Abstract
Major progress in deciphering the role of the E3 ligase, ITCH, in animal physiology has come from the generation and identification of Itch loss-of-function mutant mice (itchy). Mutant mice display an autoimmune-like phenotype characterized by chronic dermatitis, which has been attributed to increased levels of ITCH target proteins (e.g. transcription factors JUNB and CJUN) in T cells. Autoimmune disorders also exist in humans with Itch frameshift mutations resulting in loss of functional ITCH protein. Recent phenotypic analysis of male itchy mice revealed reduced sperm production, although cross breeding experiments showed no difference in litter size when male itchy mice were bred to wild type females. However, a reduction in litter sizes did occur when itchy females were bred to wild type males. Based on these results, characterization of female reproductive function in itchy mice was performed. Developmental analysis of fetuses at gestational day 18.5, cytological evaluation of estrous cyclicity, histopathological analysis of ovaries, and protein analysis were used to investigate the itchy reproductive phenotype. Gross skeletal and soft tissue analysis of gestational day 18.5 itchy fetuses indicated no gross developmental deformities. Itchy females had reduced implantation sites, decreased corpora lutea, and increased estrous cycle length due to increased number of days in estrus compared to controls. Alterations in the expression of prototypical ITCH targets in the ovaries were not indicated, suggesting that an alteration in an as yet defined ovary-specific ITCH substrate or interaction with the altered immune system likely accounts for the disruption of female reproduction. This report indicates the importance of the E3 ligase, ITCH, in female reproduction.
Keywords: Itch, itchy, E3 ligase, ubiquitin, reproduction, fertility, estrous cycle, ovulation, development
Introduction
The itchy E3 ubiquitin ligase, ITCH, was first described in a mutant mouse as an unusual allele of Agouti that affected not only coat color but also immune function.1 It was subsequently determined that this phenotype was due to a chemically and radiation-induced pericentric inversion of distal mouse chromosome 2 whose breakpoints mapped to the regulatory region of Agouti, causing a down regulation of the mRNA and lighter fur color.1 This mutation also resulted in the Itch gene coding exons becoming separated from the promoter region. Initially, this mutation arose on a C3H/HeH background, however, subsequent backcrossing for 28 generations was performed resulting in an inbred itchy mutant on a C57BL/6 J background.1,2 The Itch gene was named after the apparent chronic dermatitis exhibited by the mutant mice. These mice are characterized by severe inflammation and infiltration of immune cells into various organs that ultimately result in shortened lifespans due to pulmonary inflammation.1,3
E3 ligases are involved in the process of ubiquitination, an important post-translational protein modification utilized by cells to instigate signaling for a variety of functions. The labeling of target proteins with the 8.5 kDa ubiquitin molecule, multimono- or poly ubiquitinylation, requires a three-step enzymatic process.1,3 The final step involves an E3 ubiquitin ligase that catalyzes the transfer of the ubiquitin molecule to the target protein. Hundreds of different E3 ligases are known, each only able to ubiquitinate a narrow range of specific protein substrates. Poly-ubiquitin chains on lysine 48 (K48) are the best characterized, and lead to proteasomal degradation of the ubiquitinated target protein. However, poly-ubiquitin chains on different residues and multimono- ubiquitin have a variety of additional functions ranging from cellular trafficking to DNA repair.4 ITCH ubiquitination, specifically, has been shown to lead to the proteasomal degradation of the transcription factors CJUN and JUNB,5 as well as modulate cell signaling in non-degradation-dependent mechanisms.6
Since its identification, ITCH has been demonstrated to be essential in the regulation of target proteins within a variety of tissues and cellular pathways; most well understood in the immune system during adulthood.3 In the immune system, the loss of ITCH in T cells results in increased levels of the transcription factors JUNB and CJUN, which induce their preferential differentiation into T helper type 2 (TH2) cells.7 The pervasiveness of the autoimmune phenotype can be attributed to a cluster of ITCH target proteins that are deregulated in the itchy mice all acting in parallel to disturb homeostatic T cell differentiation, proliferation, and anergy.8 Other ITCH targets contribute to various cellular functions, such as P73, which contributes to apoptotic signaling.8 In addition to cellular functions, ITCH target proteins contribute to a plethora of known physiologic functions including stem cell maintenance, hematopoiesis, bone formation, and stress response.9
Understanding of the role of ITCH has been advanced through detailed analysis of the pathophysiology of itchy mice. Several reports suggest that the functions of ITCH in humans are analogous to that of the itchy mouse model system.9–12 A frameshift mutation resulting in the loss of ITCH was identified in a group of Amish children that had severe health concerns, including autoimmune like cell infiltrate into various organs.10 Further evidence showed that tissue alterations such as abnormal dermal proliferation and tumor proliferation first identified in itchy mice, manifest similarly in humans with Itch polymorphisms.9,11,12 These findings justify further pathophysiological analyses of itchy mice and identifications of novel physiologic pathways affected by ITCH.
