Abstract
Hypoxia-induced radioresistance has been well known as the main obstacle in cancer radiotherapy. Lysyl oxidase (LOX) was previously demonstrated to play an important role in hypoxia-induced biological behaviors, such as metastasis and angiogenesis, through hypoxia-inducible factor-1α (HIF-1α), which is an important contributing factor to radioresistance in tumor cells. However, how LOX plays a role in hypoxia-induced radioresistance has yet to be determined. Here, we found that LOX expression was in accordance with HIF-1α expression, and LOX expression at the mRNA and protein level, and enzymatic activity were remarkably upregulated in the hypoxic A549 cells, compared with normoxic A549 cells. Inhibition of LOX resulted in the reduction of the ability to repair double-stranded breaks (DSBs), promotion of apoptosis, relief of G2/M cycle arrest, and eventually reduction of hypoxia-induced radioresistance in the hypoxic A549 cells. This suggests that LOX may play an important role in hypoxia-induced radioresistance. Together, our results might suggest a novel potential therapeutic target in the management of non-small cell lung cancer (NSCLC).
Keywords: NSCLC, lysyl oxidase, hypoxia, radiosensitivity
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide, in which non-small cell lung cancer (NSCLC) accounts for 75–80%.1 Most NSCLC patients are diagnosed in the advanced stages of disease due to its insidious symptoms. Therefore, radiotherapy plays a central role in the management of locally advanced NSCLC.2 However, radioresistance exists in many NSCLC patients (intrinsic radioresistance) and patients often fail to respond to treatment when exposed to repeated radiotherapy sessions (induced radioresistance), rendering the overall curative effect unsatisfactory.
Hypoxia is a universal phenomenon in solid tumors such as NSCLC.3,4 Abundant evidence documents that it is a key factor in mediating tumor radioresistance.4,5 Tumor cells are more radioresistant in hypoxic conditions, which may act as potential sources for subsequent tumor metastasis and recurrence after radiotherapy.6–8 Thus, it is of great importance to overcome hypoxia-induced radioresistance.
Lysyl oxidase (LOX) is a copper-dependent amine oxidase which catalyzes cross-linking between collagen and elastin, and stabilizes the extracellular matrix (ECM). Numerous reports have shown that over-expression of LOX is clinically correlated with metastasis and poor outcome in patients with breast cancer, head and neck squamous cancer, prostate cancer and oropharyngeal cancer.9–11 Microarray analysis has demonstrated that LOX expression was significantly elevated in many hypoxic tumor cells and regulated by HIF-1α, an intrinsic marker of tumor hypoxia and linked to poor response to radiotherapy.12 In addition, LOX has been reported to be responsible for the invasive properties of hypoxic breast cancer cells and essential for hypoxia-induced metastasis.10 Meanwhile, HIF-1α is an important contributing factor to hypoxia-mediated radioresistance. For these reasons, we hypothesize that LOX may be associated with hypoxia-induced tumor radioresistance.
In order to determine whether LOX mediates hypoxia-induced radioresistance, we investigated the difference in LOX expression and activity in hypoxic and normoxic conditions, and then down regulated the LOX level by a specific inhibitor and then analyzed the cell’s ability to repair DSBs. Our results may provide an alternative strategy to overcome hypoxia-induced radioresistance in NSCLC.
Materials and methods
Cell culture and treatment
A549 and H460 cells were cultured in RPMI1640 culture medium (Gibco Grand Island, NY) supplemented with 10% fetal bovine serum in a humidified incubator at 37℃ and 5% CO2. As the cells reached the exponential growth phase, they were moved to the respective humidified incubator: hypoxic group, 1% O2 with different β-aminopropionitril (βAPN; Σ-Aldrich, Steinheim, Germany) concentration (0, 50 and 200 μM); normoxic group, 20% O2 for 18 h.
RT-PCR analysis
After being treated in hypoxic or normoxic conditions for 18 h, the RNA of A549 cells were extracted with Trizol. Reverse transcription and PCR amplification were performed using methods described previously.13 The Taqman polymerase chain reaction primer sequences used were: (LOX: forward: 5′-AGATGAGCTTCCTACAGCACAAC-3′; reverse: 5′-CTTTCCTGGTGAGAGATCTGCA-3′) and (GAPDH:5′-forward:ACCACAGTCCATGCCATCAC-3′; reverse: 5′-TCCACCACCCTGTTGCTGTA-3′).
Western blot analysis
After the hypoxic and normoxic groups were treated for 18 h, western blot analysis was performed as previously described.14 Briefly, cells were lysed with cell lysis buffer (RIPA buffer and 1% PMSF) and whole-cell lysates, and the protein concentrations were quantified using BCA reagent (Applygen Technologies, Beijing, China). Protein concentrations were separated in 10% sodium dodecyl sulfate-polyacrylamide gels and electrotransferred to PVDF membranes. The membranes were blocked with blocking buffer (5% bovine serum albumin (BSA) in PBS with 0.1% Tween-20) for 1 h at room temperature (RT), probed with primary antibodies overnight at 4C, incubated with secondary antibodies for 1 h at RT, and developed with the ECL AdvanceTM Western blotting detection kit. The antibodies used were anti-LOX (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), anti-actin (1:10,000, Chemicon, Temecula, CA) and the appropriate horse radish peroxidase-labeled secondary antibody.
