Abstract
The aim of the present work was to explore possible protective effects of sulforaphane (SFN) against atherosclerosis development and endothelial dysfunction in hypercholesterolemic rabbits. Rabbits were assigned to three groups of five: group I fed normal chow diet for four weeks, group II fed 1% high cholesterol diet (HCD) and group III fed HCD + SFN (0.25 mg/kg/day). Blood samples were collected for measurement of serum triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), lactate dehydrogenase (LDH) and C-reactive protein (CRP). Aortic malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD) and total nitrite/nitrate (NOx) were measured. Vascular reactivity and intima/media (I/M) ratio were analyzed. Nuclear factor-kappa B (NF-κB) activation in aortic endothelial cells was identified immunohistochemically. HCD induced significant increases in serum TGs, TC, LDL-C, LDH, and CRP, and aortic MDA and SOD. Moreover, HCD caused significant reductions in serum HDL-C, aortic GSH and NOx. SFN administration significantly decreased HCD-induced elevations in serum TC, LDL-C, CRP, and LDH. while significantly increased HDL-C and GSH levels and normalized aortic SOD and NOx. Additionally, SFN significantly improved rabbit aortic endothelium-dependent relaxation to acetylcholine. Moreover, SFN significantly reduced the elevation in I/M ratio. This effect was confirmed by aortic histopathologic examination. The expression of NF-κB in aortic tissue showed a marked reduction upon treatment with SFN. In conclusion, this study reveals that SFN has the ability to ameliorate HCD-induced atherosclerotic lesions progression and vascular dysfunction, possibly via its lipid-lowering and antioxidant effects and suppression of NF-κB-mediated inflammation.
Keywords: Hypercholesterolemia, sulforaphane, lipid profile, endothelial dysfunction, oxidative stress, NF-κB, nitric oxide
Introduction
Hypercholesterolemia is a major risk factor for atherosclerosis and related occlusive vascular disease,1 which have come to represent a major cause of morbidity and mortality throughout the world. Several lines of evidence have shown that hypercholesterolemia may increase production of oxygen free radicals leading to oxidation of low-density lipoprotein cholesterol (LDL-C), which sets the stage for development of atherosclerosis.2 Thus, apart from avoiding high-cholesterol intake, protecting LDL-C against oxidative modification by antioxidant supplementation should be effective in inhibiting or at least suppressing atherosclerosis.
The potential use of antioxidants as a therapeutic strategy for prevention or treatment of atherosclerosis has received considerable attention.3–5 However, clinical trials of dietary antioxidant supplementation did not reveal beneficial effects of antioxidants in reducing the risk of cardiovascular disease in the general population.6–8 Given the disappointing results of direct antioxidants in clinical trials, therapeutic approaches aimed at upregulation of endogenous antioxidants may be more effective.
Sulforaphane (SFN) is a naturally occurring isothiocyanate contained in cruciferous vegetables such as broccoli, brussel sprouts, cabbage, etc.9 Increased consumption of cruciferous vegetables has been associated with a decreased risk of cardiovascular disease mortality.10–12 SFN has recently attracted a great interest as an indirect antioxidant due to its extraordinary ability to induce expression of multiple endogenous enzymes against oxidative stress, namely NADPH quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1), glutathione-S-transferase, superoxide dismutase (SOD), catalase (CAT), and γ-glutamylcysteine synthetase via activation of nuclear factor-erythroid 2-related factor 2 (Nrf2), a key redox-sensitive transcription factor.13–15 Upregulation of these Nrf2-dependent antioxidants promotes detoxification and anti-inflammatory function.16 Therefore, the present study aimed to investigate whether the use of SFN can prevent the development of atherosclerosis and endothelial dysfunction induced by atherogenic diet in male rabbits. Possible mechanisms involved in SFN-mediated effects were also explored.
Materials and methods
Materials
SFN was purchased from EMD Millipore (Billerica, MA, USA). Cholesterol, thiobarbituric acid, reduced glutathione and pyrogallol were purchased from Sigma Chemical Co. (Saint Louis, Mo, USA). All other chemicals used in this study were of fine analytical grade.
Animals
Adult male New-Zealand white rabbits (eight weeks old; 1.30 ± 0.40 kg weight) were obtained from Urology and Nephrology Center, Mansoura University, Egypt. The animals were individually housed in cages with food and water available at all times, maintained under standard conditions of temperature about 25 ± 2℃ with regular 12 h light/12 h dark cycle. Standard diet pellets were prepared weekly (El Nasr Lab Chem. Co., Egypt). Animal care and handling were conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85–23, revised 1985) and had been approved by the committee on animals' experimentation of Mansoura University.
Experimental protocol
Rabbits were randomly distributed in three groups (five in each) as follow: Group I (control), fed with standard rabbit chow; Group II (high cholesterol diet, HCD), fed with 1% cholesterol-enriched chow for four weeks; Group III (HCD-SFN), fed with 1% cholesterol-enriched chow and treated with SFN (0.25 mg/kg/day) orally for four weeks. SFN was given to rabbits as a suspension in 0.5% carboxymethyl cellulose (CMC). Both control and HCD groups received 0.5% CMC (1 mL/kg/day, orally) during the treatment period.
Blood samples were collected from marginal ear vein before (0 time), after 2 weeks and at the end of experiment (after 4 weeks) for measurement of serum triglycerides (TGs), total cholesterol (TC), and high-density lipoprotein–cholesterol (HDL-C). Blood samples were allowed to clot for 90 min before centrifugation at 3000 × g to obtain serum. For aortic assessment, rabbits were euthanized by an overdose of sodium pentobarbital and exsanguinated. At the end of the experiment, the aorta was removed for measurement of aortic malondialdehyde (MDA), reduced glutathione (GSH) and SOD, vascular reactivity and intima/media (I/M) ratio.
Assessment of serum lipid profile
Commercial kits (Stanbio, USA) were used to assess serum levels of TC, TGs, and HDL-C according to previously described methods.17–19 LDL-C was calculated according to the method described by Friedewald and coworkers.20 The atherogenic LDL-C/HDL-C ratio was calculated as previously reported.21
Determination of serum C-reactive protein (CRP)
Rapid latex agglutination test was used for the quantitative determination of CRP in serum using a commercial kit (Biomed Diagnostics, Badr city, Egypt). The test is based on an immunologic reaction between CRP antigen and the corresponding antibody coated on the surface of biologically inert latex particles. The results were considered positive when agglutination of the latex particle suspension occurred within 2 min, showing a CRP level of more than 6 mg/dL. Since negative results may be caused by antigen excess, the test was repeated using diluted serum samples. Serum CRP concentration were calculated by multiplying the dilution factor by the detection limit (6 mg/dL).
Estimation of serum lactate dehydrogenase (LDH) activity
The measurement of LDH activity was based on its ability to catalyze the reduction of pyruvate, in the presence of NADH, to form lactate and NAD+ using a commercial kit (Biosystems S.A., Barcelona, Spain). The catalytic concentration was determined from the rate of reduction of NADH in the reaction medium, measured at 340 nm.