E3 ligases also play an extensive role in spermatogenesis.13 The role of ITCH in spermatogenesis has recently been identified but remains poorly understood.9 Previously published results found that male itchy mice have reduced sperm production, disorganization of late stage spermatozoa, and an increase in germ cell apoptosis.14 Despite this observed testicular pathology, no decreased testicular function was observed when cross breeding itchy male mice to wild type females compared to wild type male counterparts. However, a reduction in litter size was observed in itchy females bred to wild type males compared to wild type females. This subfertility of itchy females suggests that the E3 ligase ITCH may be significantly involved in female reproduction, however, the physiologic mechanism that is disrupted in these mice remains unknown.
Dwyer and Richburg discuss several possible mechanisms that could result in the subfertility seen in the itchy colonies.14 Developmental dysfunction of itchy fetuses, conception, or pregnancy was identified as physiologic processes that may be disrupted in itchy mice and account for the subfertility observed.14 The present study was designed to identify the basis of female itchy pathology through late-stage fetal developmental analysis and characterization of the reproductive phenotype in itchy females. The findings described herein illustrate the significance of ITCH in female reproduction, suggesting Itch has a functional role in controlling estrous cycling and ovulation in female mice.
Materials and methods
Animals
This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All mice were used according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Texas at Austin (PHS Animal Welfare Assurance Number: A4107-01). The mice were housed in the AAALAC accredited Animal Resource Center at The University of Texas at Austin. The mouse room was kept at a constant temperature (22 ± 0.5℃) and humidity (35–70%) with a 12L: 12D photoperiod. Mice were fed a breeder diet containing 9% fat (5P06 Prolab RMH 2000) and tap water ad libitum. Mating pairs of C57BL/6 J were purchased from The Jackson Laboratory (Bar Harbor, ME). Itchy mice that were inbred to a C57BL/6 J background were provided by Dr Lydia Matesic at The University of South Carolina, Columbia, SC. These non-agouti-lethal 18H (a18H) mice were originally generated through a radiation-induced chromosomal inversion.1 Itchy and wild type colonies were established as previously described.14
Genotyping PCR and primers
Wild type and itchy mouse genotypes were confirmed using PCR with primers specific for the Itch gene (protocol from Dr Lydia Matesic). Tail clippings were collected and digested overnight with proteinase K. Total genomic DNA was precipitated with ethanol and PCR was performed using Taq polymerase. The primers used included individual wild type (5′-ATC GTC TAC TCA CCC CAC ATA AGG-3′) and itchy (5′-AAG AAG CAG CAG AGA CAA CGA GTG-3′) forward primers that share a common reverse primer (5′-TCT ATG CTC TGT TGT CTC CCA TGC-3′). The wild type primer and common primer results in a 194 bp band, while the itchy primer mixed with the common primer results in a 294 bp band.
Fetal bone and tissue analysis
When virgin females reached proestrus, they were introduced to a proven sire. Copulation plugs were checked every morning shortly after the lights came on (07:00). If a plug was present, midnight was considered gestational day (GD) 0. Pregnant dams were sacrificed on GD 18.5 by CO2 asphyxiation followed by cervical dislocation (n = 5 per genotype). The uterus was opened then the fetuses and resorptions were quantified. Fetuses were weighed and observed for gross malformations, then half the litter was placed in Bouin’s fixative for tissue analysis and half the litter was placed in 4% NaCl (in ddH2O) for skeletal analysis. Fetuses were incubated in Bouin’s fixative for at least one week, with constant agitation. After two washes with 70% ethanol, the fetuses were analyzed by Wilson’s freehand cross-sectioning15 under a dissection microscope. Major organs were compared to the “Atlas of Mouse Development”16 and either demarcated as normal, or deformities were noted. Fetuses placed in 4% NaCl were agitated overnight, skinned, eviscerated, and placed in 0.14% Alcian Blue (Alfa Aesar, Ward Hill, MA, cat# J60122) plus acetic acid staining solution for four days with constant agitation. The fetuses were then transferred into 0.5% Alizarin Red (Fisher, Nazareth, PA, cat# S25131) plus KOH staining solution where the solution was changed at least daily for five days. The fetuses were then incubated in a clearing solution containing benzyl alcohol in glycerol and 70% ethanol overnight, after which they were maintained and examined in a 1:1 glycerol to 70% ethanol preserving solution.17
Uteri and implantation site examination
Virgin female mice were mated 1:1 with adult males and allowed to have a normal and undisturbed birth. On postnatal day (PND) 5, the dams were sacrificed by CO2 asphyxiation. For uteri examinations, the uterine horns along with any contents were completely removed and documented pictorially. The uterine horns were then completely submersed in a 10% ammonium sulfide solution (Fisher, cat# A705-225) for 1 h. Sites of fetal implantation, which appear as black spots, were quantified (n = 5 per genotype).
Estrous cycle staging and tissue collection
Estrous cycles were staged according to histological evaluation of crystal violet stained vaginal lavages daily (between 10:00 and 12:00) starting in early adulthood, PND 42 for both genotypes (n = 4 per genotype).18 All females of the same genotype were housed together in cages that sat between cages that housed adult males to minimize housing effects on cyclicity. Estrus cycle monitoring continued until at least four consecutive cycles had consistent cytology. Females were sacrificed between 14:00 and 16:00 during diestrus, the stage of the estrus cycle when the number of corpora lutea (CL) are maximal. One ovary was fixed in 1:10 neutral buffered formalin and embedded in paraffin for ovarian histology, the other was snap frozen in liquid nitrogen and stored in −80℃ for protein analyses. Additionally, the pituitary gland was harvested at 16:00 on the day of proestrus, from additional mice at approximately PND 56 (n = 3 per genotype), snap frozen, and stored at −80℃ until protein analyses. Pituitary glands were collected at this time to capture the stage when hormonal surges that occur from the pituitary (specifically luteinizing hormone [LH]). Thymi were collected and snap frozen from one retired breeding female mouse of each genotype at unspecified age and cycle stage, where the itchy female had not yet developed dermatitis, for positive and negative controls in the protein analysis.