LOX activity assays
Extracellular LOX enzymatic activity was measured using the Amplex Red fluorescence method. The fluorescence mix consisted of 10 μM Amplex Red, 10 mM 5-diaminopentane, 1.2 M urea, 50 mM NaBorate, and 0.1 U/mL horse radish peroxidase (HRP). A549 cells were first cultured in a flask at a density of 70%, and the cells were fed with serum-free and phenol red-free DMEM. The cells were then divided into four groups: normoxic and hypoxic+βAPN (0 μM, 50 μM, and 200 μM) groups, and treated for 18 h. Then 250 μl of media was collected and added to 750 μl of fluorescence mix and incubated at 37℃ for 30 min. Subsequently, they were placed on ice and the fluorescence was measured with an excitation and emission wavelength of 563 nm and 587 nm, respectively. Control assays were conducted with 500 μM βAPN to completely inhibit the activity of LOX, and the difference in fluorescence was recorded, which represented the extracellular LOX activity.
Radiation treatment
After incubation in hypoxic or normoxic conditions for 18 h, the A549 and H460 cells were immediately irradiated using a linear accelerator on 0.5 cm compensation. The appropriate output dose was delivered by 6 MV X-rays.
Colony-forming assay
Cells were divided into four groups: normoxia, cells in 20% O2 condition; hypoxia, cells in 1% O2 condition; normoxia+ βAPN (200 μM βAPN and 20% O2); hypoxia+ βAPN (200 μM βAPN and 1% O2). After incubation for 18 h, the cells were irradiated at doses of 2, 4, 6 or 8 Gy, trypsinized, and were aliquot into 6-well plates. All the plates were cultured for 7–14 days in the cell incubator to allow for colony formation. Cells were stained with crystal violet, and colonies containing at least 50 cells were counted. Plating efficiency was calculated as a ratio of the number of formed colonies to the seeding cells without radiation. The surviving fraction at each radiation dose was calculated as number of colonies/(number of seeding cells × plating efficiency). The survival curve was fit into a multi-target single-hit model, and radiobiological parameters were calculated including quasi-threshold dose (Dq), mean lethal dose (D0), surviving fraction at 2 Gy (SF2), and extrapolation number (N).
Flow cytometry analysis of cell cycle
A549 cells were divided into four groups using the above conditions. After the 18-h incubation, the cells were irradiated at a dose of 2 Gy. After 24 h, a single cell suspension was made using 0.25% trypsin. Cells were placed in precooled 70% ethanol at −20℃ for fixation overnight. Cells were then washed in phosphate buffered saline (PBS) and digested with RNA enzyme. Propidium iodide (PI) was added to the cells at a final concentration of 60 µg/mL. Cells were incubated in the dark, and the percentage of cells in G0/G1, S, or G2/M phases was counted and compared by flow cytometry. All experiments were conducted in triplicate.
Flow cytometry analysis of apoptosis
Cells were divided into four groups as before. After an 18 h-incubation period, the cells were irradiated at doses of 2 Gy. After 24 h, a single cell suspension was made using 0.25% trypsin without EDTA. Cell suspension were washed with PBS twice and resuspended in 250 µL binding buffer at a density of 5 × 106 cells/mL. The cell suspension was analyzed by flow cytometry after they were stained with 5 µL Annexin V-FITC and 5 µL PI solution (20 µg/mL).
Immunocytofluorescence
Cells were divided into four groups as previously mentioned. After an 18-h incubation period, the cells were irradiated at a dose of 2 Gy. The cells were fixed with 4% paraformaldehyde for 30 min, 2 h, 6 h, and 12 h. They were then treated with 1% Triton X-100 at RT for 5 min. After blocking with 2% BSA (in PBS) for 30 min at RT, the cells were stained with FITC-conjugated anti-γ-H2AX for 12 h at 4℃. After washing, they were incubated with the secondary antibody for 1 h at RT. After another washing process, the cells were treated with hoechst for 10 min, and slides were mounted using 5% glycerol. Cell morphology was examined under a laser confocal microscope (Olympus optical Co., Tokyo, Honshu, Japan).
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde in 0.2 M phosphate buffer (pH 7.4) for 48 h, followed by embedding into paraffin sections. The 4-mm thick tissue sections were prepared and further subjected to LOX and HIF-1α staining. The endogenous peroxidase activity was inactivated in a solution containing 3% hydrogen peroxide (H2O2) in methanol. Subsequently, the sections were blocked for 2 h at RT with 1.5% blocking serum. The sections were incubated with anti-HIF-1α (1:200, Abcam, Cambridge, MA) and anti-LOX antibody (1:500, Abcam) overnight at 4℃. Labelled horseradish peroxidase was applied for 30 min at RT, followed by application of diaminobenzidine solution until color developed. The slides were counterstained with haematoxylin. Negative control slides were performed without primary antibody. The staining results for HIF-1α protein were classified as follows: 0, no staining; 1, nuclear staining in <1% of cells; 2, nuclear staining in 1–10% of cells and/or weak cytoplasmic staining; 3, nuclear staining in >10% of cells and/or distinct or strong cytoplasmic staining. For LOX protein, the staining was evaluated with the same criteria. Samples graded as 0 and 1 were considered negative, and those graded as 2 and 3 were considered positive.15
Statistical analysis
All the data came from at least three independent experiments and were shown in the form of mean±standard error mean (SEM). Statistical comparisons were made using Student’s t test, in which P < 0.05 was considered as statistically significant.
