Abstract
Store-operated Ca2+ entry (SOCE) is mediated by the store-operated Ca2+ channel (SOC) that opens upon depletion of internal Ca2+ stores following activation of G protein-coupled receptors or receptor tyrosine kinases. Over the past two decades, the physiological and pathological relevance of SOCE has been extensively studied. Recently, accumulating evidence suggests associations of altered SOCE with diabetic complications. This review focuses on the implication of SOCE as it pertains to various complications resulting from diabetes. We summarize recent findings by us and others on the involvement of abnormal SOCE in the development of diabetic complications, such as diabetic nephropathy and diabetic vasculopathy. The underlying mechanisms that mediate the diabetes-associated alterations of SOCE are also discussed. The SOCE pathway may be considered as a potential therapeutic target for diabetes-associated diseases.
Keywords: Store-operated Ca2+ channel, store-operated Ca2+ entry, calcium, diabetes, diabetic complications
Introduction
Store-operated Ca2+ entry (SOCE), previously known as Ca2+ release activated Ca2+ influx1,2 or capacitative calcium entry3, is an essential Ca2+ entry mechanism in both excitable and non-excitable cells. This Ca2+ entry is mediated by store-operated Ca2+ channel (SOC) which is activated by depletion of internal Ca2+ stores i.e. endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR).4 Therefore, circulating or locally produced hormones that activate either G protein-coupled receptors or receptor tyrosine kinases can open SOC through activation of the phospholipase C/inositol 1, 4, 5-triphosphate (IP3) pathway.5 It is important to note that any channel that exhibits Ca2+ store-dependent activity can be referred to as a SOC. Electrophysiological studies of cells with depleted ER stores have shown membrane currents with diverse properties, indicating that different classes of cells express distinct SOC.4 The most studied and best-characterized SOC is the Ca2+ release-activated Ca2+ channel (CRAC) that is mainly expressed in the immune cells.1,2 In this review, we do not specify Ca2+ entry mediated by different types of SOC (CRAC or general SOC). Instead, we use SOCE to refer Ca2+ entry through any type of SOC.
Although SOCE was discovered about 30 years ago, its molecular players were not identified with certainty until recently. By high throughput RNAi screening, two protein families, stromal interaction molecule (STIM)6,7 and Orai,8–10 were identified as required components of SOCE. STIM1 is a single-pass transmembrane protein located primarily in the ER membrane and functions as an ER Ca2+ sensor to sense ER luminal Ca2+ concentration. Orai1 is a small plasma membrane protein, which constitutes the pore-forming unit of SOC. Upon depletion of ER Ca2+, STIM1 aggregates and translocates to ER-plasma membrane junctions, where it physically associates and subsequently activates Orai1 causing Ca2+ entry into the cytosol.11,12 In addition to STIM1 and Orai1, STIM2 (a mammalian homolog of STIM1), and Orai2 and 3 (two mammalian homologs of Orai1) may also constitute/regulate SOC, but with distinct functional properties.13–18 The Orai/STIM family-constituted SOCE pathway has become more complicated with the recent identification of splicing variants of Orai1 (Orai1α and Orai1β)19,20 and STIM1 (STIM1L),21–24 which generate SOC with distinct signaling and regulatory properties. Furthermore, several isoforms of canonical transient receptor potential (TRPC) proteins, which had been proposed as the molecular components of SOC prior to the discovery of Orai1 and STIM1, may also contribute to SOCE by interacting with STIM1 and/or Orai1.25–33 Readers are referred to recent outstanding reviews for more information on the molecular components and gating/regulatory mechanisms of SOC.34–39
SOCE was initially considered as a major mechanism of Ca2+ entry in non-excitable cells, such as immune cells, platelets, and endothelial cells.4,40 Later, this Ca2+ entry pathway was also found in many excitable cells, such as neurons,17 cardiac myocytes,41 skeletal muscle cells,42 and vascular smooth muscle cells.43 It is now widely accepted that SOCE is a ubiquitous Ca2+ signaling pathway that regulates diverse cellular functions in a variety of tissues and organ systems.44–51 Therefore, it is not surprising that dysfunction of SOC can lead to a series of disorders, such as immunodeficiency, myopathy, and vascular diseases.44,51–60 Over the past decade, accumulating evidence has demonstrated that many diabetic complications involve alterations of SOCE and its signaling pathways.60–64 Since diabetes and its complications are becoming epidemic worldwide and there is no curative therapy currently available for diabetic complications,65–67 continued exploration of the basic pathophysiology of diabetic complications and of new therapeutic approaches is in need. This review summarizes the published studies on the associations of abnormal SOCE with the pathology of diabetic complications. The aim of this review is to provide information that the SOCE pathway may be a potential therapeutic target for various organ and system disorders associated with diabetes.
SOCE and the development of diabetic complications
SOCE and diabetic nephropathy
Diabetic nephropathy, one of the most common complications of diabetes mellitus, is a major cause of end stage renal disease.68,69 Early features of diabetic nephropathy include glomerular hypertrophy with thickening of the glomerular basement membrane and expansion of the glomerular mesangium, which eventually develop into glomerulosclerosis and renal insufficiency.70–74 Glomerular mesangial cells are the major contributor to these structural changes in diabetic kidney.75–77 Mesangial cell function is controlled by intracellular Ca2+ signaling which involves several types of Ca2+ channels, including SOC.78 The SOC in mesangial cells was first reported by Menè et al.79 and later was electrophysiologically and pharmacologically characterized by Ma et al.50 Several protein components, such as TRPC1, TRPC4, Orai1, and STIM1, which are required for SOCE were identified in human mesangial cells.62,80,81 Furthermore, studies demonstrated that SOC participated in hormone-stimulated Ca2+ responses in mesangial cells.82–85
Alterations of SOC function in mesangial cells under conditions of diabetes have been extensively studied in both in vitro and in vivo settings. In earlier studies conducted in cultured rat and human mesangial cells, Mene et al. demonstrated that arginine vasopressin- and angiotensin II-induced SOCE was attenuated by high glucose treatment (30 mM for five days).84,86 However, both vasopressin and angiotensin II not only activate SOC, but activate the receptor-operated Ca2+ channel as well.87,88 Therefore, the attenuation of the Ca2+ response by high glucose in that study might be due to impairment of the receptor-operated Ca2+ channel. Nutt and coworkers examined the effects of high glucose on endothelin-1- (activates both SOC and receptor-operated Ca2+ channel) and thapsigargin- (selectively activates SOC)-induced Ca2+ response in cultured rat mesangial cells. They revealed that high glucose treatment at 30 mM for 5–7 days significantly reduced endothelin 1-induced Ca2+ entry, but had no effect on thapsigargin induced Ca2+ response.85 Their study suggests that in a time period of five days, high glucose treatment did not impair SOCE, but significantly inhibited the Ca2+ entry through the receptor-operated channel. Because diabetic nephropathy is a progressive disease and biological processes in kidney cells are altered along with the disease development,70,73,89 we recently examined the time course effect of high glucose treatment on SOC activity in cultured human mesangial cells. Prolonged treatment with high glucose (25 mM for >7 days) significantly enhanced cyclopiazonic acid-induced SOCE and IP3-induced SOC current while a short-term treatment (<3 days) had a tendency to reduce SOC activity.62 Although the mechanism for the initial suppression of SOC by high glucose was not clear, the augmentation of SOCE in a later phase was attributed to upregulation of STIM1 and Orai1.62 Importantly, the abundance of STIM1 and Orai1 proteins was also significantly increased in the renal glomeruli/cortices of 4-week, but not 2-week streptozotocin (STZ)-treated rats (type 1 diabetes) and of high fat diet diabetic rats (type 2 diabetes) which manifested overt diabetic nephropathy.62 Taken together, studies from different groups suggest that high glucose/diabetes effects on SOCE in mesangial cells are time course dependent. Although the mechanism and significance of the changes are not known at present, these studies have at least established an association of abnormal SOCE with diabetic kidney disease.