Ovarian histological analysis
Paraffin embedded ovaries were serially sectioned at 5 µm with 15 slides of eight sections each obtained per sample. Sections were stained with hematoxylin and eosin (H&E), and images were obtained using a Nikon Cool Snap camera and BR Elements software. Follicles in each section were identified as primary, secondary, antral, or CL based on morphology. Atretic follicles, as characterized by pyknotic granulosa cells and/or a grossly misshapen oocyte were omitted from counts. Sections were then combined in the third dimension (stacked) to analyze total count numbers of each follicular subtype in each sample (600 µm total thickness of ovary analyzed).
Western analyses of ITCH targets
Frozen ovaries, pituitary glands, and thymi were homogenized on ice in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors. Protein concentrations were determined using BioRad DC protein assays, with a bovine serum albumin (BSA) standard curve. Fifteen micrograms of total protein were resolved on a 4–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The protein was then transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was probed with monoclonal mouse anti-ITCH (BD Biosciences Pharmingen, San Diego, CA, cat# 611199, 1:1000 in 5% milk), monoclonal rabbit anti-CJUN (Cell Signaling, Danvers, MA, cat# 9165, 1:1000), monoclonal rabbit anti-JUNB (Cell Signaling, cat# 3753S, 1:1000), rabbit polyclonal anti-P73 (Cell Signaling, cat# 4662, 1:1000), or polyclonal goat anti-ACTIN (Santa Cruz Biotechnology, Dallas, TX cat# 1616, 1:1000) as a loading control. The chemiluminescent reagent Amersham ECL prime (GE Healthcare, cat# RPN2232) was used to image the protein to film.
Statistical analysis
Statistical results are expressed as the mean ± SEM. JMP Pro10 statistical analysis software was used. After distribution was analyzed for normalcy, the appropriate parametric or non-parametric test was run. For pairs of parametric data, a Student’s t-test was used. Significance is considered with P ≤ 0.05 unless otherwise stated.
Results
Itchy fetuses display no signs of developmental malformations on GD 18.5
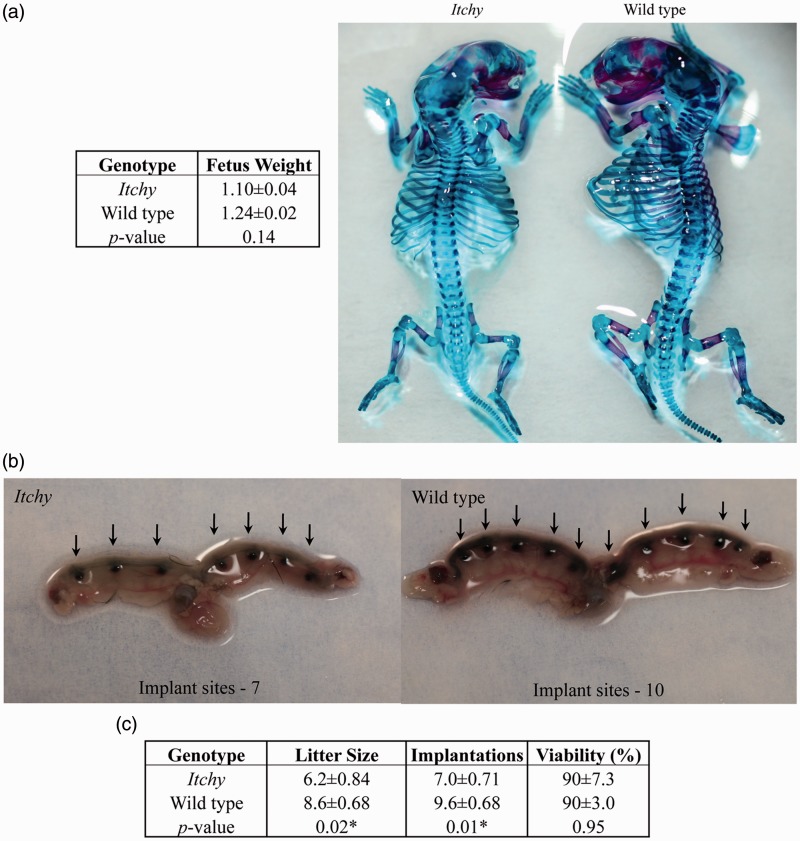
Previous cross-mating experiments suggested that ITCH plays an important role during embryonic development.14 To examine this further, gross analysis of development of itchy and wild type fetuses prior to parturition was performed. Half of each litter was subjected to bone and cartilage double staining, the other half was fixed followed by Wilson’s free hand sectioning.10 One case of hydrocephaly16 was observed in an itchy fetus, with significantly reduced body weight of 0.413 g. Representative images of skeletal staining are shown in Figure 1(a). No statistical differences in fetal weights were observed between genotypes (Figure 1a). Gross analysis of skeletal structures revealed no overt differences between genotypes (Figure 1a).