Results
LOX expression correlated with HIF-1α in NSCLC patients
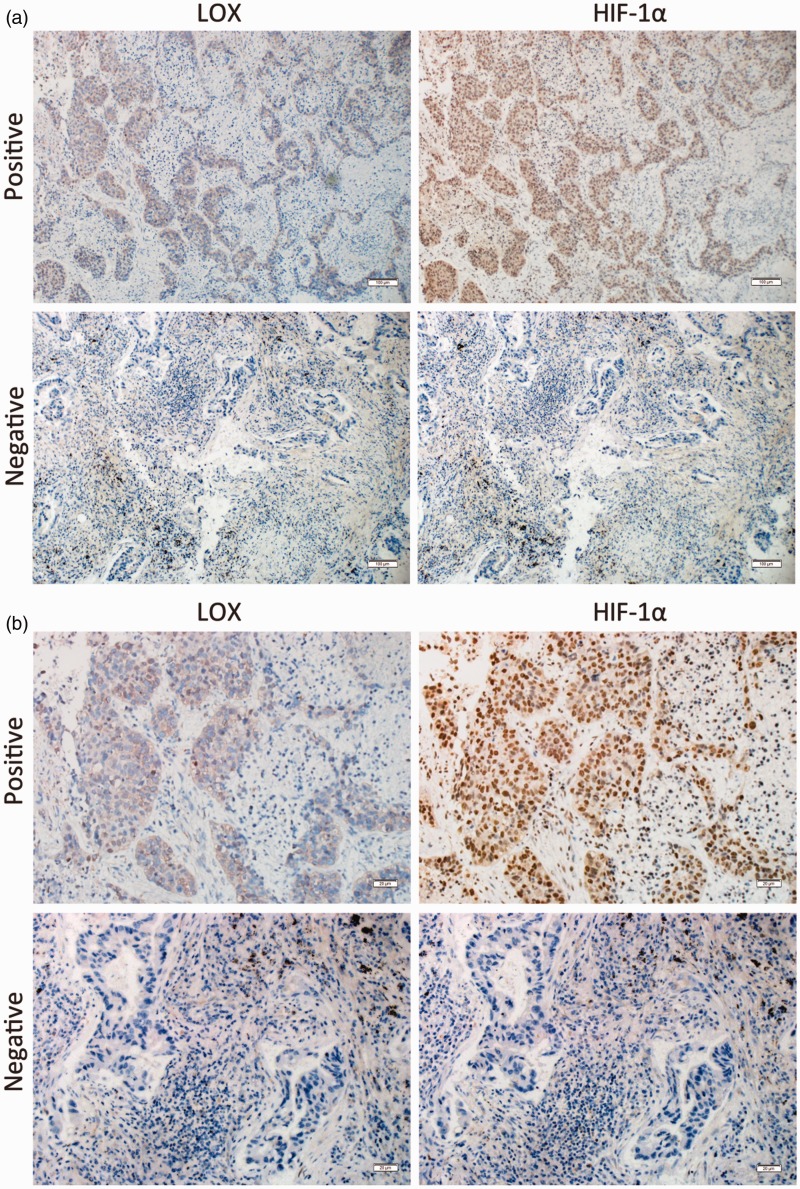
To ascertain the relationship between LOX expression and hypoxia in NSCLC, LOX and HIF-1α expression were analyzed in 20 NSCLC specimens in continuous sections. As shown in Figure 1, for most NSCLC patients, LOX expression was in accordance with HIF-1α expression. Cross table test confirmed that the expression of LOX is highly correlated with HIF-1α expression (Table 1).
Figure 1.
LOX was correlated with HIF-1α expression in patients with NSCLC. Continuous section of NSCLC was obtained and subjected to anti-LOX and anti-HIF-1α antibodies. (a) Representative micrographs for immunohistochemical staining of HIF-1α (right) and LOX (left) were shown (Magnification: 100×). (b) Details of immunohistochemical staining were shown (Magnification: 400×). (A color version of this figure is available in the online journal.)
Table 1.
The correlation between the expression of LOX and HIF-1α in NSCLC tumor tissues
| LOX expression |
χ2 | P value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| HIF-1α expression | 4.615 | 0.032 | ||
| Positive | 7 | 7 | ||
| Negative | 0 | 6 | ||
Hypoxia induces up-regulation and increases enzymatic activity of LOX in A549 cells
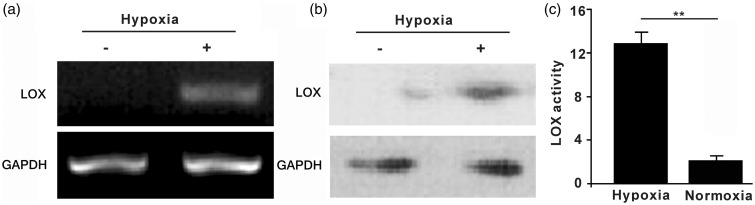
To probe the role of LOX in hypoxia-induced radioresistance, we investigated LOX expression in A549 cells in response to hypoxic conditions. As shown in Figure 2(a), RT-PCR determined that LOX mRNA expression was upregulated in hypoxic A549 cells, compared with the normoxic group. Consistent with the mRNA expression level, LOX protein expression also increased after incubation in the hypoxic conditions (Figure 2b). In addition to mRNA and protein levels, LOX enzyme activity was determined using the Amplex Red assay. As shown in Figure 2(c), LOX activity was significantly increased in hypoxic conditions. The above data indicate that hypoxia resulted in upregulation and enhanced activity of LOX in A549 cells.