Preparation of aortic homogenate
A 5% w/v aortic homogenate was made in ice-cold 1.15 % KCl, pH 7.4 using a mini handheld homogenizer (Omni international, USA). Tissue homogenates were centrifuged (1000 × g, 4℃, 10 min) and supernatants were collected to measure the following parameters:
Determination of aortic MDA levels
Lipid peroxidation was determined by MDA concentration as thiobarbituric acid reactive substances (TBARS) in the supernatant. Briefly, 1.5 mL of 20% acetic acid adjusted to pH 3.5 with NaOH, 200 µL of 8.1% sodium dodecyl sulfate and 1.5 mL of 0.8% aqueous solution of TBA were added to 200 µL of aortic homogenate. The reaction mixture was completed to 4 mL and then incubated at 95℃ for 60 min. After cooling with tap water, 1 mL of distilled water was added to each sample and then samples were centrifuged at 1000 × g for 10 min and the absorbance of the supernatant was read at 532 nm using a colorimeter WPA colourwave (Model CO 7500, Cambridge, UK). TBARS results were expressed as MDA equivalents using 1, 1, 3, 3 tetramethoxypropane as a standard.22
Determination of aortic GSH levels
The level of acid-soluble thiols, mainly GSH, in the aorta was assayed colorimetrically, based on its reaction with Ellman’s reagent [5,5′ -dithio-bis(2-nitrobenzoic acid)] according to the method earlier described by Ellman23 after protein precipitation with trichloroacetic acid. The absorbance was measured at 412 nm and the concentrations were expressed as µmol/g wet tissue.
Determination of aortic SOD activity
The enzymatic activity of SOD was assessed as previously reported.24 SOD activity was expressed as U/g wet tissue. One unit of SOD activity is defined as the amount of the enzyme causing 50% inhibition of auto-oxidation of pyrogallol.
Measurement of total nitrite/nitrate (NOx) in aortic homogenates
Total aortic nitrite and nitrate, the stable metabolites of nitric oxide (NO), were determined as previously described25,26 using a commercial assay kit (R and D Systems, Minneapolis, MN, USA). Briefly, the nitrate content of the aortic homogenate was converted to nitrite by incubation of samples with nitrate reductase in the presence of NADH at 37℃ for 30 min. Total nitrite was then detected colorimetrically as an azo dye product of the Griess Reaction. The absorbance of each sample was measured at 540 nm and total NOx levels were determined using linear regression of sodium nitrate standard curve.
Preparation of aortic rings
The descending thoracic aorta was quickly separated and placed in a cold oxygenated physiological salt solution (PSS) of the following composition (mM): NaCl 118, CaCl2 2.5, KCl 4.7, MgSO4·7H2O 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11.1; pH 7.4. The vessels were dissected free of connective tissue and fat, then cut into 2–4 mm rings.
In vitro vascular reactivity
Aortic rings were mounted between two stainless steel hooks in an organ bath filled with 10 mL of PSS at 37℃ and bubbled with a mixture of 95% O2 and 5% CO2. Rings were allowed to equilibrate under 1.5 g resting tension for 60 min, and during that time the bath solution was replaced every 15 min. Isometric tension was measured using a force displacement transducer (K30, Hugosachs Elektronik, March, Germany) and recorded with a Powerlab unit linked to a PC running Chart v4.2 software (ADInstruments Pty Ltd., Australia). After the equilibration period, the responsiveness of arterial ring was assessed by measuring contraction to KCl (80 mM). To measure vasorelaxation, rings were first preconstricted with 1 µM phenylephrine and after reaching a steady state contraction (plateau), cumulative concentration–response curves to acetylcholine (10−7–10−5 M) were constructed.
Histopathological analysis
The entire aorta was rapidly dissected out and fixation was done by immersion in 10% neutral buffered formalin solution (pH 7.4). After fixation, the arterial tissues were embedded in paraffin, sectioned transversely (5 µm) and then stained with hematoxylin and eosin (H&E) stain. H&E-stained sections of thoracic aortas were also analyzed for I/M ratios. The analyses were performed microscopically (Leica Imaging Systems®, Cambridge, UK) and the images were analyzed with a specific software (ImageQuant®, Leica®). The intimal cross-sectional area was determined by subtracting the lumen area from the area enclosed by the internal elastic lamina. The medial area was determined by subtracting the area enclosed by the internal elastic lamina from that enclosed by the external elastic lamina. Mean areas were calculated to deduce I/M ratios. Histological assessment was performed by a pathologist, who was unaware of the experimental data in this study.
Immunohistochemical (IHC) analysis for nuclear factor-kappa B (NF-κB)
The expression and localization of NF-κB was determined by detection of the p65 subunit of NF-κB with IHC analysis. Formalin-fixed, paraffin-embedded aortic slices were serially sectioned at 5 µm. Immunostaining was performed using avidin–biotin complex method. In brief, slides were deparaffinized in xylol and rehydrated in descending grades of alcohol. Blocking of endogenous peroxidase using 30% hydrogen peroxide in methanol for 10 min was done followed by washing in phosphate-buffered solution (PBS). Antigen retrieval is performed, when required, in citrate buffer solution at 95℃. Slides were allowed to cool and washed in PBS. Serum blocking solution was added for 10 min and rinsed without washing. Rabbit anti-NF-κB p65 antibody (Thermo Scientific, USA) was applied for 1 h at room temperature followed by washing in PBS three times. Secondary anti-rabbit antibody was added for 10 min and washed in PBS. The slides then covered by avidin enzyme conjugate for 10 min and then washed in PBS. Diaminobenzidine was added as a chromogen for 5 min at room temperature. Slides were counterstained with Meyer’s hematoxylin, dehydrated, and covered. The expression and localization of the p65 subunit of NF-κB was determined by microscopic observation of the brown peroxidase reaction product on the vascular wall of the thoracic aorta. The immunostained sections were examined by an observer blinded to the treatments using light microscope (Olympus).
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM), where n equals the number of rabbits. Vascular relaxation was calculated as a percentage of the maximal contraction induced by 1 µM phenylephrine. The highest response obtained was considered as the maximum response (Emax). pEC50 (negative log the concentration producing 50% of maximal response) was determined from non-linear regression analysis (four-parameter curve fit). Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test. Serum lipid profiles were compared by two-way ANOVA followed by Bonferroni test. Differences were considered significant at P < 0.05. Statistical analyses were carried out using Graphpad Prism software (GraphPad Software Inc. V4.03, San Diego, CA, USA).
Results
Body weight
The final body weight of the HCD group (2.82 ± 0.10 kg) was significantly greater than that of the normal control (2.17 ± 0.03 kg) rabbits (P < 0.05). The HCD and HCD-SFN groups did not differ in body weight (Table 1). Neither HCD nor SFN treatment had any significant effects on food or water consumption in all groups (data were not shown).