It is well known that over-production of extracellular matrix proteins by mesangial cells contributes to glomerular damage in diabetic nephropathy.75–77 In general, SOCE promotes protein synthesis and cell growth, for instance contributing to cardiac hypertrophy.51,90 However, a recent study revealed that the SOC-mediated Ca2+ influx suppressed cell growth in mouse embryonic fibroblasts and rat uterine leiomyoma cells through inhibition of AKT1.91 Thus, the effect of SOC on protein production is cell type specific and/or cell context-dependent. We recently found that activation of SOC abrogated high glucose- and TGF-β1-induced fibronectin protein expression in cultured human mesangial cells.92 Consistently, downregulation of SOC function in mesangial cells significantly increased extracellular matrix protein expression in cultured mesangial cells or in glomeruli/renal cortices in animals.92 Thus, SOC in mesangial cells is an anti-fibrotic mechanism in kidney. It is possible that the early attenuation of SOCE in mesangial cells caused by high glucose described above62 contributes to the early pathological changes in diabetic glomerulus (deposition of extracellular matrix proteins and mesangial expansion), but the later enhancement of SOCE is a compensatory response to counteract detrimental pathways in diabetic kidneys.
It should be noted that in addition to mesangial cells, several other types of kidney cells are also involved in the development of diabetic nephropathy, such as podocytes,93–95 tubular epithelial cells,70,71,96,97 and smooth muscle cells in the renal arterioles.98–101 Studies have shown that SOC is present in some of those cells.102–106 However, an association of SOC in those cells with the development of diabetic kidney disease has not been established.
SOCE and diabetic vasculopathy
An early indicator for the development of microvascular and macrovascular complications of diabetes is endothelial dysfunction, defined as a reduction in the vasodilatation response to an endothelium-dependent vasodilator (such as acetylcholine) or to flow-mediated vasodilatation.107–110 Studies have linked the diabetes-associated dysfunction of the vascular endothelium to disturbances in Ca2+ homeostasis.61,111 Prolonged exposure (4 days) of human umbilical vein endothelial cells to high glucose medium (30 mM) resulted in a significant increase in apoptosis, which was associated with increased SOC activity (assessed by whole cell patch clamp).112 Furthermore, blockade of SOC with 2-aminoethoxydiphenyl borate (2-APB) and La3+ reversed the hyperglycemia-induced apoptosis. Similarly, in bovine aortic endothelial cells, Bishara and Ding showed that high glucose treatment at 25 mM for 24 and 72 h resulted in a sustained increase in SOCE following activation of the P2Y receptor by ATP.113 They proposed that the TRPC1 protein contributed to the enhanced SOCE because TRPC1 protein expression was elevated after 72-h high glucose treatment, and antisense TRPC1 treatment attenuated the ATP-induced Ca2+ response. However, it is not clear whether the TRPC1 protein functions as an SOC itself or as a regulator/modulator of SOC in that study. Recently, Daskoulidou et al. provided a molecular basis for high glucose-enhanced SOCE in vascular endothelial cells. They demonstrated that hyperglycemia (25 mM for three days) augmented SOCE which was accompanied by increased abundance of Orai1-3 and STIM1-2 proteins.63 Expression levels of the Orai1-3 and STIM1-2 mRNAs were significantly increased in the abdominal aortae of Akita diabetic mice and STZ-diabetic mice.63 Furthermore, expression levels of Orai1-2 and STIM1-2 mRNAs were also significantly higher in the aortae in type 2 diabetic patients.63 However, an intriguing question which was not addressed in that study is whether the increases in SOCE and Orais/STIMs are the consequence of diabetes or the cause of diabetic vascular disease. Contrary to the Daskoulidou study, Estrada et al. recently reported that STIM1 protein expression was significantly reduced in coronary endothelial cells from STZ-diabetic mice.114 The decrease in STIM1 protein abundance impaired ER Ca2+ refilling by disrupting the interaction between STIM1 and the ER/SR Ca2+-ATPase, and consequently attenuated endothelium-dependent relaxation in diabetic coronary arteries.114 Importantly, the endothelial dysfunction could be rescued by restoring the expression level of STIM1 in diabetic coronary endothelial cells.114 Surprisingly, SOCE was not significantly different between control and diabetic endothelial cells in that study.114 The discrepancies between the Daskoulidou and Estrada studies in the same diabetic model (STZ mouse) at similar stages of diabetes (6-8 weeks after STZ injection) may be derived from differences in the segments of vessels prepared (aortae vs. coronary artery), the samples studied (entire vesicular tissues vs. endothelial cells), and the molecular levels analyzed (mRNA vs. protein). Furthermore, the Estrada study did not examine the expression level of STIM2 which is also present in the coronary endothelial cells and may play a major role in regulating resting Ca2+ level in the ER (refilling).17
It is known that vascular complications of diabetes are produced, at least in part, by increased contraction of vascular smooth muscle cells due to elevated intracellular Ca2+ concentration.115–117 Store-operated Ca2+ influx was substantially reduced in retinal microvascular smooth muscle from STZ diabetic rats. The attenuated SOCE was reversed by insulin treatment (to normalize blood glucose level).118 Using the same diabetic model, Ma et al. also found an attenuated SOCE in aortic smooth muscle cells of STZ rats.119 Importantly, the contractile response of the vessel was significantly reduced in the diabetic rats compared to that in control rats.119 Similar results were also reported in type 2 diabetic animals. Mita and colleagues demonstrated that SOCE (activated by cyclopiazonic acid)-induced contraction of caudal artery smooth muscle strips isolated from Goto-Kakizaki rats (a type 2 diabetes model) was compromised. Interestingly, the expression levels of TRPC1 and TRPC6 were about two-fold greater in the vascular myocytes from Goto-Kakizaki rats than in those from non-diabetic rats.120 In addition, the vascular smooth muscle from the diabetic rats expressed the TRPC4 protein, which was not present in the muscle cells from control rats.120 The authors proposed that these contradictory findings of increased TRPCs with decreased SOC activity were due to changes in TRPC protein expression in Goto-Kakizaki rats. Increase in some TRPC proteins may specifically affect the assembly of the homo- and heterotetramers building the TRPC channels, resulting in channels with different electrophysiological activity.120 On the contrary, the saphenous veins from patients with type 2 diabetes showed exaggerated cyclopiazonic acid-induced SOCE and contraction compared to the vessels from subjects without diabetes.60 Apparently, the diabetes/high glucose effect on SOCE in vascular smooth muscle cells is complex and may be dependent on the species (human vs. rat/mouse), the stage of diabetes/duration of high glucose treatment, and the segments of the vessels. If SOCE in vascular smooth muscle cells is attenuated in diabetes as demonstrated by most studies discussed above, it is difficult to interpret the enhanced contractile response of vascular myocytes, a characteristic of diabetic vasculopathy.115–117 One possible explanation is that a decrease in SOCE is a compensatory response, which protects vascular smooth muscle from over-reactive contraction in diabetes.