Figure 1.
Implantation and litter analysis. a) Representative photographs of skeletal staining. No significant differences in fetal weight, gross or skeletal structure between genotypes of GD 18.5 fetuses were found. b) Representative images of stained uteri. Arrows indicate sites of implantation. Significant differences between genotypes were observed. c) Summary of implantation and litter data (mean ± SEM), asterisks denote significant differences between genotypes (P < 0.05). (A color version of this figure is available in the online journal.)
Itchy females have fewer implantation sites and fetuses as compared to wild type females
Because no developmental deformities could be linked to genotype, and therefore do not account for the observed reduction in itchy litter sizes, implantation sites were quantified. Itchy dams averaged seven implantation sites per uteri whereas wild type dams averaged 9.6 implantation sites per uteri (P = 0.01) (Figure 1b, c). These data coincide with the decreased number of itchy fetuses compared to wild type (P = 0.02). No differences in litter viability were found (90%, Figure 1c).
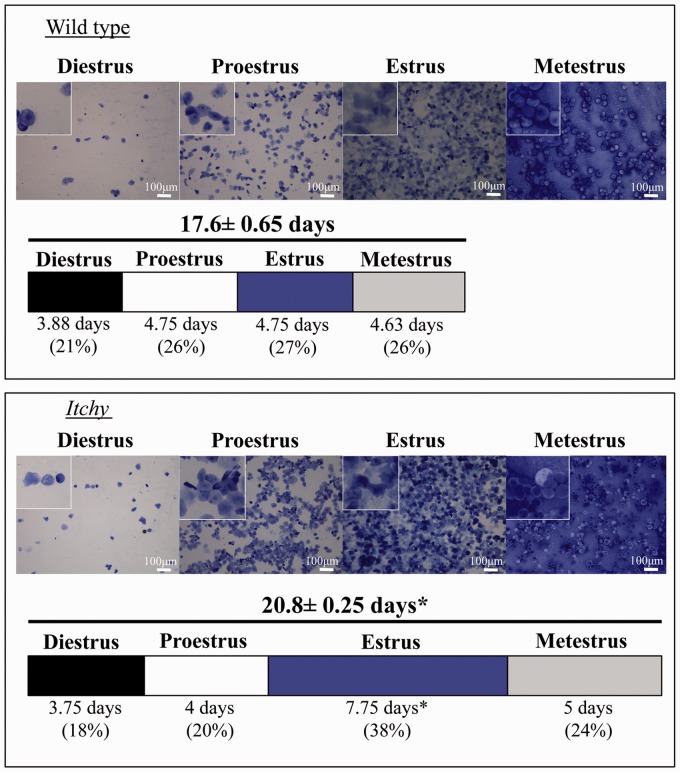
Increased estrous cycle length and number of days in estrus
Starting at PND 42, after females have begun to cycle regularly, the estrous cycles of females from each genotype were monitored. Cytology was consistent between genotypes in each stage (Figure 2). Itchy females exhibited significantly longer estrous cycles (P = 0.004), taking an average of 20.8 days to complete four cycles, compared to wild type that completed four cycles in 17.6 days on average (Figure 2). This extended cycle length was due to significantly increased number of days in estrus (P = 0.004), where itchy females averaged 7.75 days in estrus during four cycles, and wild type on average spent only 4.75 days in estrus during four cycles (Figure 2).
Figure 2.
Estrus cycle monitoring. Representative photomicrographs of vaginal lavages for wild type and itchy females stained with crystal violet. Insets are magnified 150% and the scale bar is 100 µm. The bar graph represents four consecutive estrous cycles, and each sub-section represents the number of days in the specified stage as a percent of total length of estrous. Length of bar graph suggests total number of days to complete four estrous cycles. Asterisks denote significant differences (P < 0.05). (A color version of this figure is available in the online journal.)