Table 1.
Effect of sulforaphane (SFN, 0.25 mg/kg/day) on body weight (BW) in high cholesterol diet (HCD)-fed rabbits
| Groups | Initial BW (kg) | Final BW (kg) |
|---|---|---|
| Control | 1.57 ± 0.06 | 2.17 ± 0.03 |
| HCD | 1.36 ± 0.10 | 2.82 ± 0.10* |
| HCD-SFN | 1.31 ± 0.04 | 2.58 ± 0.10* |
Note: Data are represented as means ± SEM of five rabbits from each group.
Significantly different when compared with the control group, using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (P < 0.05).
Serum lipid profile
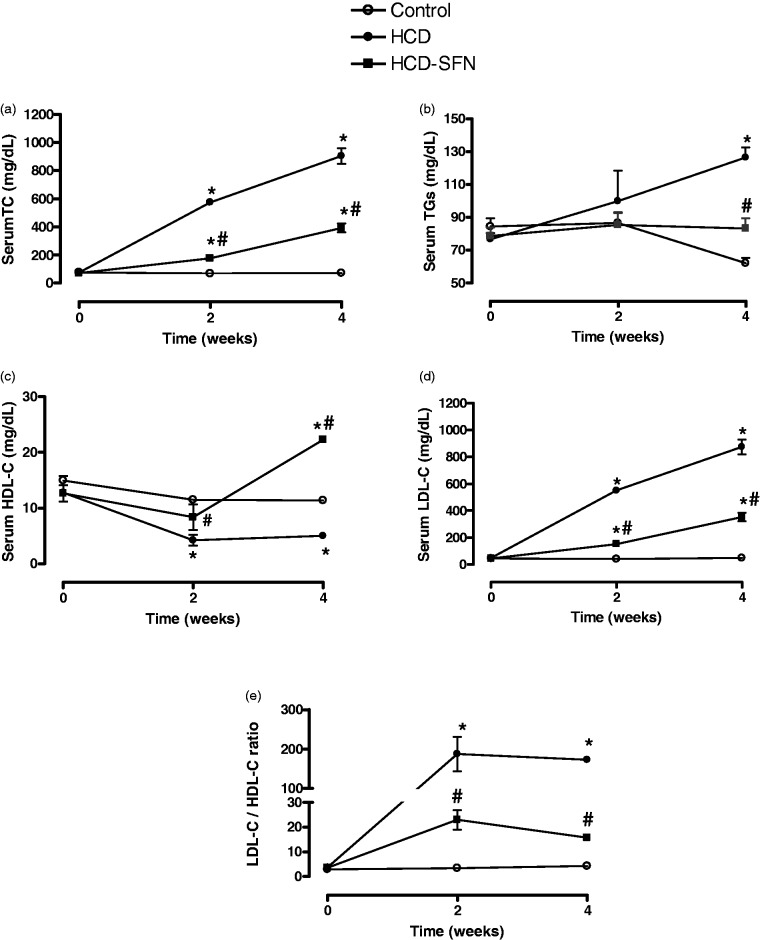
The serum lipid profiles in the three groups of animals are shown in Figure 1(a) to (e). Initial serum levels of lipid profile were not significantly different before diets administration in all groups. HCD induced significant increases in TC, LDL-C and LDL-C/HDL-C ratio and a significant reduction of HDL-C at two and four weeks compared to control group at the same time points (P < 0.05). TG level was only significantly increased in the HCD group compared to control rabbits after four weeks. Treatment with SFN significantly (P < 0.05) decreased levels of TC, TGs, and LDL-C by 43, 70, and 40%, respectively compared to HCD group at four weeks. The protective HDL-C was significantly enhanced (P < 0.05) by SFN treatment to above-normal level at four weeks. Moreover, the atherogenic LDL-C/HDL-C index was brought to near-normal level of control group by chronic SFN treatment.
Figure 1.
Effect of sulforaphane (SFN, 0.25 mg/kg/day) on serum total cholesterol (TC, panel a), triglycerides (TGs, panel b), high density lipoprotein-cholesterol (HDL-C, panel c), low density lipoprotein-cholesterol (LDL-C, panel d) and LDL-C/HDL-C ratio (panel e) in high cholesterol diet (HCD)-fed rabbits. Data are expressed as means ± SEM, n = 5 in each group. * and # Significantly different when compared with the control and HCD groups, respectively, at same experimental time, using two-way ANOVA followed by Bonferroni test (P < 0.05)
Serum CRP
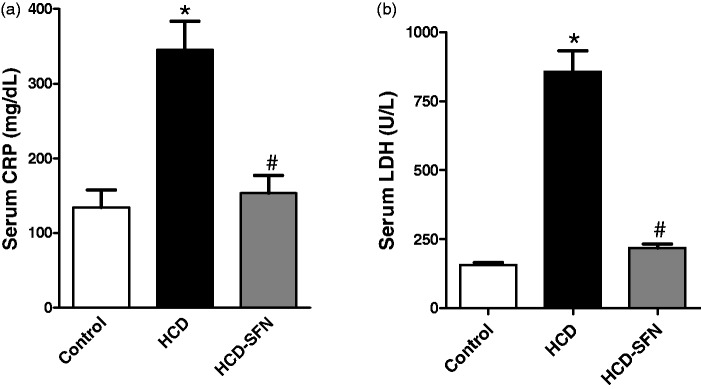
Cholesterol feeding significantly (P < 0.05) increased serum CRP by 157% at four weeks compared to control group at the same time (Figure 2a). SFN treatment significantly (P < 0.05) decreased CRP level by 52% at four weeks compared with HCD group.
Figure 2.
Effect of sulforaphane (SFN, 0.25 mg/kg/day) treatment on serum (a) C-reactive protein (CRP) and (b) lactate dehydrogenase (LDH) in high cholesterol diet (HCD)-fed rabbits. Values are expressed as means ± SEM, n = 5 in each group. * and # Significantly different when compared with the control and HCD groups, respectively using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (P < 0.05)
Serum LDH
Cholesterol feeding significantly (P < 0.05) increased LDH by 446% at four weeks compared to control group at similar time (Figure 2b). SFN treatment significantly (P < 0.05) reduced LDH by 74% at four weeks compared with HCD group.