SOCE and platelet disorder in diabetes
Diabetes mellitus is a well-known risk factor for atherosclerotic disorders. In diabetes, exaggerated aggregation of platelets is one of the key factors for the initiation and progression of atherosclerosis.121,122 Abnormality of Ca2+ mobilization was observed in platelets from diabetic patients.123,124 Platelets have two separate agonist-sensitive Ca2+ stores and SOCE is the major mechanism of Ca2+ entry in platelets. Earlier studies revealed that SOCE stimulated by thrombin, thapsigargin or ionomycin was significantly greater in platelets from type 2 diabetic patients than in those from healthy controls.125,126 Treatment with catalase and trolox almost completely abolished the increased SOCE response, suggesting a reactive oxygen species (ROS)/reactive nitrogen species-mediated mechanism.125 A later study from the same group provided molecular evidence for the diabetes-induced enhancement of SOCE in platelets. They found that the STIM1 and Orai1 proteins were significantly increased in platelets from patients with type 2 diabetes mellitus.127 Interestingly, in a recent study this group used Mn2+ entry as an indication of SOC activity and found that SOCE in platelets from type II diabetic patients was actually reduced even though the overall Ca2+ entry was increased.128 The authors reasoned that the enhanced SOCE observed previously might be derived from other Ca2+ entry mechanisms secondary to store depletion, such as reverse Na+/Ca2+ exchange, secretion of autocrine signaling molecules, and TRPC channels. Nevertheless, their studies provided evidence that SOCE in platelets is altered in diabetes and the abnormality of SOCE could contribute to increased adhesiveness and aggregation of platelets, a prothrombotic state leading to micro and macroangiopathy in diabetes. Apparently, further study is needed to establish a cause–effect relationship between an abnormal SOCE in platelets and diabetic cardiovascular complications.
SOCE and diabetic cardiomyopathy
Diabetic cardiomyopathy is characterized by hypertrophy and it often deteriorates into a loss of cardiac mass.129 In cardiomyocytes, SOCE has been shown to play an important role in regulating hypertrophic signaling pathways.51,90 An increased amount of STIM1 protein as well as its variant STIM1L, in cardiomyocytes contributed to pathological cardiac hypertrophy by enhancing SOCE.22 However, studies on the role of SOCE in diabetes-derived cardiac hypertrophy are scarce. In a study of cultured neonatal rat ventricular myocytes, Pang et al. demonstrated that short-term hyperglycemia (30 mM for 20 h) significantly decreased SOCE stimulated by angiotensin II or thapsigargin.130 Hyperglycemia also significantly blunted the Ca2+-dependent hypertrophic response as well as the Ca2+-sensitive nuclear translocation of nuclear factor of activated T-cells (NFAT),130 a well-known signaling pathway for cardiac hypertrophy.131 However, it is uncertain whether this short-term hyperglycemia effect is beneficial or detrimental for the heart in diabetes. Although a prolonged hypertrophy may eventually lead to chronic cardiac failure, an initial cardiac hypertrophy may be an adaptive mechanism to hemodynamic stress at the early stage of diabetes. Further study is needed to clarify the significance and pathological relevance of altered SOCE in the development of diabetic cardiomyopathy.
Mechanisms for altered SOCE in diabetes
Because of the ubiquitous distribution and diverse functions of SOC and the sophisticated molecular and biological processes of diabetes, it is impossible to delineate a common mechanism for abnormal SOCE in different organs/tissues in diabetes. However, several factors, as described below, have been proposed to mediate the diabetes-associated alterations of SOCE.
Impairment of interactions among the molecular components of SOCE pathway
Shortly after the discovery of SOCE, the “conformational coupling model” was proposed to delineate how SOC was activated. In this model, depletion of the internal Ca2+ stores induces a conformational change of a particular protein on the ER membrane (an IP3 receptor in the original hypothesis), and consequently elicits the opening of SOC through a direct physical coupling between the ER protein and the channel proteins in the plasma membrane.132–136 With the recent breakthrough findings about the STIM1 and Orai1 proteins,6–10 this protein–protein interaction model has been modified to a currently widely accepted model in which STIM1 protein on the ER membrane aggregates and translocates to ER-plasma membrane junctions upon depletion of ER Ca2+, where it physically associates and subsequently activates Orai1/TRPCs, resulting in SOCE.11,12,25–33 Therefore, interactions between STIM1 and Orai1/TRPCs are required for the initiation of SOCE, and impairment of those interactions is expected to attenuate SOCE. Diabetes may influence SOCE by disrupting the physical interactions among these essential protein components of the SOCE pathway. In a recent study by Jardin et al., SOCE was reduced in platelets from type II diabetic subjects.128 However, the expression levels of several proteins in the SOCE pathway were increased (STIM1 and Orai1) or not altered (TRPC1).127 A further study demonstrated that associations between STIM1 and Orai1/TRPC1 were attenuated in platelets from diabetic donors.128 Therefore, the attenuation of SOCE in diabetic platelets is due to impairment of functional coupling between the gating protein (STIM1) and the channel proteins (Orai1/TRPCs). An interesting question is whether the diabetes-induced alteration of protein coupling is specific to platelets or is a common mechanism for other cell types. If it is platelet specific, what is the underlying mechanism for this cell context-dependent pathway?