Figure 2.
Hypoxia induced upregulation of LOX expression and increased enzymatic activity in A549 cells. (a) A549 cells were cultured in either hypoxic conditions at 1% O2 or normoxic condition at 20% O2 for 18 h. LOX mRNA expression level was determined by RT-PCR and quantitative real-time PCR. (b) Determination of LOX protein level in response to various O2 condition using western blots; (c) Detection of LOX enzymatic activity by the Amplex Red fluorescence method. Data were from three independent experiments and were presented as mean ± SEM. *p < 0.05, **p < 0.01
LOX mediates hypoxia-induced radioresistance in A549 cells
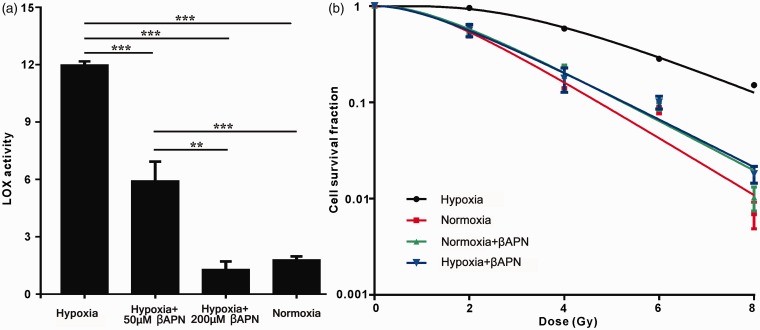
Since hypoxia-induced radioresistance is commonly present in various types of cancer, we next investigated whether this process is mediated via LOX. In this setting, A549 cells were treated with different concentrations of βAPN (an irreversible inhibitor of LOX enzymatic activity) in hypoxic conditions and LOX enzymatic activity was determined. Compared with the hypoxia control, the addition of 50 μM βAPN resulted in a decreased activity of LOX (Figure 3a). When 200 μM βAPN was added, the LOX activity returned to the normoxia level. Thus, we selected the βAPN concentration of 200 μM for use in the following studies. Next, we tested the radiosensitivity of the cells treated with or without βAPN under different conditions (normoxia or hypoxia) using a clonogenic assay. The radiosensitive parameters were calculated (Table 2). We found that the values of D0, Dq, N and SF2 decreased in the hypoxia group compared with the normoxic group, indicating that the radiosensitivity of hypoxic A549 cells was reduced. Interestingly, inhibition of LOX by βAPN abrogated this reduction. The sensitization enhancement ratio (SER) of hypoxic A549 cells were 1.76 (normoxia), 1 (hypoxia), 1.65 (hypoxia + βAPN) and 1.70 (normoxia + βAPN). As shown in Figure 3(b), hypoxia increased survival of A549 cells, further confirming that hypoxia induces radioresistance in A549 cells. Notably, inhibition of LOX reduced hypoxia-induced radioresistance, suggesting that the resulting hypoxia-induced radioresistance was mediated by LOX activity. Moreover, inhibition of LOX did not affect the cell survival rate in normoxic conditions, indicating that only in hypoxia-induced radioresistance does LOX mediate radiosensitivity but not in normoxic conditions. In addition, we further strengthened the above results in another NSCLC cell line H460 cells and obtained similar results (Figure S1 and Table S1, Supplementary material). The sensitization enhancement ratio (SER) of hypoxic H460 cells were 1.49 (normoxia), 1 (hypoxia), 1.48 (hypoxia + βAPN), and 1.48 (normoxia+ βAPN).
Figure 3.
LOX mediates the radioresistance of hypoxic A549 cells. (a) A549 cells were cultured in either hypoxic conditions at 1% O2 or normoxic conditions with or without βAPN. LOX enzymatic activity was determined by the Amplex Red fluorescence method; (b) Radiation survival curves for A549 cells. Cells were cultured in hypoxic or normoxic conditions for 18 h before radiation at doses of 2, 4, 6 or 8 Gy. 200 μM βAPN was added for the inhibition of LOX enzymatic activity as indicated. Data were from three independent experiments and are presented as mean ± SEM. *p < 0.05, **p < 0.01. (A color version of this figure is available in the online journal.)
Table 2.