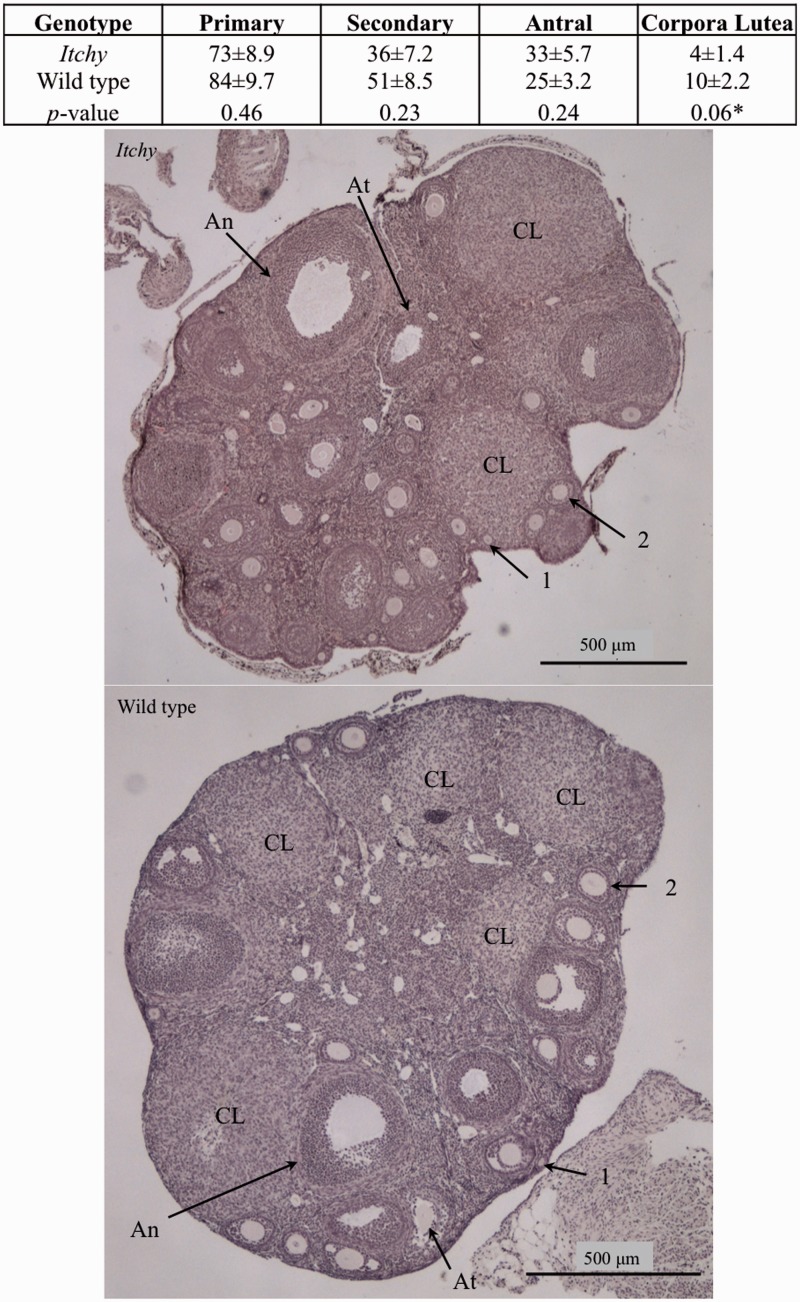
Decreased corpora lutea in adult itchy ovaries
Folliculogenesis was investigated through ovarian histology to further identify the mechanism of subfertility in the itchy female. Age- and cycle-matched females’ primary, secondary, and antral follicles as well as CL were quantified for each genotype. Quantification demonstrated that itchy females had no significant changes in number of follicles, but tended to have fewer CL compared to wild type controls (P = 0.06) (Figure 3).
Figure 3.
Follicular analysis of cycle matched ovaries. Morphological quantification of wild type and itchy ovaries during diestrus. Significant differences denoted by asterisks. Images are representative photomicrographs of an ovary stained with H&E from a wild type female in diestrus (top) and itchy female in diestrus (bottom). Each type of follicle is indicated as such: primary (1), secondary (2), antral (An), atretic (At), and corpus luteum (CL). Images illustrate the similarity between the number of follicles from the mice of the two genotypes, and the greater number of CL in the wild type ovary. Scale bar is 500 µm. (A color version of this figure is available in the online journal.)
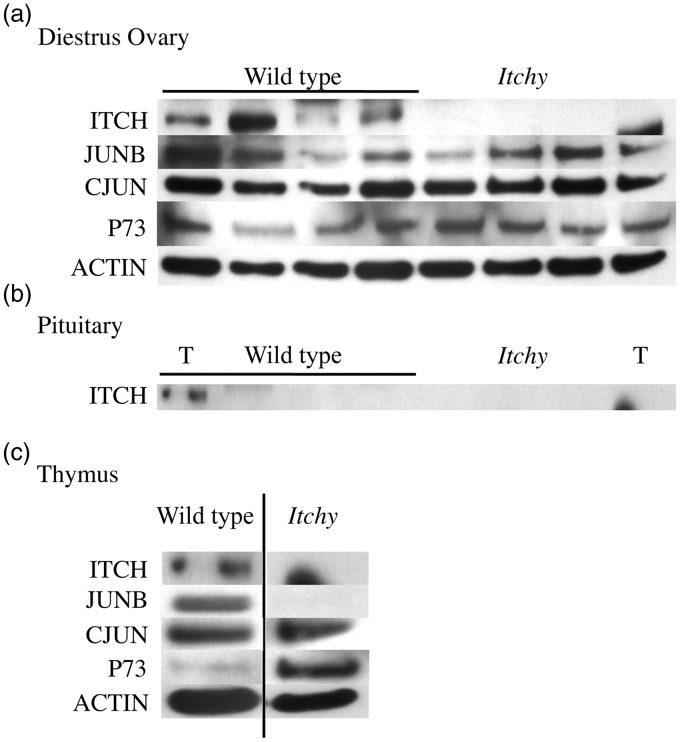
No change in levels of ITCH target protein in adult itchy ovary
To further investigate the mechanism behind the phenotype seen in itchy females, protein levels of ITCH targets in cycle matched ovaries and pituitaries were quantified. It was confirmed that itchy females lacked ITCH in the ovary (Figure 4a), although ITCH expression varied greatly in between wild type females. No statistical differences occurred in the protein levels of CJUN, JUNB, or P73 in the ovaries of itchy females compared to wild type (Figure 4a), with significant variation in the levels of expression of these proteins observed between individual mice, despite the fact that the tissues were cycle matched. The pituitary is a key player in the hormonal control of the ovary. ITCH was not found to be expressed in the pituitary in either wild type or itchy mice (Figure 4b), therefore further investigation into ITCH targets was not performed in this tissue. Thymi from each genotype were collected and probed as positive controls, where ITCH expression was confirmed in wild type but not itchy thymus. P73 expression appears to be higher in the itchy thymus than in the wild type, which is expected based on literature19 (Figure 4c). However, inconsistencies with previous literature7 were observed as CJUN expression was found to be similar between the genotypes, and JUNB was increased in the wild type thymus compared to the itchy thymus (Figure 4c).
