5.Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord 2003; 3: 1–30. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene Ther 2000; 7: 451–7. [DOI] [PubMed] [Google Scholar]
- 7.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000; 97: 3422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 2001; 98: 10344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–6. [DOI] [PubMed] [Google Scholar]
- 10.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004; 428: 668–73. [DOI] [PubMed] [Google Scholar]
- 11.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 2002; 106: 3009–17. [DOI] [PubMed] [Google Scholar]
- 12.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003; 361: 45–6. [DOI] [PubMed] [Google Scholar]
- 13.Gowdak LH, Schettert IT, Baptista E, Lopes NL, Rochitte CE, Vieira ML, Grupi CJ, Cesar LA, Krieger JE, de Oliveira SA. Intramyocardial injection of autologous bone marrow cells as an adjunctive therapy to incomplete myocardial revascularization—safety issues. Clinics (São Paulo) 2008; 63: 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gowdak LH, Schettert IT, Rochitte CE, Lisboa LA, Dallan LA, Cesar LA, de Oliveira SA, Krieger JE. Early increase in myocardial perfusion after stem cell therapy in patients undergoing incomplete coronary artery bypass surgery. J Cardiovasc Transl Res 2011; 4: 106–13. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 2004; 287: H2670–6. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol 2007; 42: 88–97. [DOI] [PubMed] [Google Scholar]
- 17.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res 2009; 104: 1410–20. [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 2011; 42: 2045–53. [DOI] [PubMed] [Google Scholar]
- 19.Henning RJ, Abu-Ali H, Balis JU, Morgan MB, Willing AE, Sanberg PR. Human umbilical cord blood mononuclear cells for the treatment of acute myocardial infarction. Cell Transplant 2004; 13: 729–39. [DOI] [PubMed] [Google Scholar]
- 20.Hirata Y, Sata M, Motomura N, Takanashi M, Suematsu Y, Ono M, Takamoto S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem Biophys Res Commun 2005; 327: 609–14. [DOI] [PubMed] [Google Scholar]
- 21.Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS ONE 2008; 3: e3336–e3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J, Yu P, Li C, Liu X, Huang Z, Wang D, Li H, Gu Z, Liu B, Li Z. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther 2010; 12: R210–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suuronen EJ, Price J, Veinot JP, Ascah K, Kapila V, Guo XW, Wong S, Mesana TG, Ruel M. Comparative effects of mesenchymal progenitor cells, endothelial progenitor cells, or their combination on myocardial infarct regeneration and cardiac function. J Thorac Cardiovasc Surg 2007; 134: 1249–58. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Wei M, Zhu W, Han B. Combined transplantation of endothelial progenitor cells and mesenchymal stem cells into a rat model of isoproterenol-induced myocardial injury. Arch Cardiovasc Dis 2008; 101: 333–42. [DOI] [PubMed] [Google Scholar]
- 25.Senegaglia AC, Barboza LA, Dallagiovanna B, Aita CA, Hansen P, Rebelatto CL, Aguiar AM, Miyague NI, Shigunov P, Barchiki F, Correa A, Olandoski M, Kreiger MA, Brofman PR. Are purified or expanded cord blood-derived CD133+ cells better at improving cardiac function? Exp Biol Med (Maywood) 2010; 235: 119–29. [DOI] [PubMed] [Google Scholar]
- 26.Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008; 233: 901–13. [DOI] [PubMed] [Google Scholar]
- 27.Zornoff LAM, Paiva SAR, Minicucci MF, Spadaro J. Infarto do miocárdio experimental em ratos: análise do modelo. Arq Bras Cardiol 2009; 93: 403–8. [Google Scholar]
- 28.Zhao M, Zhang W, Xing D, Li P, Fu J, Gong K, Hage FG, Oparil S, Chen YF. Endothelial cells overexpressing IL-8 receptor reduce cardiac remodeling and dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 2013; 305: H590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP, Xiao YF. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg 2002; 74: 1568–75. [DOI] [PubMed] [Google Scholar]
- 30.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002; 105: 93–8. [DOI] [PubMed] [Google Scholar]
- 31.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol 2003; 35: 1113–9. [DOI] [PubMed] [Google Scholar]
- 32.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 2005; 102: 11474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation 2005; 112: 214–23. [DOI] [PubMed] [Google Scholar]
- 34.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, Watt SM, Martin-Rendon E. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS ONE 2012; 7: e37373–e37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henning RJ, Burgos JD, Vasko M, Alvarado F, Sanberg CD, Sanberg PR, Morgan MB. Human cord blood cells and myocardial infarction: effect of dose and route of administration on infarct size. Cell Transplant 2007; 16: 907–17. [DOI] [PubMed] [Google Scholar]
- 36.Taylor DA, Zenovich AG. Cardiovascular cell therapy and endogenous repair. Diabetes Obes Metab 2008; 10(Suppl 4): 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botta R, Gao E, Stassi G, Bonci D, Pelosi E, Zwas D, Patti M, Colonna L, Baiocchi M, Coppola S, Ma X, Condorelli G, Peschle C. Heart infarct in NODSCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J 2004; 18: 1392–4. [DOI] [PubMed] [Google Scholar]
- 38.Ma N, Stamm C, Kaminski A, Li W, Kleine HD, Muller-Hilke B, Zhang L, Ladilov Y, Egger D, Steinhoff G. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res 2005; 66: 45–54. [DOI] [PubMed] [Google Scholar]
- 39.Wu KH, Zhou B, Mo XM, Cui B, Yu CT, Lu SH, Han ZC, Liu YL. Therapeutic potential of human umbilical cord-derived stem cells in ischemic diseases. Transplant Proc 2007; 39: 1620–2. [DOI] [PubMed] [Google Scholar]
- 40.Park DH, Lee JH, Eve DJ, Borlongan CV, Sanberg PR, Chung YG, Cho TH. Human umbilical cord blood stem cells rescue ischemic tissues. In: Wislet-Gendebien S. (ed.). Advances in regenerative medicine, Rijeka, Croatia: InTech, 2011. [Google Scholar]
- 41.Henning RJ, Burgos JD, Ondrovic L, Sanberg P, Balis J, Morgan MB. Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplant 2006; 15: 647–58. [DOI] [PubMed] [Google Scholar]
- 42.Hu CH, Wu GF, Wang XQ, Yang YH, Du ZM, He XH, Xiang P. Transplanted human umbilical cord blood mononuclear cells improve left ventricular function through angiogenesis in myocardial infarction. Chin Med J (Engl) 2006; 119: 1499–506. [PubMed] [Google Scholar]
- 43.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 2005; 80: 229–36. [DOI] [PubMed] [Google Scholar]