The radiosensitive parameters of A549 cells with or without the treatment of βAPN in different conditions
| Parameter | Hypoxic group | Normoxic group | βAPN group | βAPN+hypoxia group |
|---|---|---|---|---|
| D0 | 2.18 | 1.46 | 1.67 | 1.74 |
| Dq | 9.23 | 2.37 | 2.28 | 1.95 |
| SF2 | 0.95 | 0.54 | 0.58 | 0.56 |
| N | 5.24 | 2.62 | 2.37 | 2.12 |
| SER | 1 | 1.76 | 1.65 | 1.70 |
D0, mean lethal dose; Dq, quasi-threshold dose; SF2, surviving fracion at 2 Gy; N, extrapolation number; SER, sensitization enhancement ratio.
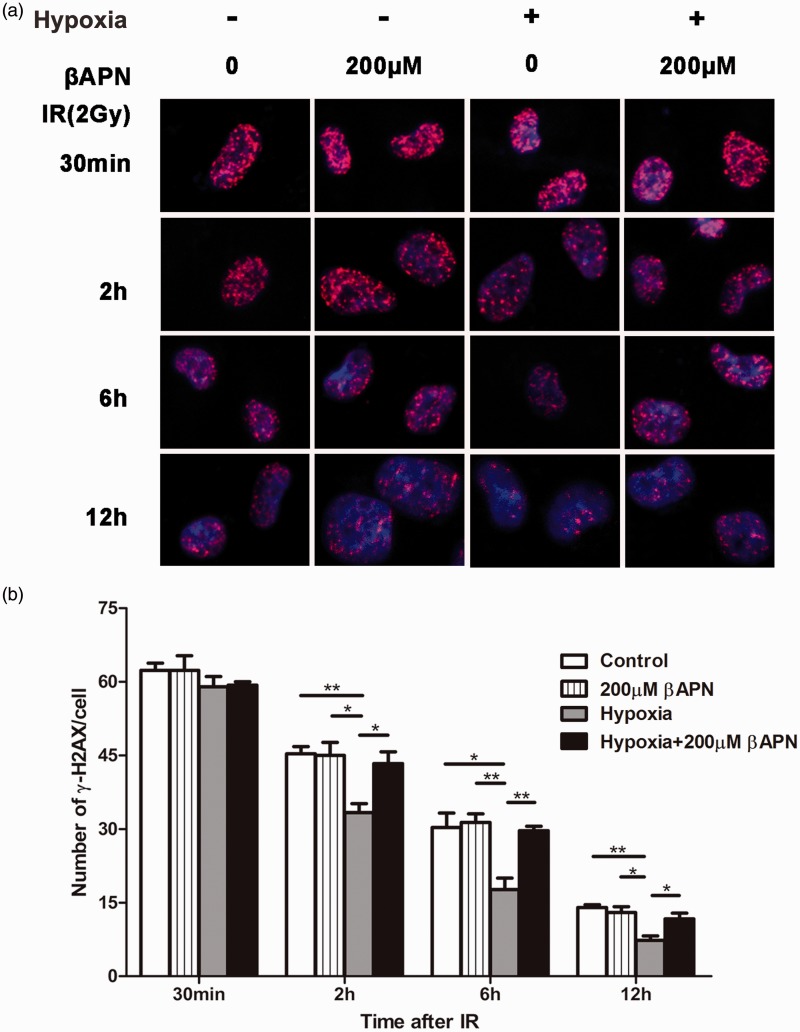
Inhibition of LOX reduces DNA repair activity in hypoxic A549 cells
To further confirm the above results, we then studied the effect of βAPN on radiation-induced DNA repair. Following 2 Gy irradiation, we tracked the number of γ-H2AX foci (an indicator of DNA DSBs) under various conditions during a period of 12 h. As shown in Figure 4 and Figure S2, the number of γ-H2AX foci per nucleus gradually decreased over time. At 12 h, the residual level of γ-H2AX foci per nucleus in the hypoxia group was significantly lower than that of the LOX inhibitor (hypoxia+200 μM βAPN) group. Moreover, LOX inhibitor did not affect the DNA repair capability in normoxic conditions. These results suggest that hypoxia can enhance the repair capability of lung cancer cells and inhibition of LOX can significantly impair DNA damage repair, thus alleviating the hypoxia-induced radioresistance.
Figure 4.
Inhibition of LOX reduced DNA repair in hypoxic A549 cells. (a) A549 cells were cultured in hypoxic or normoxic conditions with or without 200 μM βAPN as indicated. Representative images of nuclei (blue) and γ-H2AX (red) complexes are shown. (b) Quantification of the H2AX foci per nucleus from at least three independent measurements are presented as mean ± SEM.*p < 0.05; **p < 0.01. (A color version of this figure is available in the online journal.)