Figure 4.
Western blot of ITCH targets. a) Top row confirms ITCH expression in wild type but not itchy mouse cycle-matched diestrus ovaries. CJUN, JUNB, and P73 were measured in wild type and itchy mice. No statistical differences were found in these target proteins, however, individual variation in both genotypes can be seen. b) ITCH is not expressed in cycle-matched pituitary, T represents thymus from respective genotype (wild type or itchy). c) Thymi from one mouse of each genotype was used as positive and negative control based on previously published literature. ITCH expression was confirmed in wild type but lost in itchy thymus. Target proteins CJUN, JUNB, and P73 were measured with varying results
Discussion
Previous observations of the tissue pathology of the itchy mouse model system have provided essential insights into the function of ITCH in human physiology. Recent studies of the testis of male itchy mice determined that the loss of ITCH is associated with several types of abnormalities in sperm development.14 Despite these abnormalities, itchy males were able to produce similar size litters as wild type males. Itchy females, however, had reduced litter sizes compared to their wild type counterparts, suggesting that the loss of functional ITCH lead to a pathologic subfertility in itchy female mice. The work presented here suggests the underlying physiologic mechanism behind the subfertility is a measurable alteration in estrous cycling, decreased number of implantation sites, and a trend toward decreased CL formation in itchy mice. Taken together, these findings suggest that ITCH is functionally important for fertility in female mice.
Humans that express a truncated, non-functional ITCH protein have physiological similarities to those of itchy mice, where autoimmune infiltration occurs into various organs. Children with non-functional ITCH also have severe cranio-facial deformities accompanied by motor and cognitive deficits.10 Similar to these observations, increased incidences of cranio-facial deformities have also been observed in itchy mice at adulthood. Previous studies have also implicated ITCH in bone formation.20 The analysis of GD 18.5 itchy fetuses in this study did not reveal significant developmental cranio-facial (Supplemental Figure 1) or other gross skeletal deformities (Figure 1a). As cranio-facial deformities were previously observed in adult mice, there may be differences in the age of onset in humans and mice with mutated ITCH. The developmental regulation of ITCH may offer interesting avenues for comparison of the function of human ITCH with murine ITCH.
Reduced litter sizes were previously observed by Dwyer and Richburg14 and confirmed in this report. Further investigations into the cause for reduced litter sizes demonstrated that there were significantly less implantation sites per uteri from itchy females than wild type females (Figure 1b, c). Viability of the litters was similar between genotypes at 90% (Figure 1c). Taken together, these results suggest that the origin of the pathologic subfertility in itchy females is prior to implantation.
The estrous cycle and ovarian histology were characterized to provide insight into the cause of the difference in litter size and reduced implantation sites between the two genotypes. Itchy females maintained a significantly longer estrous cycle than wild type females, due to an increased number of days in estrus (Figure 2). Timing and progression through the estrous cycle occur in response to hormonal events that occur along the hypothalamic–pituitary–gonadal axis. Estrus begins in mice when estrogen levels are maximal.21 Maximal estrogen triggers the gonadotropin-releasing hormone (GnRH) to surge from the hypothalamus, consequentially, LH surges from the pituitary. The LH surge induces ovulation.22 Histological analysis indicated that itchy females tend to have fewer CL present in cycle-matched ovaries compared to wild type (Figure 3). This suggests itchy females are ovulating less than wild type, which could be responsible for both the fewer implantation sites and the smaller litter sizes birthed from itchy females (Figure 1b, c). Neither direct measurement of ovulation, nor hormone levels were measured, so it remains unclear whether the results here are primarily from a failure to ovulate, or a disruption in the underlying neuroendocrine control mechanisms such as the LH surge. Understanding the interdependence between estrus and ovulation, both of which are altered in itchy mice, may be key to uncovering the role of ITCH in female reproduction. Further studies are needed to address how the loss of functional ITCH may affect regulation of reproductive functioning at either the hormonal or somatic level.
ITCH targets have broad cellular functions that contribute to a variety of physiological functions. In reproductive tissues ITCH targets are responsible for proliferation and follicle stimulating hormone (FSH) responsiveness in granulosa cells (CJUN, JUNB),23–27 meiotic arrest in oocytes (p63),28,29 mitosis in blastocysts (P73),30 and regression of the CL (CJUN, JUNB).31 There were no significant alterations in total protein levels of any of the targets investigated in the ovary (Figure 4a). However, it is possible that an as yet undiscovered ITCH protein target specific for the ovary accounts for the underlying pathological mechanism. Thymi showed conflicting results where some targets such as JUNB and CJUN were inconsistent with previous literature,7 and other targets such as P73 were consistent with previous literature.19 These observations confound interpretation of the results and make it difficult to draw any concrete conclusions about target protein involvement in the itchy reproductive phenotype.