Oxidative stress
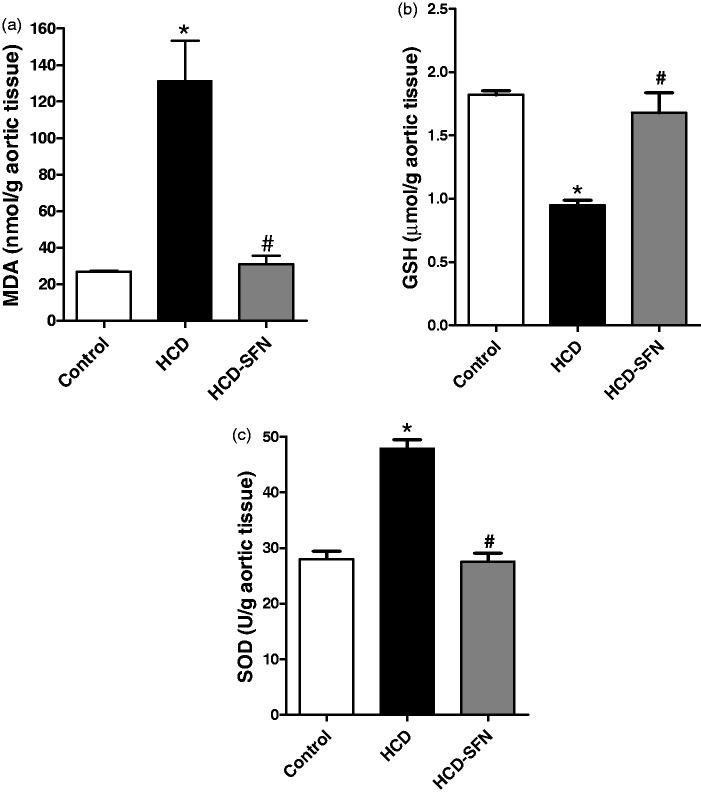
HCD significantly (P < 0.05) increased aortic MDA and SOD levels by 390% and 71%, respectively (Figure 3(a) and (c)) while there was a significant reduction in GSH levels by 48% compared to the control group (Figure 3b). SFN treatment in rabbits receiving HCD significantly (P < 0.05) reduced MDA and SOD levels by 76% and 42%, respectively, while increased GSH levels by 78% compared to the HCD group.
Figure 3.
Effect of sulforaphane (SFN, 0.25 mg/kg/day) treatment on aortic (a) malondialdehyde (MDA), (b) reduced glutathione (GSH), and (c) superoxide dismutase (SOD) in high cholesterol diet (HCD)-fed rabbits. Values are expressed as means ± SEM, n = 5 in each group. * and # Significantly different when compared with the control and HCD groups, respectively using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (P < 0.05)
Aortic NOx
Chronic cholesterol feeding resulted in a significant reduction of aortic NOx levels (P < 0.05), when compared to the control group (Figure 4). SFN treatment of HCD-fed rabbits restored NOx in aortic tissue to similar levels (P > 0.05) of control rabbits.
Figure 4.
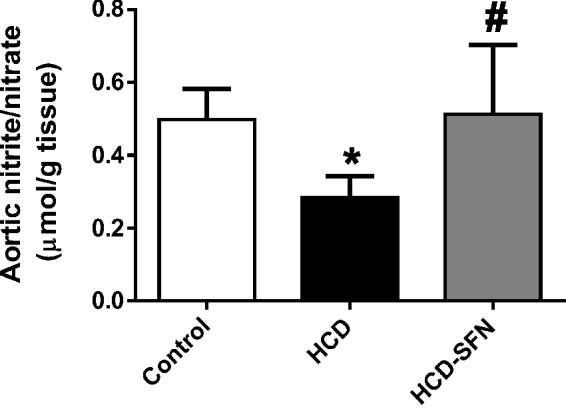
Effect of sulforaphane (SFN, 0.25 mg/kg/day) treatment on aortic nitrite/nitrate (NOx) levels in high cholesterol diet (HCD)-fed rabbits. Values are expressed as means ± SEM, n = 5 in each group. * and # Significantly different when compared with the control and HCD groups, respectively using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (P < 0.05)
Vascular reactivity
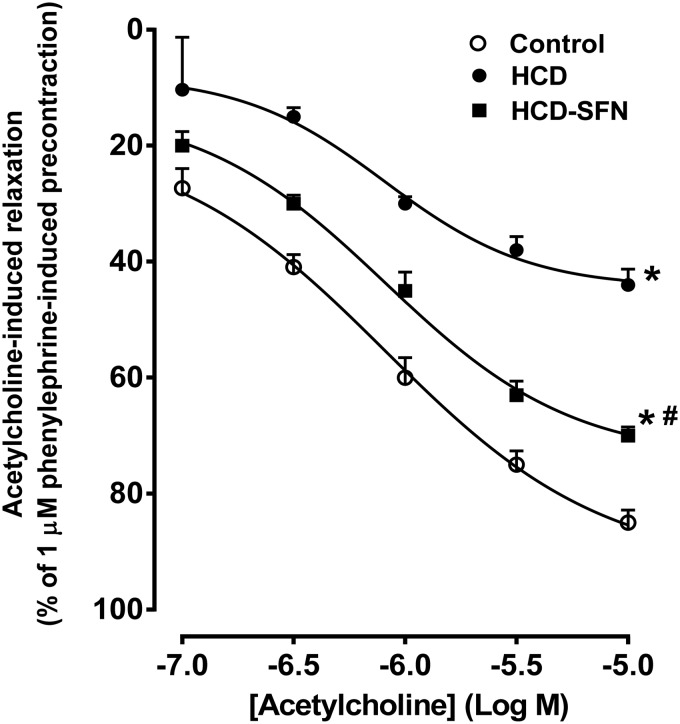
Acetylcholine induced concentration-dependent relaxation in aortic rings from all groups (Figure 5). In the HCD group, relaxations to acetylcholine were significantly impaired as manifested by the decrease in Emax (from 92.37 ± 2.53% to 44.43 ± 3.44%) compared to control group. Treatment of hypercholestrolemic rabbits with SFN significantly (P < 0.05) enhanced acetylcholine-induced relaxation as indicated by the increase of Emax to 74.40 ± 3.67 %.
Figure 5.
Effect of sulforaphane (SFN, 0.25 mg/kg/day) treatment on acetylcholine-induced relaxations of isolated aortic rings in high cholesterol diet (HCD)-fed rabbits. Values are expressed as means ± SEM, n = 5 in each group. Cumulative dose-response curves to acetylcholine (10−7–10−5 M) were measured after precontraction with 1 µM phenylephrine. P < 0.05 for Emax value compared with control group (*) and compared with HCD group (#). One-way ANOVA followed by Tukey–Kramer’s multiple comparisons post hoc test
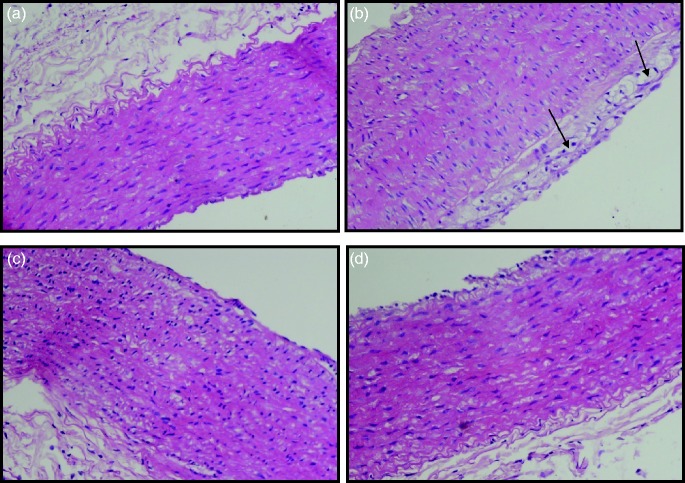
Histopathological analysis of aorta
Histopathological examination of the aorta using H&E stain in control rabbits revealed normal wall of aorta with intact endothelial lining without interruption (Figure 6a). HCD group showed endothelial cell atrophy with subendothelial inflammation and foamy histeocyte infiltrate (Figure 6b), edema and mild fibrosis in the tunica media (Figure 6c). Aorta from SFN-treated rabbits showed very mild edema dissecting the tunica media (Figure 6d).