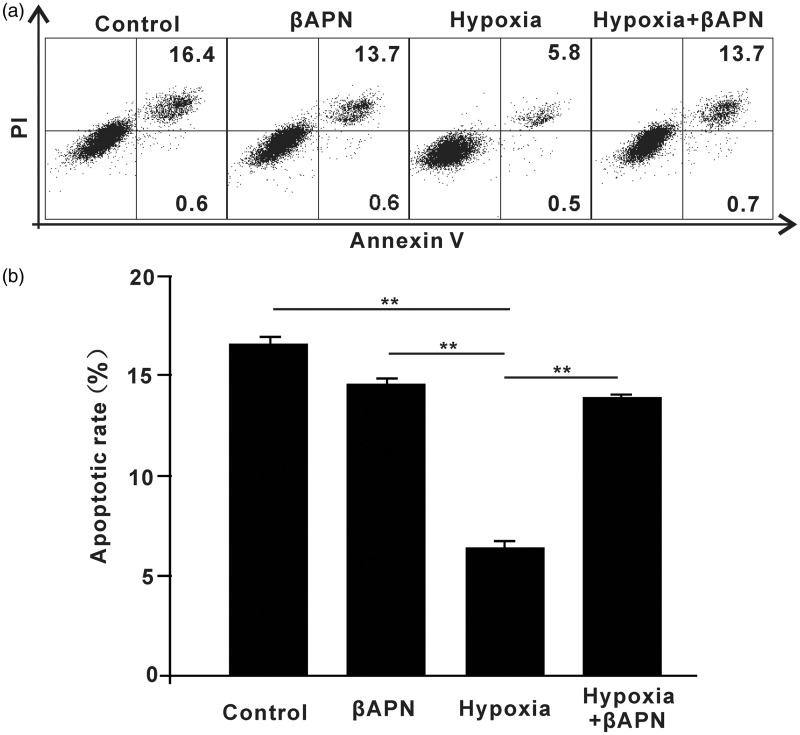
Inhibition of LOX promotes radiation-induced apoptosis
Next, we determined the role of LOX in radiation-induced apoptosis. A549 cells were cultured in hypoxic or normoxic conditions with or without βAPN, and then subjected to irradiation as indicated. As shown in Figure 5(a) and (b), hypoxic conditions decreased apoptosis of A549 cells and inhibition of LOX further promoted radiation-induced apoptosis. This suggests that LOX mediates hypoxia-induced radioresistance in A549 cells. In addition, we performed these studies in H460 cells. As expected, βAPN promoted the radiation-induced apoptosis in H460 cells (Figure S3).
Figure 5.
Inhibition of LOX promoted radiation-induced apoptosis. A549 cells were cultured in hypoxic or normoxic conditions with or without βAPN. A549 cells were then subjected to irradiation at a dose of 2 Gy. Apoptotic status was analyzed by flow cytometry at 24 h. *p < 0.05; **p < 0.01
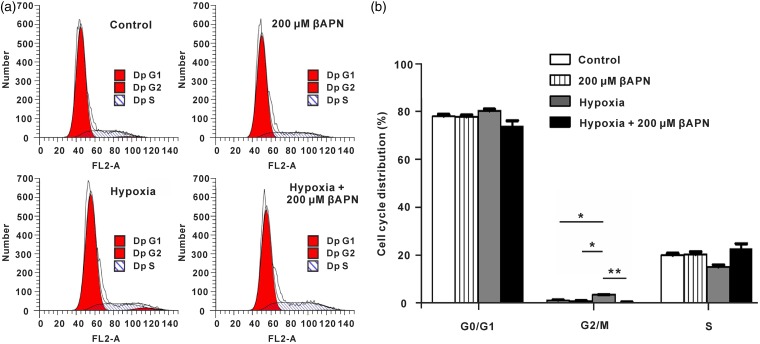
LOX regulates A549 cell cycle in hypoxic conditions
After demonstrating the role of LOX in mediating hypoxia-induced radioresistance, we further determined the effects of LOX in regulation of the cell cycle. As shown in Figure 6(a) and (b) and Figure S4, hypoxia significantly increased the frequency of the G2/M phase in A549 and H460 cells, while inhibition of LOX relieved hypoxia induced-G2/M phase arrest, indicating that LOX regulates A549 and H460 cell cycle in hypoxic conditions.
Figure 6.
LOX mediates G2/M phase in A549 cells. A549 cells were cultured in hypoxic or normoxic conditions with or without βAPN for 18 h. A549 cells were then subjected to irradiation at a dose of 2 Gy. Cell cycle analysis was performed by flow cytometry at 24 h. *p < 0.05; **p < 0.01. (A color version of this figure is available in the online journal.)
Discussion
LOX catalyzes cross-linking of collagen and elastin in the ECM. This reaction plays an important role in maintaining the ECM stability, embryo development and tissue remodeling.16 Studies suggested that LOX could promote cell progression and tumor metastasis.17–19 Erler et al. reported that LOX with enzymatic activity could enhance cell invasion in human breast cancer MDA-231 cells.9 Over-expression of the LOX protein was correlated with tumor size, lymph node involvement, and a poorer progression-free survival (PFS) in NSCLC.20 Furthermore, recent research has shown that LOX is a hypoxia-responsive gene and regulated by HIF-1,21 which is an important contributing factor to hypoxia-induced biological behaviors in tumor cells.22 Prospective head and neck cancer studies revealed that the LOX expression level was significantly correlated with hypoxia in patients.9 Moreover, LOX expression was responsive to hypoxia in pulmonary artery smooth muscle cells, and in some cases over expression of LOX resulted in vascular remodeling and even pulmonary arterial hypertension.20 Consistent with previous reports, our results showed that LOX expression in patients is correlated with HIF-1α expression. Moreover, our findings revealed that after exposure to hypoxic conditions, cellular LOX mRNA expression and enzymatic activity were significantly higher than that of the normoxic group in A549 cells, indicating that hypoxia may induce LOX expression via HIF-1α.