ROS
ROS is a critical pathogenic factor in the development of diabetic complications.77,137–139 Accumulating evidence has indicated that ROS contributes to the abnormality of SOCE in diabetes. In cultured human umbilical vein endothelial cells, high glucose treatment (30 mM for 4 days) enhanced SOCE, and consequently resulted in apoptosis.112 Both responses were significantly inhibited by catalase, an enzyme that activates the decomposition of H2O2 into water and oxygen.140 Thus, H2O2 is a mediator of high glucose-enhanced SOCE. Redondo et al. also reported an association of H2O2 with increased SOCE and aggregation in platelets from type 2 diabetic patients.125 As discussed above,130 short-term hyperglycemia (30 mM for 20 hours) reduced SOCE and hypertrophy in cardiomyocytes.130 This heart-protective effect of hyperglycemia was partially restored by inhibition of superoxide production with thenoyltrifluoroacetone (an inhibitor of electron transport complex II) and aminooxyacetic acid (an inhibitor of the malate-aspartate shuttle), suggesting a ROS-mediated response.130 Therefore, ROS can increase SOCE (in endothelial cells and platelets), which is detrimental, and decrease SOCE (cardiomyocytes), which is beneficial, in diabetes depending on the tissues/organs. The dual effects of ROS on SOCE may reflect the complicated roles of ROS in cell signaling, i.e. being both an intracellular secondary messenger in many cellular signal transduction pathways and a major contributor to a variety of diseases.77,137–139,141–145
pp60src
In an earlier study, King et al. found that the activity of tyrosine kinase pp60src was elevated in STZ-induced diabetic rats.146 Recent studies have shown that activation of this enzyme might contribute to diabetes/high glucose-induced augmentation of SOCE.112,126 In cultured human umbilical vein endothelial cells, inhibition of pp60src with PP1 significantly attenuated high glucose (30 mM for 4 days)-induced increase in SOCE.112 In platelets from patients with type 2 diabetes, SOCE was exaggerated, which was accompanied by a greater activity of pp60src upon depletion of the internal Ca2+ stores.126 However, neither study determined how diabetes/high glucose activates pp60src. It is known that pp60src is a redox sensitive tyrosine kinase and mediates H2O2-induced responses in a variety of cells.147–149 Therefore, it is possible that this enzyme is a downstream component of ROS in the regulation of SOCE in states of diabetes mellitus.
Protein kinase C
Hyperactivation of isoforms of protein kinase C (PKC) has been implicated in multiple complications associated with diabetes.150 In glomerular mesangial cells cultured in medium containing normal glucose, PKC mediated store depletion-induced SOC activation.151 However, when mesangial cells were cultured in high glucose medium (30 mM for five days), PKC suppressed SOCE.84 This glucose concentration-dependent effect may be due to distinct isoforms of PKC which are activated under different conditions. For instance, under normal glucose conditions, it is the α isoform of PKC (PKCα) that is predominantly activated and is responsible for SOC activation.152 However, under high glucose conditions, another isoform of PKC, such as PKCβ, which could be inhibitory to SOC, may play a predominant role. It has been firmly established that PKCβ is associated with the development of diabetic nephropathy.153–156 Curtis et al. also proposed a PKC-mediated pathway for the reduction of SOCE in the smooth muscle cells of retinal microvessels from STZ diabetic rats.118 In their study, the PKC antagonist staurosporine completely restored the reduced SOCE in the diabetic vascular myocytes.118 Further study suggested that the β isoform of PKC was responsible for the inhibition because PKCβII was specifically upregulated in diabetic retina, and an inhibitor of PKCβ partially reversed the attenuated SOCE in the vascular smooth muscle cells from these diabetic rats.118
O-GlcNAcylation
Dynamic cycling of N-acetylglucosamine (termed O-GlcNAcylation) on nuclear and cytoplasmic proteins serves as a nutrient sensor to regulate cellular metabolism and physiology in response to nutrients, such as glucose.157 O-GlcNAcylation regulates cellular process both independently and also via cross-talking with protein phosphorylation and other post-translational modifications.158 Recently, emerging evidence has shown that O-GlcNAcylation of proteins is a major molecular player in the development of complications associated with diabetes, such as vasculopathy,159,160 retinopathy,161,162 cardiopathy,163,164 and nephropathy.165 Although multiple mechanisms which may be tissue-specific are responsible for contributions of abnormal O-GlcNAcylation to diabetic complications,158–165 modulation of SOCE may play a role. As in a study discussed earlier, SOCE was attenuated by hyperglycemia (30 mM for 20 h) in cardiomyocytes.130 This inhibitory effect was prevented by azaserine, an inhibitor of hexosamine biosynthetic pathway.130 The hexosamine biosynthetic pathway is crucial in providing the substrate in formation of O-linked β-N-acetylglucosamine, which is needed for O-GlcNAcylation of proteins. Thus, modification of key components of SOCE pathway by O-GlcNAcylation may contribute to the impairment of SOCE in diabetes. Indeed, it has been reported that modification of STIM1 by O-GlcNAcylation attenuates SOCE in neonatal cardiomyocytes.64
Calcineurin/NFAT
It is known that the calcineurin/NFAT pathway is activated and contributes to the development of diabetic complications.76,166 In cardiomyocytes and vascular endothelial cells, this pathway has been shown to reside downstream of SOCE and to mediate SOCE-induced hypertrophy 51,130 and apoptosis.112 However, Daskoulidou et al. found that high glucose (25 mM for three days)-promoted SOCE in vascular endothelial cells and smooth muscle cells was mediated by the activation of calcineurin/NFAT signaling which upregulated the expression of Orai/STIM proteins.63 Therefore, it is possible that diabetes triggers a positive feedback loop between SOCE and the calcineurin/NFAT pathway. In this loop, an initial increase in SOCE activates calcineurin/NFAT signaling which subsequently stimulates Orai1/STIM1 protein expression and consequently enhances SOCE, amplifying the cascade.