Figure 6.
Light microscopy photomicrographs depicting aortic sections of rabbits. Aortic sections were fixed and stained with hematoxylin and eosin (H&E) stain. Images were captured at 200× magnification. (a) Control: normal aorta with intact endothelium; (b) High cholesterol diet (HCD): Endothelial cell atrophy with subendothelial inflammation and foamy histeocyte infiltrate (arrows); (c). HCD: Edema and mild fibrosis in the tunica media; (d) HCD-SFN: Very mild edema dissecting the tunica media. (A color version of this figure is available in the online journal.)
Morphometric analysis of aorta
The I/M ratios of thoracic aortas of the control group averaged 0.22 ± 0.01. The mean ratio was increased to 0.62 ± 0.04 in the HCD group (P < 0.05). In HCD-SFN group, there was a significant reduction to 0.37 ± 0.02 in I/M ratio compared to the HCD group (Figure 7).
Figure 7.
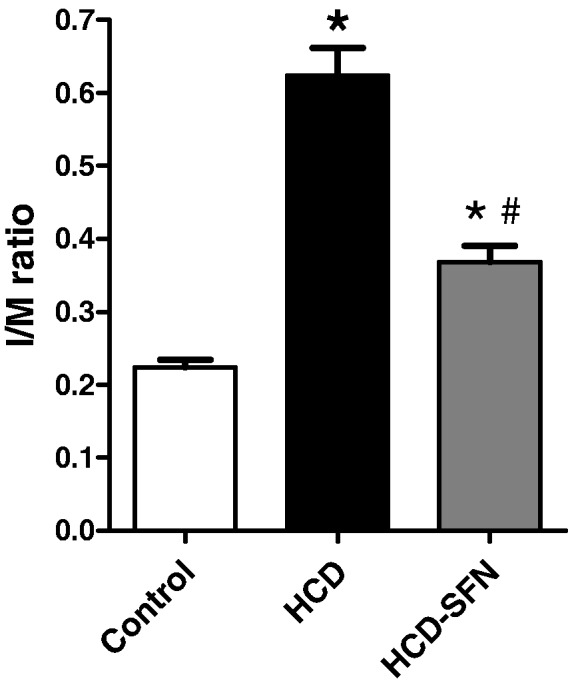
Effect of sulforaphane (SFN, 0.25 mg/kg/day) treatment on intimal/media (I/M) ratio of isolated rabbit thoracic aortas in high cholesterol diet (HCD)-fed rabbits. Data are expressed as mean ± SEM, n = 5 in each group. * and # Significantly different when compared with the control and HCD groups, respectively using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (P < 0.05)
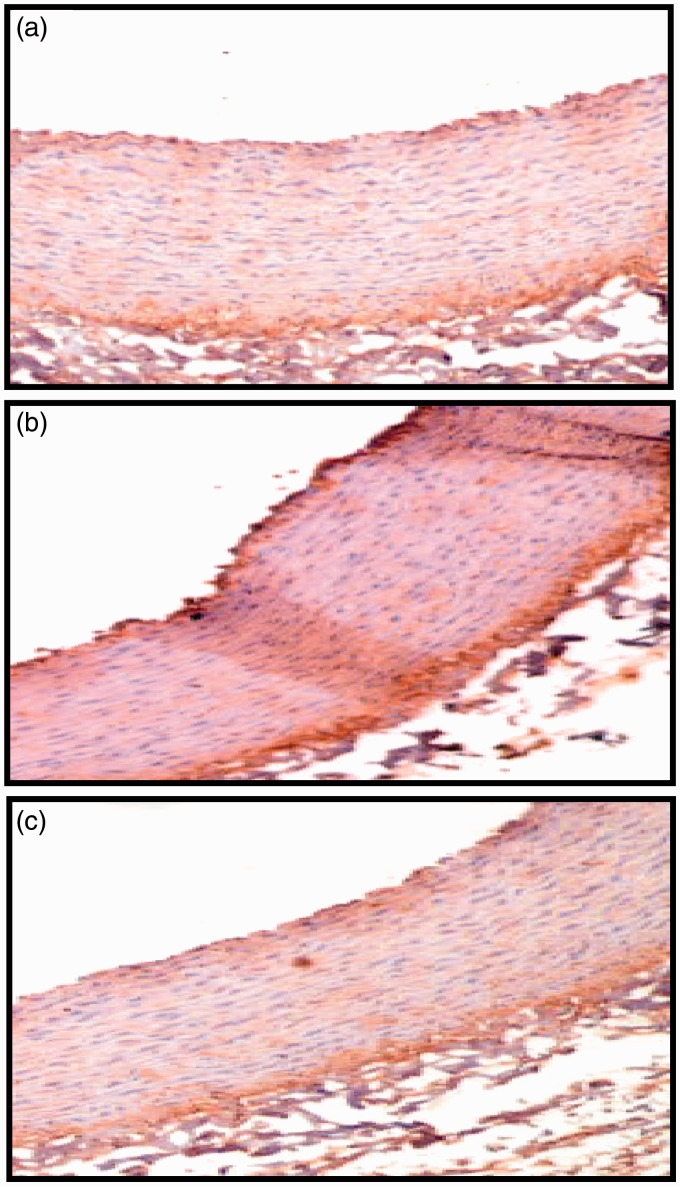
Effect of SFN on p65 subunit of NF-κB localization in thoracic aorta induced by HCD in rabbits
IHC analysis showed a relatively abundant expression of the NF-κB immunoactivity in thoracic aortic tissues after four weeks in the HCD group. However, NF-κB expression was not found in the aortic tissue of control rabbits. After treatment with SFN, the expression and localization of NF-κB in the tissue substantially declined (Figure 8).
Figure 8.
Immunohistochemical staining for p65 subunit of NF-κB in the thoracic aorta Thoracic aorta obtained from control group (a), high cholesterol diet (HCD) group (b), sulforaphane (SFN)-treated group (c). Images were captured at 20× magnification. (A color version of this figure is available in the online journal.)