Hypoxia is the key factor influencing radiation sensitivity. In light of the fact that expression of LOX is closely related to hypoxia and ionizing radiation itself could induce LOX secretion in tumor cells,23 we sought to determine the role of LOX in hypoxia-induced radioresistance. For this purpose, we treated hypoxic A549 cells with βAPN, and found that inhibition of LOX reduced hypoxia-induced radioresistance but not in normoxic conditions. Furthermore, these results were further confirmed in H460 cells. This proved our initial hypothesis that LOX with enzymatic function mediated hypoxia induced-radioresistance in NSCLC cells.
We further investigated the underlying mechanisms. As we know, DNA DSBs is the main cause of cell death by ionizing radiation. Ionizing radiation can cascade γH2AX phosphorylation, recruit repair proteins, form DSB repair complexes, and finally repair the DNA DSBs. Numerous researchers have demonstrated that hypoxia can promote DNA DSB repair, leading to attenuation of radiosensitivity.22 The γH2AX assay used in our study revealed a significant decrease in DNA DSB level in drug-free A549 cells exposed to hypoxia compared with the normoxia control group. However, this level in hypoxic A549 cells was significantly increased in the presence of LOX inhibition. We obtained similar results in another NSCLC cell line H460 cells. These results indicate that inhibition of LOX can reduce cell radiosensitivity by impairing DNA repair activity in hypoxic NSCLC cells. Of note, a recent study pointed out that recombinant lysyl oxidase propeptide (rLOX-PP), which is derived from pro-lysyl oxidase (Pro-LOX) but does not possess the lysyl oxidase enzyme activity,24 could sensitize prostate cancer cells to ionizing radiation.25 This suggests a complicated role for LOX in enhancing cell radiosensitivity, and LOX-induced radioresistance might be associated with the enzymatic activity of LOX.
A further major determinant of radiosensitivity is cell cycle arrest, which provides more time for the induction and repair of DNA DSBs, avoiding fatal damage caused by ionizing radiation. Hypoxia is a type of unique stress that induces replication arrest in the absence of DNA damage.26 Our results showed that hypoxia significantly increased the frequency of the G2/M phase in A549 and H460 cells, while inhibition of LOX relieved hypoxia induced-G2/M phase arrest. In accordance with our data, Goto et al. reported that high cell density enhances cell cycle arrest and matrix remodeling, and this phenomenon may be due to HIF-1α-induced LOX expression.27 In addition, we determined the role of LOX in radiation-induced apoptosis. We found that inhibition of LOX promoted radiation-induced apoptosis in hypoxic conditions. Newly published research demonstrated that over-expression of Lysyl oxidase-like 2 (LOXL2), a member of the lysyl oxidase gene family, could promote cell growth by interfering with MARCKSL1-induced apoptosis.28 The effect of LOXL2 on apoptosis-related components may also support our results. Notably, we also observed a slight decrease in apoptotic rate under normoxic condition when applying βAPN, although there was no significant difference between these two groups. Lövey et al.29 also reported that inhibition of LOX attenuated radioresistance upon PCa cells under hypoxic conditions but not in normoxic condition.
Some reports indicated that mature LOX catalyzes substrates and produce hydrogen peroxide which activate focal-adhesion kinase (FAK) and Src.19,30 Hehlgans et al. found that human head and neck squamous cell carcinoma cells exhibited enhanced radiosensitivity when treated with FAK inhibitor TAE226.31 Other findings indicated that the effects of LOX may be mediated via the PI3K/AKT signaling pathway.32 Kucharzewska et al. reported that hypoxia/LOX signaling activates PI3K/Akt pathway.33 As PI3k/Akt pathway per se is oncogenic, activation of PI3k/Akt leads to DSB and genomic instability,33,34 and it may also participate in angiogenesis, cell cycle arrest, and resistance to radiation-induced apoptosis.34,35 In this regard, it might not be surprised to speculate that PI3k/Akt be responsible for the LOX-induced radioresistance. The PI3K/AKT signaling pathway was also reported to be involved in the regulation of tumor radiosensitivity.36 Despite these findings, the molecular pathway of LOX-induced radioresistance might need deeper investigation.
In conclusion, here we show that LOX mediates hypoxia-induced radioresistance. It may act by promoting hypoxia-induced G2/M cycle arrest and DNA DSBs repair, as well as reducing apoptosis. Thus, our findings may provide a potential therapeutic strategy as an adjuvant to the radiotherapy of NSCLC.
Acknowledgement
This work was supported by a grant of the Hubei Provincial Natural Science Foundation (grant no. 2014CFB998).