Closing remarks
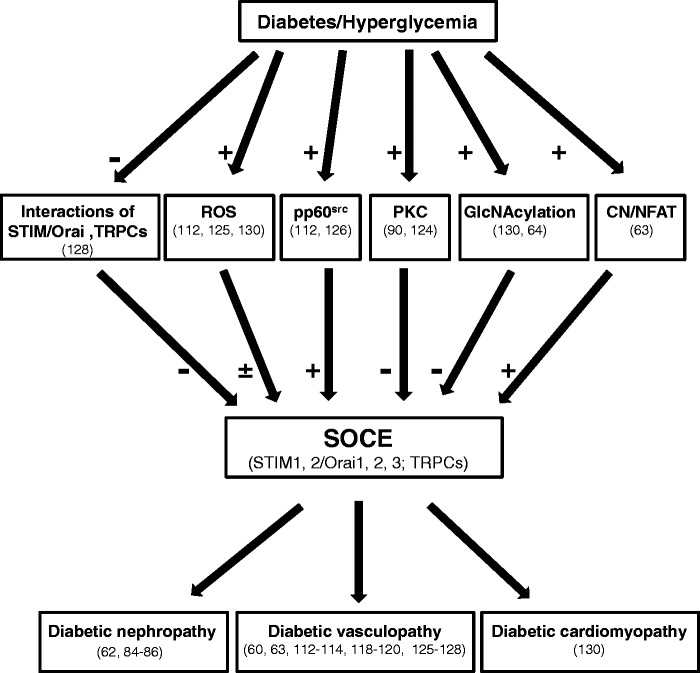
It is clear that diabetes is associated with global changes in the SOCE pathway. However, the alterations of SOCE vary among different cell types and tissues with increased activity in some cells/tissues and decreased in others. Even in the same cell type and tissue, results from different groups appear to contradict each other. Although the reason for the discrepancies is not known with certainty, it is worth noting that in many instances the animal species, cell/tissue types, stages and severity of diabetes, approaches for testing SOC activity, and glucose concentrations differ between sets of experiments. In addition, there are likely several different pathways mediating the diabetes-associated SOC dysfunction. These intracellular pathways may be cell type specific and therefore contribute to the varying SOCE responses to high glucose or diabetes in different type of cells. Furthermore, the mechanism of diabetes-associated SOCE dysregulation is currently unclear. Although several molecules, such as ROS and PKC, have been proposed to be mediators in this pathological process, how they upregulate or downregulate SOCE in diabetes is unknown. Moreover, whether the alteration of SOCE is the cause or the effect of diabetic diseases has yet to be determined. Figure 1 summarizes what is presently known regarding diabetes-associated changes in SOCE in different tissues and the possible underlying mechanisms for those changes. As a result of more information becoming known regarding abnormal SOCE and the development of diabetic complications, the development of specific regulators of SOCE could be a strategic option for various diabetic complications. Given the global pandemic of diabetes, searching for additional therapeutic agents is essential to reduce the immense burden of the disease. SOCE and its molecular components (STIM1/Orai1) may be a novel therapeutic target for patients with diabetic complications.
Figure 1.
Illustration of involvement of abnormal SOCE in diabetic complications and possible underlying mechanisms. VSMCs: vascular smooth muscle cells; ROS: reactive oxygen species; PKC: protein kinase C; CN: calcineurin; NFAT: nuclear factor of activated T cell. “+”: denotes promotion; “-”: denotes inhibition; ±: denotes either promotion or inhibition, depending on tissues and cell types. The numbers in parenthesis indicate citations
Acknowledgements
This review was supported by the National Institutes of Health (RO1 DK079968-01A2).
Authors’ contributions
SC and RM equally contributed to this manuscript preparation.
References
- 1.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol (Lond) 1993; 465: 359–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992; 355: 353–6. [DOI] [PubMed] [Google Scholar]
- 3.Putney JW, Mckay RR. Capacitative calcium entry channels. BioEssays 1999; 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 4.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev 2005; 85: 757–810. [DOI] [PubMed] [Google Scholar]
- 5.Broad LM, Braun FJ, lievremont JP, Bird G, Kurosaki T, Putney JW. Role of the phospholipase C-induced 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem 2001; 276: 15945–52. [DOI] [PubMed] [Google Scholar]
- 6.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005; 169: 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Meyer T. STIM is a Ca2+ store sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current Biol 2005; 15: 1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006; 441: 179–85. [DOI] [PubMed] [Google Scholar]
- 9.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006; 312: 1220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, Yeromin AV, Zhang XHF, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. PNAS 2006; 103: 9357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai-dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem 2009; 284: 22501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Deng X, Gill DL. Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci Signal 2010; 3: pe42–1-pe42-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Current Biol 2006; 16: 1465–70. [DOI] [PubMed] [Google Scholar]
- 14.Dehaven WI, Smyth JT, Boyles RR, Putney JW. Calcium inhibition and calcium potentiation of Orail1, Orail2 and Orail3 calcium-release-activated calcium channels. J Biol Chem 2007; 282: 17548–56. [DOI] [PubMed] [Google Scholar]
- 15.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol 2007; 17: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3. J Biol Chem 2010; 285: 19173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS ONE 2011; 6: e19285–e19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez RA, Wan J, Song S, Smith KA, Gu Y, Tauseef M, Tang H, Makino A, Mehta D, Yuan JXJ. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am J Physiol Cell Physiol 2015; 308: C581–C593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J Cell Sci 2012; 125: 4354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal 2015; 8: ra74–ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol 2011; 194: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol 2012; 52: 136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horinouchi T, Higashi T, Higa T, Terada K, mai Y, Aoyagi H, Hatate C, Nepal P, Horiguchi M, Harada T, Miwa S. Different binding property of STIM1 and its novel splice variant STIM1L to Orai1, TRPC3, and TRPC6 channels. Biochem Biophy Res Commun 2012; 428: 252–8. [DOI] [PubMed] [Google Scholar]
- 24.Saüc S, Bulla M, Nunes P, Orci L, Marchetti A, Antigny F, Bernheim L, Cosson P, Frieden M, Demaurex N. STIM1L traps and gates Orai1 channels without remodeling the cortical ER. J Cell Sci 2015; 128: 1568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem 2009; 284: 9733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 2007; 42: 205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 2007; 6: 636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong E, Nesin V, Long CL, Bai CX, Guz JL, Ivanov IP, Abramowitz J, Birnbaumer L, Humphrey MB, Tsiokas L. A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J Biol Chem 2013; 288: 22219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduced store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 2009; 137: 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. PNAS 2009; 106: 3202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. PNAS 2008; 105: 2895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. PNAS 2007; 104: 4682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. J Biol Chem 2007; 282: 9105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis RS. The molecular choreography of a store-operated calcium channel. Nat 2007; 446: 284–7. [DOI] [PubMed] [Google Scholar]
- 35.Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 2007; 42: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 2007; 42: 173–82. [DOI] [PubMed] [Google Scholar]
- 37.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium 2007; 42: 133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. TRENDs Biochem Scien 2007; 32: 235–45. [DOI] [PubMed] [Google Scholar]
- 39.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW. Emerging perspectives in store-operated Ca2+ entry: Roles of Orai, Stim and TRP. Biochim Biophys Acta 2006; 1763: 1147–60. [DOI] [PubMed] [Google Scholar]