Discussion
Previous studies of cultured vascular cells showed that SFN can reduce inflammatory activation of endothelial cells.27,28 However, the protective effects of SFN against atherosclerosis have not been studied directly. The present study demonstrates that SFN can suppress the progression of atherosclerosis and improves endothelial dysfunction in HCD-fed rabbits. Possible underlying mechanisms are discussed below.
SFN protects against HCD-induced aortic oxidative stress
Chronic consumption of HCD resulted in an elevation of aortic MDA and a reduction of aortic GSH compared to control rabbits. This is compatible with previous studies.29–31 The increased level of aortic MDA, a well-known index of lipid peroxidation, confirms the occurrence of vascular oxidative stress due to excess generation of reactive radical species,32 which are released by the endothelial cells and infiltrated monocytes.33,34 The decreased aortic GSH level, an intracellular direct antioxidant, may be related to its consumption in detoxifying reactive oxygen species (ROS) or due to impaired GSH synthesis.30
Moreover, HCD-fed rabbits showed an increased aortic SOD activity. This supports the findings of other investigators.4,35,36 Induction of aortic SOD activity may be a defensive mechanism of the endothelial cells against oxidative stress. Elevation of SOD activity with different forms of oxidative stress has been reported.37 Moreover, cultured endothelial cells increase the biosynthesis of SOD in the presence of generators of superoxide radical.38 Failure of activated SOD to defend the vascular wall against the damaging effects of free radical may be due to depletion of other antioxidant mechanisms, which inevitably results in increased vascular susceptibility to oxidative injury.35
SFN treatment prevented HCD-induced increase of MDA, suggesting that it could reduce oxidative stress and lipid peroxidation, which potentially leads to a reduction of hypercholesterolemia-induced atherosclerosis. Several previous reports have shown that SFN prevents vascular inflammation-associated accumulation of ROS.13,39,40 SFN also restored levels of GSH and SOD to normal levels of control rabbits. The SFN-mediated normalization of SOD activity is likely due to decreased defensive synthesis of SOD by endothelial cells in response to reduced oxidative stress. SFN exerts its antioxidant effects through activation of the transcription factor, Nrf2,13,14 which induces gene expression of endogenous antioxidant proteins, resulting in the enhancement of cellular antioxidant capacity.40,41
SFN improves serum lipid profile of hypercholesterolemic rabbits
HCD-fed rabbits exhibited abnormal increases of TC, LDL-C and TG levels and a reduction of HDL-C. Moreover, LDL-C/HDL-C ratio significantly increased. These results are in accordance with previous studies.29,42,43 HCD causes excess radical production, followed by oxidative stress.44 Free radical-mediated lipoprotein oxidation, particularly LDL, is a central event in the pathophysiology of atherosclerosis.45,46 Oxidized LDL species are taken up by macrophages, which accumulate in the endothelial wall as lipid-filled foam cells in the early phases of atherosclerotic lesion formation.46 Oxidized LDL particles can also drive atherosclerosis through induction of intracellular ROS47 and pro-inflammatory molecules.48
Administration of SFN to HCD-fed rabbits showed a beneficial lowering effect on serum lipid levels (TGs, TC and LDL-C). Moreover, SFN significantly increased HDL-C to above-normal level, resulting in a decrease of atherogenic LDL-C/HDL-C index. SFN has been shown to inhibit endothelial lipase expression, which reduces HDL-C level during vascular inflammation.49 Moreover, SFN could also induce hepatic paraoxonase-1, an HDL-C-associated enzyme that protects LDL-C from oxidation.50 Interestingly, rabbits express cholesteryl ester transfer protein (CETP),51 a plasma protein that can elevate the atherogenic LDL-C/HDL-C ratio.51 Whether SFN-mediated increase in serum HDL-C occurs through an effect on CETP expression remains to be determined.
SFN exerts anti-inflammatory effect in hypercholesterolemic rabbits
Cholesterol-fed rabbit showed increased serum levels of LDH activity and CRP relative to control rabbits. Abnormal high activity of serum LDH indicates cellular damage caused by the atherogenic diet.52 CRP, a hepatic pro-inflammatory protein that can also be produced locally by monocytes and smooth muscle cells in atherosclerotic lesions, has been shown to promote atherosclerosis by increasing the expression of adhesion proteins on endothelial cells,53 recruiting monocytes into the arterial wall and increasing the production of inflammatory cytokines by monocytes.54,55
Treatment of HCD-fed rabbits with SFN prevented HCD-induced elevations of serum CRP level and LDH activity, suggesting that SFN possesses anti-inflammatory effects that potentially contributed to the prevention of the progression of atherosclerotic lesions in the current study. SFN also reduced the HCD-induced activation of the transcription factor NF-κB in aortic tissue, supporting previous studies on cultured endothelial cells.39,56,57 This may explain, at least in part, SFN-mediated anti-inflammatory activities. NF-κB regulates expression of multiple genes involved in vascular inflammation including adhesion molecules and chemotactic cytokines.58–60 Coincident with this notion, it has been suggested that SFN suppresses pro-inflammatory activation of endothelial cells, endothelial lipase production, and monocyte adhesion to endothelial cells through reduction of NF-κB-mediated signaling.39,49,61,62 However, NF-κB-independent mechanisms of the anti-inflammatory potential of SFN have also been suggested.27
SFN improves HCD-induced vascular dysfunction
Chronic consumption of cholesterol resulted in a reduced response to acetylcholine- induced vascular relaxation. This is compatible with earlier reports under similar experimental conditions.63–65 Several mechanisms of HCD-induced impairment of endothelium-dependent relaxation have been proposed. Hypercholesterolemia has been shown to be associated with a reduced arterial expression of endothelial NO synthase (eNOS).66 Moreover, increased plasma LDL inhibits the active transport of l-arginine, the substrate of eNOS, by endothelial cells, hence reducing NO synthesis. This leads to increased superoxide anion production,67 which also reacts with NO forming peroxynitrite that further reduces NO bioactivity.68,69 This notion is strongly supported by decreased levels of aortic NOx, an indicator of NO availability, in HCD-fed rabbits in the current investigation. Depletion of vascular GSH also contributes to impaired NO-mediated relaxation in hypercholesterolemia.30,31
SFN-mediated protective effect on endothelial function in the present study may be linked to its ability to lower serum LDL, increase vascular GSH and prevent lipid peroxidation within the vascular wall. These combined effects may reduce the oxidative environment within the vessel wall and thus inhibit intracellular degradation of NO, which may explain restoration of normal aortic NOx levels in SFN-treated HCD-fed rabbits. Whether SFN influences eNOS activity/expression remains to be investigated. Interestingly, Nrf2 activation in redox-stressed endothelial cells resulted in an increase of bioavailable NO, while paradoxically decreased eNOS protein expression.70
Effect of SFN on HCD-induced aortic pathologic change
After four weeks of HCD, morphometric analysis of rabbit aorta confirmed the development of atherosclerosis, in accordance with previous studies.21,29 SFN significantly attenuated atherosclerotic lesions and the I/M layer area ratio of thoracic aortas compared with HCD group. Similarly, SFN inhibited TNF-α-induced structure change in the intima layer of mouse aortas.57 The atheroprotective effect of SFN could be attributed to lowering of serum cholesterol levels. Moreover, SFN has been shown to reduce aortic damage induced by high-fat diet in mice through nrf2-dependent mechanisms.71
Relevance to nutritional studies
Dietary intake of broccoli, a source of sulforaphane,72 was associated with reduced risk of coronary heart disease mortality.11 Moreover, ingestion of broccoli sprouts by the spontaneously hypertensive stroke-prone rats resulted in decreased vascular oxidative stress and inflammation and improvement of aortic endothelium-dependent relaxation.10 The findings of the current study suggest that SFN may have been involved, at least in part, in broccoli-mediated cardiovascular protection. The dose used in the current study was based on the efficacy of SFN established in previous animal studies,73,74 which provides plasma concentration close to that produced by dietary consumption of broccoli.75 The present study thus supports the notion that it would be very beneficial for hypercholesterolemic patients to consider the intake of SFN-rich foods for preventing vascular complications.