It is appreciated that loss of ITCH may affect reproductive function through indirect action, due to other tissue alterations in itchy mice and interaction between different physiologic systems. Both helper T cells9 and macrophages32 are alternatively polarized in itchy mice. The biased differentiation of leukocytes in itchy mice may account for the pathologic female reproductive function. Leukocytes have been indicated to play an integral role in ovulation.33 Depletion of macrophages, either through administration of clodronate liposomes or genetic knockout of colony stimulating factor-1 (CSF-1) resulted in reduced ovulation.34,35 Specific T helper cell type 1 (TH1) cytokines have been recognized to amplify ovulation rate in rats36–38 and antibody absorption of these cytokines in the ovary reduces ovulation rate. The TH1 cytokines work in concert to amplify hormonal function and they also cause several somatic changes that enhance ovulation.39 The biased polarization of leukocytes towards TH2 exhibited by itchy mice may offer an alternative explanation for the reproductive phenotypes seen in itchy females and a possible avenue for future research.
In summary, the findings of this manuscript suggest that the reduced litter sizes of itchy breeding pairs are preceded by reduced implantations and can be attributed to alterations in ovulation and estrous cycling and not to disruptions in fetal development. No statistical differences between genotypes were observed in total levels of ITCH target proteins, however the role of leukocyte polarization in reproductive function offers an alternative mechanism by which loss of ITCH could mediate the observed phenotype. Future investigations into hormone levels in itchy mice, cytokine levels and cell populations in the ovary of itchy females may further aid in unraveling the function of ITCH in female reproduction. The results presented here suggest that itchy mice have altered reproductive function compared to the background C57BL/6 J strain, and Itch is functionally important for fertility.
Acknowledgments
The authors would like to thank Dr Bogdan Wlodarczyk for providing expert advice in fetal development and Ms Rashin Ghaffari for her editorial expertise.
Authors’ contribution
All authors participated in the interpretation of the studies, analysis of the data, and writing of the manuscript. ARS, JLM, CJM, and JHR designed the studies, ARS conducted the experiments, and LM supplied the Itchy mice.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part, by The Center for Molecular and Cellular Toxicology and grants from the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS 5R01 ES016591 & T32 ES07247 JHR), and the National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) grant (DCE-0549428 JM).
References
- 1.Hustad CM, Perry WL, Siracusa LD, Rasberry C, Cobb L, Cattanach BM, Kovatch R, Copeland NG, Jenkins NA. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics 1995; 140: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry WL, Hustad CM, Swing DA, O'Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18h mice. Nat Genet 1998; 18: 143–6. [DOI] [PubMed] [Google Scholar]
- 3.Matesic L, Copeland N, Jenkins N. Itchy mice: the identification of a new pathway for the development of autoimmunity. In: Beutler B. (ed). Immunology, phenotype first: How mutations have new principles and pathways in immunology, Berlin: Springer, 2008, pp. 185–97. [DOI] [PubMed] [Google Scholar]
- 4.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2001; 2: 195–201. [DOI] [PubMed] [Google Scholar]
- 5.Gao M, Labuda T, Ying X, Gallager E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science 2004; 306: 270–75. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, Yang C, Hu K, Elly C, Liu YC. Itch E3 ligase-mediated regulation of TGF-beta signaling by modulating Smad2 phosphorylation. Mol Cell 2004; 15: 825–31. [DOI] [PubMed] [Google Scholar]
- 7.Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol 2002; 3: 281–7. [DOI] [PubMed] [Google Scholar]
- 8.Melino G, Gallagher E, Aqeilan RI, Knight R, Peschiaroli A, Rossi M, Scialpi F, Malatesta M, Zocchi L, Browne G, Ciechanover A, Bernassola F. Itch: a HECT-type E3 ligase regulating immunity, skin and cancer. Cell Death Differ 2008; 15: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 9.Aki D, Zhang W, Liu YC. The E3 ligase Itch in immune regulation and beyond. Immunol Rev 2015; 266: 6–26. [DOI] [PubMed] [Google Scholar]
- 10.Lohr NJ, Molleston JP, Strauss KA, Torres-Martinez W, Sherman EA, Squires RH, Rider NL, Chikwava KR, Cummings OW, Morton DH, Puffenberger EG. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet 2010; 86: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardini S, Gravina P, Croce N, Perricone R, Knight RA, Valentini A, Melino G, Federici G. Itch gene polymorphisms in healthy population and in patients affected by rheumatoid arthritis and atopic dermatitis. Cell Cycle 2014; 7: 3607–9. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara T, Tsuda H, Hotta A, Kozaki K, Yoshida A, Noh JY, Ito K, Imoto I, Inazawa J. ITCH is a putative target for a novel 20q11.22 amplification detected in anaplastic thyroid carcinoma cells by array-based comparative genomic hybridization. Cancer Sci 2008; 99: 1940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richburg JH, Myers JL, Bratton SB. The role of E3 ligases in the ubiquitin-dependent regulation of spermatogenesis. Semin Cell Dev Biol 2014; 30: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer JL, Richburg JH. Age-dependent alterations in spermatogenesis in itchy mice. Spermatogenesis 2012; 2: 104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes AW, Kruger CL. Hayes' principles and methods of toxicology, 6th ed London: CRC Press, 2014. [Google Scholar]