- 40.Targos B, Baranska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim Pol 2005; 52: 397–409. [DOI] [PubMed] [Google Scholar]
- 41.Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium 2002; 31: 89–96. [DOI] [PubMed] [Google Scholar]
- 42.Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 2014; 47: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung FP, yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol 2007; 153: 846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feske S. Calcium signalling in lympjocyte activation and disease. Nat Rev Immunol 2007; 7: 690–702. [DOI] [PubMed] [Google Scholar]
- 45.Darbellay B, Amaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Ora1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem 2009; 284: 5370–80. [DOI] [PubMed] [Google Scholar]
- 46.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol 2008; 586: 4815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 2008; 103: 1289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 2010; 298: C993–C1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 2009; 23: 2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma R, Smith S, Child A, Carmines PK, Sansom SC. Store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol 2000; 278: F954–F961. [DOI] [PubMed] [Google Scholar]
- 51.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 2010; 48: 1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in Orai1 and STIM1. Clin Immunol 2010; 135: 169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feske S. Orai1 and STIM1 deficiency in human and mice: role of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev 2009; 231: 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picard C, McCarl C, Papolos A, Khalil S, Lüthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 2009; 360: 1971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duke AM, Hopkins PM, Calaghan SC, Halsall JP, Steele DS. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J Biol Chem 2010; 285: 25645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008; 7: 561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarl C, Picard C, Khalil S, Kawasaki T, Röther J, Papolos A, Kutok J, Hivroz C, LeDeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. Orai1 deficiency and lack of store-oeprated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol 2009; 124: 1311–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JXJ. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 2011; 302: C405–C411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agrotis A, Koulis C. STIM1: a new therapeutic target in occlusive vascular disease? Cardiovasc Res 2009; 81: 627–8. [DOI] [PubMed] [Google Scholar]
- 60.Chung AW, Yeung KA, Chum E, Okon EB, van Breemen C. Diabetes modulates capacitative calcium entry and expression of transient receptor potential canonical channels in human saphenous vein. Eur J Pharmacol 2009; 613: 114–18. [DOI] [PubMed] [Google Scholar]
- 61.Ding H, Triggle CR. Endothelial dysfunction in diabetes: Multiple targets for treatment. Pflugers Arch 2010; 459: 977–94. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J, Begg M, Ma R. High glucose and diabetes enhanced store-operated Ca2+ entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol 2014; 306: F1069–F1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daskoulidou N, Zeng B, Berglund LM, Jiang H, Chen GL, Kotova O, Bhandari S, Ayoola J, Griffin S, Atkin SL, Gomez MF, Xu SZ. High glucose enhances store-operated calcium entry by upregulating Orai/STIM via calcineurin-NFAT signaling. J Mol Med 2015; 93: 511–21. [DOI] [PubMed] [Google Scholar]
- 64.Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC. Modification of STIM1 by O-linked N-acetylglucosamine (O-GlcNAc) attenuates store-operated calcium entry in neonatal cardiomyocytes. J Biol Chem 2012; 287: 39094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasinath BS. Diabetic nephropathy: challenges remain. NephSAP 2012; 11: 303–7. [Google Scholar]
- 66.US Renal Data System USRDS 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States, NIH/NIDDK. Bethesda, MD, 2011, 2011. [Google Scholar]
- 67.Molitch ME, Adler AI, Flyvbjerg A, Nelson RG, So WY, Wanner C, Kasiske BL, Wheeler DC, de, Zeeuw D, Mogensen CE. Diabetic kidney disease: a clinical update from kidney disease: Improving global outcomes. Kidney Int 2015; 87: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L. Excerpts from the US renal data system 2009 annual data report. Am J Kidney Dis 2010; 55: S1–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foley RN, Collins AJ. End-stage renal disease in the Unites States: An update from the Unites States renal data system. J Am Soc Nephrol 2007; 18: 2644–8. [DOI] [PubMed] [Google Scholar]
- 70.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med 2008; 233: 4–11. [DOI] [PubMed] [Google Scholar]
- 71.Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 2007; 71: 846–54. [DOI] [PubMed] [Google Scholar]
- 72.Mason RM, Wahab A. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 2003; 14: 1358–73. [DOI] [PubMed] [Google Scholar]
- 73.Kanwar YS, Akagi S, Sun L, Nayak B, Xie P, Wada J, Chugh SS, Danesh FR. Cell biology of diabetic kidney disease. Nephron Exp Nephrol 2005; 101: e100–e110. [DOI] [PubMed] [Google Scholar]
- 74.Ziyadeh FN, Sharma K. Role of transforming growth factor-β in diabetic glomerulosclerosis and renal hypertrophy. Kidney Int 1995; 48: S34–S36. [PubMed] [Google Scholar]
- 75.Abboud HE. Mesangial cell biology. Exp Cell Res 2012; 318: 979–85. [DOI] [PubMed] [Google Scholar]
- 76.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol 2003; 284: F144–F154. [DOI] [PubMed] [Google Scholar]
- 77.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 2005; 280: 39616–26. [DOI] [PubMed] [Google Scholar]
- 78.Ma R, Pluznick JL, Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology 2005; 20: 102–11. [DOI] [PubMed] [Google Scholar]
- 79.Menè P, Teti A, Pugliese F, Cinotti GA. Calcium release-activated calcium influx in cultured human mesangial cells. Kidney Int 1994; 46: 122–8. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol 2004; 287: C357–C364. [DOI] [PubMed] [Google Scholar]
- 81.Sours-brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med 2009; 234: 673–82. [DOI] [PubMed] [Google Scholar]
- 82.Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J Am Soc Nephrol 2001; 12: 47–53. [DOI] [PubMed] [Google Scholar]
- 83.Menè P, Pugliese F, Cinotti GA. Regulation of capacitative calcium influx in cultured human mesangial cells: Roles of protein kinase C and calmodulin. J Am Soc Nephrol 1996; 7: 983–90. [DOI] [PubMed] [Google Scholar]
- 84.Mené P, Pugliese G, Pricci F, DiMario U, Cinotti GA, Pugliese F. High glucose level inhibits capacitative Ca2+ influx in cultured rat mesangial cells by a protein kinase C-dependent mechanism. Diabetologia 1997; 40: 521–7. [DOI] [PubMed] [Google Scholar]
- 85.Nutt LK, O'Neil RG. Effect of elevated glucose on endothelin-induced store-operated and non-store-operated calcium influx in renal mesangial cells. J Am Soc Nephrol 2000; 11: 1225–35. [DOI] [PubMed] [Google Scholar]