Conclusion
Our findings suggest that SFN can attenuate atherosclerosis progression and improve endothelial dysfunction in HCD-fed rabbits, possibly by lowering circulating cholesterol levels and reducing vascular oxidative stress and associated inflammation, the cornerstones of pathogenesis of hypercholestrolemia-induced atherosclerosis.
Acknowledgement
The authors thank Dr Azza Abdel-Aziz, Department of Pathology, Faculty of Medicine, Mansoura University for providing assistance.
Authors’ contributions
Both authors participated in the design of the study, conduction of the experiments, interpretation and analysis of data, and writing and review of manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Castelli WP. Cardiovascular disease in women. Am J Obstet Gynecol 1988; 158: 1553–60, 66–7. [DOI] [PubMed] [Google Scholar]
- 2.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88: 1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad K, Kalra J. Oxygen free radicals and hypercholesterolemic atherosclerosis: Effect of vitamin E. Am Heart J 1993; 125: 958–73. [DOI] [PubMed] [Google Scholar]
- 4.Mantha SV, Kalra J, Prasad K. Effects of probucol on hypercholesterolemia-induced changes in antioxidant enzymes. Life Sci 1996; 58: 503–9. [DOI] [PubMed] [Google Scholar]
- 5.Duell PB. Prevention of atherosclerosis with dietary antioxidants: fact or fiction? J Nutr 1996; 126: 1067S–71S. [DOI] [PubMed] [Google Scholar]
- 6.Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr 2009; 28: 3–9. [DOI] [PubMed] [Google Scholar]
- 7.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 2008; 101: 75D–86D. [DOI] [PubMed] [Google Scholar]
- 8.Raghavamenon A, Garelnabi M, Babu S, Aldrich A, Litvinov D, Parthasarathy S. Alpha-tocopherol is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes: a potential explanation for the failure of antioxidants to affect human atherosclerosis. Antioxid Redox Signal 2009; 1.48–1: 1237–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 2008; 52: S128–38.. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Noyan Ashraf MH, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BH. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci U S A 2004; 101: 7094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol 1999; 149: 943–9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Shu XO, Xiang YB, Yang G, Li H, Gao J, Cai H, Gao YT, Zheng W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr 2011; 94: 240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 2008; 57: 2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002; 62: 5196–203. [PubMed] [Google Scholar]
- 15.Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann N Y Acad Sci 2011; 1229: 184–9. [DOI] [PubMed] [Google Scholar]
- 16.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 2003; 278: 21592–600. [DOI] [PubMed] [Google Scholar]
- 17.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20: 470–5. [PubMed] [Google Scholar]
- 18.Finley PR, Schifman RB, Williams RJ, Lichti DA. Cholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurement. Clin Chem 1978; 24: 931–3. [PubMed] [Google Scholar]
- 19.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins – an integrated approach to mechanisms and disorders. N Engl J Med 1967; 276: 34–42. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 21.Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation 1999; 9: 9.62–1355. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–8. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70–7. [DOI] [PubMed] [Google Scholar]
- 24.Marklund SL. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res 1985; 148: 129–34. [DOI] [PubMed] [Google Scholar]
- 25.Granger DL, Taintor RR, Boockvar KS, Hibbs JB., Jr Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Meth Enzymol 1996; 268: 142–51. [DOI] [PubMed] [Google Scholar]
- 26.Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin Chem 1995; 41: 904–7. [PubMed] [Google Scholar]
- 27.Chen XL, Dodd G, Kunsch C. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res 2009; 58: 513–21. [DOI] [PubMed] [Google Scholar]
- 28.Kwon JS, Joung H, Kim YS, Shim YS, Ahn Y, Jeong MH, Kee HJ. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 2012; 225: 41–9. [DOI] [PubMed] [Google Scholar]
- 29.Prasad K, Mantha SV, Kalra J, Lee P. Prevention of hypercholesterolemic atherosclerosis by garlic, an antixoidant. J Cardiovasc Pharmacol Ther 1997; 2: 309–20. [DOI] [PubMed] [Google Scholar]
- 30.Adachi T, Cohen RA. Decreased aortic glutathione levels may contribute to impaired nitric oxide-induced relaxation in hypercholesterolaemia. Br J Pharmacol 2000; 129: 1014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma XL, Lopez BL, Liu GL, Christopher TA, Gao F, Guo Y, Feuerstein GZ, Ruffolo RR, Jr., Barone FC, Yue TL. Hypercholesterolemia impairs a detoxification mechanism against peroxynitrite and renders the vascular tissue more susceptible to oxidative injury. Circ Res 1997; 80: 894–90. [DOI] [PubMed] [Google Scholar]
- 32.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993; 91: 2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubanyi GM. Vascular effects of oxygen-derived free radicals. Free Radic Biol Med 1988; 4: 107–20. [DOI] [PubMed] [Google Scholar]
- 34.Hennig B, Chow CK. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med 1988; 4: 99–106. [DOI] [PubMed] [Google Scholar]
- 35.Del Boccio G, Lapenna D, Porreca E, Pennelli A, Savini F, Feliciani P, Ricci G, Cuccurullo F. Aortic antioxidant defence mechanisms: time-related changes in cholesterol-fed rabbits. Atherosclerosis 1990; 81: 127–35. [DOI] [PubMed] [Google Scholar]
- 36.Mantha SV, Prasad M, Kalra J, Prasad K. Antioxidant enzymes in hypercholesterolemia and effects of vitamin E in rabbits. Atherosclerosis 1993; 101: 135–44.. [DOI] [PubMed] [Google Scholar]
- 37.Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem 1991; 266: 24398–403. [PubMed] [Google Scholar]
- 38.Hassan HM. Biosynthesis and regulation of superoxide dismutases. Free Radic Biol Med 1988; 5: 377–85. [DOI] [PubMed] [Google Scholar]