- 16.Kaufman M. The atlas of mouse development, London: Academic Press, 1992. [Google Scholar]
- 17.Wlodarczyk B, Spiegelstein O, Gelineau-Van Waes J, Vorce RL, Lu X, Le CX, Finnell RH. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol Appl Pharmacol 2001; 177: 238–46. [DOI] [PubMed] [Google Scholar]
- 18.McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. JoVE 2012; 67: 4389–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, Cesareni G, Melino G. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J 2005; 24: 836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Xing L. Ubiquitin E3 ligase itch negatively regulates osteoblast differentiation from mesenchymal progenitor cells. Stem Cells 2013; 31: 1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snell G. Biology of the laboratory mouse, New York, NY: Dover Publications, 1956. [Google Scholar]
- 22.Senger P. Pathways to pregnancy and parturition, 2nd ed Pullman: Current Conceptions, 2005. [Google Scholar]
- 23.Ness JM, Kasson BG. Gonadotropin regulation of c-fos and CJUN messenger ribonucleic acids in cultured rat granulosa cells. Mol Cell Endocrinol 1992; 90: 17–25. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SC, Richards JS. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 2000; 275: 33718–28. [DOI] [PubMed] [Google Scholar]
- 25.Son DS, Arai KY, Roby KF, Terranova PF. Tumor necrosis factor alpha (TNF) increases granulosa cell proliferation: dependence on c-Jun and TNF receptor type 1. Endocrinology 2004; 145: 1218–26. [DOI] [PubMed] [Google Scholar]
- 26.Oktay K, Oktay MH. Immunohistochemical analysis of tyrosine phosphorylation and AP-1 transcriptional factors C-Jun, Jun D, and Fos family during early ovarian follicle development in the mouse. Appl Immunohistochem Mol Morphol 2004; 12: 364–9. [DOI] [PubMed] [Google Scholar]
- 27.Oktem O, Buyuk E, Oktay K. Preantral follicle growth is regulated by c-Jun-N-terminal kinase (JNK) pathway. Reprod Sci 2011; 18: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 2008; 135: 3–12. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch GB, Zielonka EM, Coutandin D, Weber TA, Schafer B, Hannewald J, Luh LM, Durst FG, Ibrahim M, Hoffmann J, Niesen FH, Senturk A, Kunkel H, Brutschy B, Schleiff E, Knapp S, Acker-Palmer A, Grez M, McKeon F, Dotsch V. DNA damage in oocytes induces a switch of the quality control factor TAp63alpha from dimer to tetramer. Cell 2011; 144: 566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasini R, Tsuchihara K, Wilhelm M, Fujitani M, Rufini A, Cheung CC, Khan F, Itie-Youten A, Wakeham A, Tsao MS, Iovanna JL, Squire J, Jurisica I, Kaplan D, Melino G, Jurisicova A, Mak TW. TAP73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 2008; 22: 2677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz FJ, Luo W, Wiltbank MC. Prostaglandin F2alpha regulation of mRNA for activating protein 1 transcriptional factors in porcine corpora lutea (CL): lack of induction of JUN and JUND in CL without luteolytic capacity. Domest Anim Endocrinol 2013; 44: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino A, Menghini R, Fabrizi M, Casagrande V, Mavillo M, Stoehr R, Candi E, Mauriello A, Moreno-Navarrete JM, Gomez-Serrano M, Peral B, Melino G, Lauro R, Fernandez Real JM, Frederici M. ITCH deficiency protects from diet-induced obesity. Diabetes 2014; 63: 550–61. [DOI] [PubMed] [Google Scholar]
- 33.Brannström M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol 2002; 57: 47–60. [DOI] [PubMed] [Google Scholar]
- 34.Cohen PE, Zhu L, Pollard JW. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrus cycles and ovulation. Biol Reprod 1997; 56: 110–18. [DOI] [PubMed] [Google Scholar]
- 35.Van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA, Norman RJ. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod 2000; 62: 1059–66. [DOI] [PubMed] [Google Scholar]
- 36.Brannström M, Mayrhofer G, Robertson SA. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod 1993; 48: 277–86. [DOI] [PubMed] [Google Scholar]
- 37.Brannström M, Wang L, Norman RJ. Ovulatory effect of interleukin-lβ on the perfused rat ovary. Endocrinology 1993; 132: 399–404. [DOI] [PubMed] [Google Scholar]
- 38.Bonello N, McKie K, Jasper M, Andrew L, Ross N, Braybon E, Brannstrom M, Norman RJ. Inhibition of nitric oxide: effects of interleukin-1β-enhanced ovulation rate, steroid hormones, and ovarian leukocyte distribution at ovulation in the rat. Biol Reprod 1996; 54: 436–45. [DOI] [PubMed] [Google Scholar]
- 39.Gerard N, Caillaud M, Martoriati A, Goudet G, Lealmanach AC. The interleukin-1 system and female reproduction. J Endocrinol 2004; 180: 203–12. [DOI] [PubMed] [Google Scholar]