- 86.Menè P, Pascale C, Teti A, Bernardini SV, Cinotti GA, Pugliese F. Effects of advanced glycation end products on cytosolic Ca2+ signaling of cultured human mesangial cells. J Am Soc Nephrol 1999; 10: 1478–86. [DOI] [PubMed] [Google Scholar]
- 87.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wnag Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 2011; 286: 31799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 2006; 577: 479–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–20. [DOI] [PubMed] [Google Scholar]
- 90.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouillé A, Dupuis M, Hadri L, Jeong D, Mühlstedt S, Schmitt J, Braun A, Bénard L, Saliba Y, Laggerbauer B, Nieswandt B, Lacampagne A, Hajjar RJ, Lompré AM, Engelhardt S. Critical role for stromal interactionmolecule 1 in cardiac hypertrophy. Circulation 2011; 124: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng H, Liu J, Chen R, Wang Y, Duan J, Li C, Li B, Jing Y, Chen X, Mao Q, Xu KF, Walker CL, Li J, Wang J, Zhang H. mTORC1 enhancement of STIM1-mediated store-operated Ca2+ entry constrains tuberous sclerosis complex-related tumor development. Oncogene 2013; 32: 4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu P, Wang Y, Davis ME, Zuckerman JE, Chaudhari S, Begg M, Ma R. Store-operated Ca2+ channel in mesangial cells inhibits matrix protein expression. J Am Soc Nephrol 2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reddy GR, Kotlyarevska K, Ransom RF, Menon RK. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens 2008; 17: 32–6. [DOI] [PubMed] [Google Scholar]
- 94.Spurney RF, Coffman TM. Stressed-out podocytes in diabetes? J Am Soc Nephrol 2008; 19: 2035–42. [DOI] [PubMed] [Google Scholar]
- 95.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 2009; 58: 1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vallon V, Thomson SC. Renal function in daibetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012; 74: 351–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Persson P, Hansell P, Palm F. NADPH oxidase inhibition reduces tubular sodium transport and improves kidney oxygenation in diabetes. Am J Physiol Regul Integr Comp Physiol 2012; 302: R1443–R1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int 1991; 40: 792–807. [DOI] [PubMed] [Google Scholar]
- 99.Carmines PK, Fujiwara K. Altered electromechanical coupling in the renal microvasculature during the early stage of diabetes mellitus. Clin Exp Pharmacol Physiol 2002; 29: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Troncosco BCM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 2008; 295: F171–F178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inazu M, Sakai Y, Homma I. Contractile responses and calcium mobilization in renal arteries of diabetic rats. Eur J Pharmacol 1991; 203: 79–84. [DOI] [PubMed] [Google Scholar]
- 102.Fellner SK, Arendshorst WJ. Ryanodine receptor and capacitative Ca2+ entry in fresh preglomerular vascular smooth muscle cells. Kidney Int 2000; 58: 1686–94. [DOI] [PubMed] [Google Scholar]
- 103.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arteioles: differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res 2000; 87: 551–7. [DOI] [PubMed] [Google Scholar]
- 104.Nagahama T, Hayashi K, Ozawa Y, Takenaka T, Saruta T. Role of protein kinase C in angiotensin II-induced constriction of renal microvessels. Kidney Int 2000; 57: 215–23. [DOI] [PubMed] [Google Scholar]
- 105.Facemire CS, Arendshorst WJ. Calmodulin mediates norepinephrin-induced receptor-operated calcium entry in preglomerular resistance arteries. Am J Physiol Renal Physiol 2005; 289: F127–F136. [DOI] [PubMed] [Google Scholar]
- 106.Schweda F, Riegger GAJ, Kurtz A, Kramer BK. Store-operated calcium influx inhibits renin secretion. Am J Physiol Renal Physiol 2000; 279: F170–F176. [DOI] [PubMed] [Google Scholar]
- 107.Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens 2001; 14: 475–86. [DOI] [PubMed] [Google Scholar]
- 108.Lagaud GJL, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in Murine mesenteeric resistance arteries. J Vas Res 2001; 38: 578–89. [DOI] [PubMed] [Google Scholar]
- 109.Okon EB, Chung AWY, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van Breemen C. Compromised arterial function in human type 2 diabetic patients. Diabetes 2005; 54: 2415–23. [DOI] [PubMed] [Google Scholar]
- 110.Bokoch GM, Diebold B, Kim JS, Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid Redox Signal 2009; 11: 2429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang S, Peng Q, Zhang J, Liu L. Na+/H+ exchanger is required for hyperglycemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovasc Res 2008; 80: 255–62. [DOI] [PubMed] [Google Scholar]
- 112.Tamareille S, Mignen O, Capiod T, Rucker-Martin C, Feuvray D. High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium 2006; 39: 47–55. [DOI] [PubMed] [Google Scholar]
- 113.Bishara NB, Ding H. Glucose enhances expression of TRPC1 and calcium entry in endothelial cells. Am J Physiol Heart Circ Physiol 2010; 298: H171–H178. [DOI] [PubMed] [Google Scholar]
- 114.Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JXJ, Han W, Makino A. STIM1 restores coronary endothelial function in type I diabetic mice. Circ Res 2012; 111: 1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol 2010; 298: C211–C220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A. Increased myogenic tone in skeletal muscle arteriols of daibetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovas Res 1999; 43: 1018–28. [DOI] [PubMed] [Google Scholar]
- 117.Jarajapu YPR, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol 2008; 579: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Curtis TM, Major EH, Trimble ER, Scholfield CN. Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle. Diabetologia 2003; 46: 1252–9. [DOI] [PubMed] [Google Scholar]
- 119.Ma L, Zhu B, Chen X, Liu J, Guan Y, Ren J. Abnormalities of sarcoplasmic reticulum Ca2+ mobilization in aortic smooth muscle cells from streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol 2008; 35: 568–73. [DOI] [PubMed] [Google Scholar]
- 120.Mita M, Ito K, Taira K, Nakagawa J, Walsh MP, Shoji M. Attenuation of store-operated Ca2+ entry and enhanced expression of TRPC channels in caudal artery smooth muscle from type 2 diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol 2010; 37: 670–8. [DOI] [PubMed] [Google Scholar]
- 121.Andrews KI, Pannirselvam M, Anderson TJ, Jenkins A, Triggle CR, Hill MA. The vascular endothelium in diabetes: a practical target for drug treatment? Expert Opin Ther Targets 2005; 9: 101–17. [DOI] [PubMed] [Google Scholar]
- 122.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109(23 Suppl 1): III27–III32. [DOI] [PubMed] [Google Scholar]
- 123.Sobol AB, Watala C. The role of platelets in diabetes-related vascular complications. Diabetes Res Clin Pract 2000; 50: 1–16. [DOI] [PubMed] [Google Scholar]
- 124.EI Haouari M, Rosado JA. Platelet signalling abnormalities in patients with type 2 diabetes mellitus: a review. Blood Cells Mol Dis 2008; 41: 119–23. [DOI] [PubMed] [Google Scholar]
- 125.Redondo PC, Jardin I, Hernandez-Cruz JM, Pariente JA, Salido GM, Rosado JA. Hydrogen peroxide and peroxynitrite enhances Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem Biophys Res Commun 2005; 333: 794–802. [DOI] [PubMed] [Google Scholar]