- 39.Kim JY, Park HJ, Um SH, Sohn EH, Kim BO, Moon EY, Rhee DK, Pyo S. Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-alpha-stimulated mouse vascular smooth muscle cells: involvement of the MAPK, NF-kappaB and AP-1 signaling pathways. Vasc Pharmacol 2012; 56: 131–41. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol 2008; 8: 115–25. [DOI] [PubMed] [Google Scholar]
- 41.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol 1999; 37: 973–9. [DOI] [PubMed] [Google Scholar]
- 42.Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr 2004; 34–23: 1025–1025. [DOI] [PubMed] [Google Scholar]
- 43.De La Cruz JP, Quintero L, Villalobos MA, Sanchez de la Cuesta F. Lipid peroxidation and glutathione system in hyperlipemic rabbits: influence of olive oil administration. Biochim Biophys Acta 2000; 1485: 36–44. [DOI] [PubMed] [Google Scholar]
- 44.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol 2004; 13: 125–38. [DOI] [PubMed] [Google Scholar]
- 45.Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology 2006; 13: 129–42. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989; 320: 915–24. [DOI] [PubMed] [Google Scholar]
- 47.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 2000; 275: 1263–83. [DOI] [PubMed] [Google Scholar]
- 48.Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem 2004; 279: 30175–81. [DOI] [PubMed] [Google Scholar]
- 49.Kivela AM, Makinen PI, Jyrkkanen HK, Mella-Aho E, Xia Y, Kansanen E, Leinonen H, Verma IM, Yla-Herttuala S, Levonen AL. Sulforaphane inhibits endothelial lipase expression through NF-kappaB in endothelial cells. Atherosclerosis 2010; 213: 122–8. [DOI] [PubMed] [Google Scholar]
- 50.Schrader C, Graeser AC, Huebbe P, Wagner AE, Rimbach G. Allyl isothiocyanate as a potential inducer of paraoxonase-1–studies in cultured hepatocytes and in mice. IUBMB Life 2012; 64: 162–8. [DOI] [PubMed] [Google Scholar]
- 51.Wu BJ, Shrestha S, Ong KL, Johns D, Dunn LL, Hou L, Barter PJ, Rye KA. Increasing HDL levels by inhibiting cholesteryl ester transfer protein activity in rabbits with hindlimb ischemia is associated with increased angiogenesis. Int J Cardiol 2015; 199: 204–12. [DOI] [PubMed] [Google Scholar]
- 52.El-Awady MS, Suddek GM. Agmatine ameliorates atherosclerosis progression and endothelial dysfunction in high cholesterol-fed rabbits. J Pharm Pharmacol 2014; 66: 835–43. [DOI] [PubMed] [Google Scholar]
- 53.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 2001; 103: 2531–4. [DOI] [PubMed] [Google Scholar]
- 54.Thomassen MJ, Meeker DP, Deodhar SD, Wiedemann HP, Barna BP. Activation of human monocytes and alveolar macrophages by a synthetic peptide of C-reactive protein. J Immunother Emphasis Tumor Immunol 1993; 13: 1–6. [DOI] [PubMed] [Google Scholar]
- 55.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000; 20: 2094–9. [DOI] [PubMed] [Google Scholar]
- 56.Liu YC, Hsieh CW, Weng YC, Chuang SH, Hsieh CY, Wung BS. Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-kappaB is dependent upon glutathione depletion in endothelial cells. Vasc Pharmacol 2008; 48: 54–61. [DOI] [PubMed] [Google Scholar]
- 57.Nallasamy P, Si H, Babu PV, Pan D, Fu Y, Brooke EA, Shah H, Zhen W, Zhu H, Liu D, Li Y, Jia Z. Sulforaphane reduces vascular inflammation in mice and prevents TNF-alpha-induced monocyte adhesion to primary endothelial cells through interfering with the NF-kappaB pathway. J Nutr Biochem 2014; 25: 824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem 1995; 270: 933–43. [DOI] [PubMed] [Google Scholar]
- 59.Boyle EM, Jr., Kovacich JC, Canty TG, Jr., Morgan EN, Chi E, Verrier ED, Pohlman TH. Inhibition of nuclear factor-kappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response. Circulation 1998; 98: II282–8. [PubMed] [Google Scholar]
- 60.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem 2008; 283: 26771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner AE, Boesch-Saadatmandi C, Dose J, Schultheiss G, Rimbach G. Anti-inflammatory potential of allyl-isothiocyanate–role of Nrf2, NF-(kappa) B and microRNA-155. J Cell Mol Med 2012; 16: 836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang CS, Lin AH, Liu CT, Tsai CW, Chang IS, Chen HW, Lii CK. Isothiocyanates protect against ox idized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFkappaB activation. Mol Nutr Food Res 2013; 57: 1918–30. [DOI] [PubMed] [Google Scholar]
- 63.Jeremy RW, McCarron H, Sullivan D. Effects of dietary L-arginine on atherosclerosis and endothelium-dependent vasodilatation in the hypercholesterolemic rabbit. Response according to treatment duration, anatomic site, and sex. Circulation 1996; 94: 498–506. [DOI] [PubMed] [Google Scholar]
- 64.Chappell SP, Lewis MJ, Henderson AH. Effect of lipid feeding on endothelium dependent relaxation in rabbit aortic preparations. Cardiovasc Res 1987; 21: 34–8. [DOI] [PubMed] [Google Scholar]
- 65.White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A 1996; 93: 8745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson SH, Simari RD, Best PJ, Peterson TE, Lerman LO, Aviram M, Nath KA, Holmes DR, Jr., Lerman A. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol 2001; 21: 122–8. [DOI] [PubMed] [Google Scholar]
- 67.Pritchard KA, Jr., Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res 1995; 77: 510–8. [DOI] [PubMed] [Google Scholar]
- 68.Thakur NK, Hayashi T, Sumi D, Kano H, Tsunekawa T, Iguchi A. HMG-CoA reductase inhibitor stabilizes rabbit atheroma by increasing basal NO and decreasing superoxide. Am J Physiol Heart Circ Physiol 2001; 281: H75–83. [DOI] [PubMed] [Google Scholar]
- 69.Miller FJ, Jr., Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 1998; 82: 1298–305. [DOI] [PubMed] [Google Scholar]
- 70.Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem 2009; 284: 31579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev 2014; 2014: 123963–123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A 1997; 94: 10367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr 2008; 99: 559–64. [DOI] [PubMed] [Google Scholar]
- 74.Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer 2005; 117: 356–62. [DOI] [PubMed] [Google Scholar]
- 75.Song L, Morrison JJ, Botting NP, Thornalley PJ. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal Biochem 2005; 347: 234–43. [DOI] [PubMed] [Google Scholar]