- 126.Saavedra FR, Redondo PC, Hernandez-Cruz JM, Salido GM, Pariente JA, Rosado JA. Store-operated Ca2+ entry and tyrosine kinase pp60 (src) hyperactivity are modulated by hyperglycemia in platelets from patients with non-insulin-dependent diabetes mellitus. Arch Biochem Biophys 2004; 432: 261–8. [DOI] [PubMed] [Google Scholar]
- 127.Zbidi H, Lopez JJ, Amor NB, Bartegi A, Salido GM, Rosado JA. Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis 2009; 43: 211–13. [DOI] [PubMed] [Google Scholar]
- 128.Jardin I, López J, Zbidi H, Bartegi A, Salido GM, Rosado JA. Attenuated store-operated divalent cation entry and association between STIM1, Orai1, hTRPC1 and hTRPC6 in platelets from type 2 diabetic patients. Blood Cells Mol Dis 2011; 46: 252–60. [DOI] [PubMed] [Google Scholar]
- 129.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 2000; 87: 1123–32. [DOI] [PubMed] [Google Scholar]
- 130.Pang Y, Hunton DL, Bounelis P, Marchase RB. Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 2002; 51: 3461–7. [DOI] [PubMed] [Google Scholar]
- 131.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cadiac hypertrophy. Cell 1998; 93: 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Birnbaumer L, Boulay G, Brown D, Jiang M, Dietrich A, Mikoshiba K, Zhu X, Qin N. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Hormone Res 2000; 55: 127–61. [PubMed] [Google Scholar]
- 133.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol 2001; 19: 497–521. [DOI] [PubMed] [Google Scholar]
- 134.Berridge MJ. Capacitative calcium entry. Biochem J 1995; 312: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 2000; 287: 1647–51. [DOI] [PubMed] [Google Scholar]
- 136.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 1998; 396: 478–82. [DOI] [PubMed] [Google Scholar]
- 137.Hayden MR, Sowers JR. Redox imbalance in diabetes. Antioxid Redox Signal 2007; 9: 865–7. [DOI] [PubMed] [Google Scholar]
- 138.Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type 1 diabetes. Antioxid Redox Signal 2007; 9: 879–89. [DOI] [PubMed] [Google Scholar]
- 139.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, Abboud HE. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 2015; 308: F1276–F1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem 1998; 273: 3756–64. [DOI] [PubMed] [Google Scholar]
- 141.Lassègue B, Griendling KK. Reactive oxygen species in hypertension. Am J Hypertens 2004; 17: 852–60. [DOI] [PubMed] [Google Scholar]
- 142.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 2003; 14: S211–S215. [DOI] [PubMed] [Google Scholar]
- 143.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell 2008; 19: 2984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 2008; 10: 1275–312. [DOI] [PubMed] [Google Scholar]
- 145.Schnackenberg CG. Oxygen radicals in cardiovascular-renal disease. Curr Opin Pharmacol 2002; 2: 121–5. [DOI] [PubMed] [Google Scholar]
- 146.King MJ, Pugazhenthi S, Khandelwal RL, Sharma RK. Elevated N-myristoyl transferase activity is reversed by sodium orthovanadate in streptozotocin-induced diabetic rat. Biochim Biophys Acta 1993; 1165: 259–62. [DOI] [PubMed] [Google Scholar]
- 147.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 2003; 107: 1040–5. [DOI] [PubMed] [Google Scholar]
- 148.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B invovles a sulphenyl-amide intermediate. Nature 2003; 423: 769–73. [DOI] [PubMed] [Google Scholar]
- 149.Rosado JA, Redondo PC, Salido GM, Gomez-Arteta E, Sage SO, Panente JA. Hyderogen peroxide generation induces pp60src activation in human platelets. J Biol Chem 2004; 279: 1665–75. [DOI] [PubMed] [Google Scholar]
- 150.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859–66. [DOI] [PubMed] [Google Scholar]
- 151.Ma R, Pluznick J, Kudlacek P, Sansom SC. Protein kinase C activates store-operaated Ca2+ channels in human glomerular mesangial cells. J Biol Chem 2001; 276: 25759–65. [DOI] [PubMed] [Google Scholar]
- 152.Ma R, Kudlacek PE, Sansom SC. Protein kinase Cα participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Cell Physiol 2002; 283: C1390–C1398. [DOI] [PubMed] [Google Scholar]
- 153.Meier M, Park JK, Overheu D, Kirsch T, Lindschau C, Gueler F, Leitges M, Menne J, Haller H. Deletion of protein kinase C-β isoform in vivo reduces renal hypertrophy but not albuminuria in the streptozotocin-induced diabetic mouse model. Diabetes 2007; 56: 346–54. [DOI] [PubMed] [Google Scholar]
- 154.Kelly DJ, Buck D, Cox A, Zhang Y, Gilbert R. Effects of protein kinase C-β inhibition on glomerular vascular endothelial growth factor expression and endothelial cells in advanced experimental diabetic nephropathy. Am J Physiol Renal Physiol 2007; 293: F565–F574. [DOI] [PubMed] [Google Scholar]
- 155.Tuttle KR. Protein kinase C-β inhibition for diabetic kidney disease. Diabetes Res Clin Pract 2008; 82 S: S70–S74. [DOI] [PubMed] [Google Scholar]
- 156.Whiteside CI, Dlugosz JA. Mesangial cell protein kinase C isozyme activation in the diabetic milieu. Am J Physiol Renal Physiol 2002; 282: F975–F980. [DOI] [PubMed] [Google Scholar]
- 157.Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab 2014; 20: 208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vaidyanathan K, Wells L. Multiple tissue-specific roles for the O-GlcNAc post-translational modification in the induction of and complications arising from type II diabetes. J Biol Chem 2014; 289: 34466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell'Italia LJ, Chatham JC, Wu H, Chen Y. Activation of AKT by O-linked N-Acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res 2014; 114: 1094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rogers MA, Aikawa E. Modifying vascular calcification in diabetes mellitus contribution of O-GlcNAcylation. Circ Res 2014; 114: 1074–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Semba RD, Huang H, Lutty GA, Van Eyk JE, Hart GW. The role of O-GlcNAc signaling in the pathogensis of diabetic retinopathy. Proteomics Clin Appl 2014; 8: 218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim SJ, Yoo WS, Choi M, Chung I, Yoo JM, Choi WS. Increased O-GlcNAcylation of NF-κB enhances retinal ganglion cell death in streptozotocin-induced diabetic retinopathy. Curr Eye Res 2015. in press. [DOI] [PubMed] [Google Scholar]
- 163.Aguilar H, Fricovsky E, Ihm S, Schimke M, Maya-Ramos L, Aroonsakool N, Ceballos G, Dillmann W, Villarreal F, Ramirez-Sanchez I. Role of high-glucose-induced protein O-GlcNAcylation in stimulating cardiac fibroblast collagen synthesis. Am J Physiol Cell Physiol 2014; 306: C794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Banerjee PS, Ma J, Hart GW. Diabetes-associated dysregulation of O-glcNAcylation in rat cardiac mitochondria. PNAS 2015; 112: 6050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park MJ, Kim D, Lim SK, Choi JH, Han HJ, Yoon KC, Park SH. High glucose-induced O-GlcNAcylated carbohydrate response element-binging protein (ChREBP) mediates mesangial cell lipogenesis and fibrosis, the possible role in the development of diabetic nephropathy. J Biol Chem 2014; 289: 13519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li R, Zhang L, Shi W, Zhang B, Liang X, Liu S, Wang W. NFAT2 mediates high glucose-induced glomerular podocyte apoptosis through incrased Bax expression. Exp Cell Res 2013; 319: 992–1000. [DOI] [PubMed] [Google Scholar]