Authors’ contributions
RG and JC conceived the project, interpreted the data and wrote the manuscript. RG and GW planned the experiments. CG carried out cell cycle analysis experiments, performed supplementary experiments, and helped to complete the final manuscript. HJ provided valuable suggestions on the first manuscript and on details of experiments, and helped to revise the manuscript. YS and ZL carried out western blot analysis experiments.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Chang JY, Kestin LL, Barriger RB, Chetty IJ, Ginsburg ME, Kumar S, Loo BW, Movsas B, Rimner A, Rosenzweig KE, Stinchcombe TE, Videtic GM, Willers H. ACR Appropriateness Criteria(R) nonsurgical treatment for locally advanced non-small-cell lung cancer: good performance status/definitive intent. Oncology (Williston Park) 2014; 28: 706–10, 12, 14.. [PubMed] [Google Scholar]
- 3.Wouters BG, Weppler SA, Koritzinsky M, Landuyt W, Nuyts S, Theys J, Chiu RK, Lambin P. Hypoxia as a target for combined modality treatments. Eur J Cancer 2002; 38: 240–57. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM. The hypoxic cell: A target for selective cancer therapy – eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 1999; 59: 5863–70. [PubMed] [Google Scholar]
- 5.Kondo A, Safaei R, Mishima M, Niedner H, Lin X, Howell SB. Hypoxia-induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res 2001; 61: 7603–7. [PubMed] [Google Scholar]
- 6.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91. [DOI] [PubMed] [Google Scholar]
- 7.Wouters BG, Koritzinsky M, Chiu RK, Theys J, Buijsen J, Lambin P. Modulation of cell death in the tumor microenvironment. Semin Radiat Oncol 2003; 13: 31–41. [DOI] [PubMed] [Google Scholar]
- 8.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol 1996; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 9.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006; 440: 1222–6. [DOI] [PubMed] [Google Scholar]
- 10.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 2004; 101: 811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albinger-Hegyi A, Stoeckli SJ, Schmid S, Storz M, Iotzova G, Probst-Hensch NM, Rehrauer H, Tinguely M, Moch H, Hegyi I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC). Int J Cancer 2010; 126: 2653–62. [DOI] [PubMed] [Google Scholar]
- 12.Vordermark D, Brown JM. Endogenous markers of tumor hypoxia predictors of clinical radiation resistance? Strahlenther Onkol 2003; 179: 801–11. [DOI] [PubMed] [Google Scholar]
- 13.Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res 2002; 62: 4478–83. [PubMed] [Google Scholar]
- 14.Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, Csiszar K, Hendrix MJ, Kirschmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res 2005; 65: 11429–36. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep 2009; 21: 707–12. [PubMed] [Google Scholar]
- 16.Thomassin L, Werneck CC, Broekelmann TJ, Gleyzal C, Hornstra IK, Mecham RP, Sommer P. The Pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J Biol Chem 2005; 280: 42848–55. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Song XR, Sun JJ, Wang XW, Xie L, Lv LY. Lysyl oxidase may play a critical role in hypoxia-induced NSCLC cells invasion and migration. Cancer Biother Radiopharm 2012; 27: 672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol 2001; 70: 1–32. [DOI] [PubMed] [Google Scholar]
- 19.Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene 2009; 28: 3390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ping W, Jiang WY, Chen WS, Sun W, Fu XN. Expression and significance of hypoxia inducible factor-1alpha and lysyl oxidase in non-small cell lung cancer. Asian Pac J Cancer Prev 2013; 14: 3613–8. [DOI] [PubMed] [Google Scholar]
- 21.Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene 2003; 22: 5907–14. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. [DOI] [PubMed] [Google Scholar]
- 23.Shen CJ, Sharma A, Vuong DV, Erler JT, Pruschy M, Broggini-Tenzer A. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC Cancer 2014; 14: 532–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vora SR, Guo Y, Stephens DN, Salih E, Vu ED, Kirsch KH, Sonenshein GE, Trackman PC. Characterization of recombinant lysyl oxidase propeptide. Biochemistry 2010; 49: 2962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bais MV, Ozdener GB, Sonenshein GE, Trackman PC. Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways. Oncogene 2015; 34: 1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinive P, Defresne F, Bouzin C, Saliez J, Lair F, Gregoire V, Michiels C, Dessy C, Feron O. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: Implications for anticancer therapies. Cancer Res 2006; 66: 11736–44. [DOI] [PubMed] [Google Scholar]
- 27.Goto TM, Arima Y, Nagano O, Saya H. Lysyl oxidase is induced by cell density-mediated cell cycle suppression via RB-E2F1-HIF-1alpha axis. Cell Struct Funct 2013; 38: 9–14. [DOI] [PubMed] [Google Scholar]
- 28.Kim BR, Dong SM, Seo SH, Lee JH, Lee JM, Lee SH, Rho SB. Lysyl oxidase-like 2 (LOXL2) controls tumor-associated cell proliferation through the interaction with MARCKSL1. Cell Signal 2014; 26: 1765–73. [DOI] [PubMed] [Google Scholar]
- 29.Lövey J1, Nie D, Tóvári J, Kenessey I, Tímár J, Kandouz M, Honn KV. Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett 2013; 335: 495–501. [DOI] [PubMed] [Google Scholar]
- 30.Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem 2008; 103: 1369–78. [DOI] [PubMed] [Google Scholar]
- 31.Beinke C, Van Beuningen D, Cordes N. Ionizing radiation modules of the expression and tyrosine phosphorylation of the focal adhesion-associated proteins focal adhesion kinase (FAK) and its substrates p130cas and paxillin in A549 human lung carcinoma cells in vitro. Int J Radiat Biol 2003; 79: 721–31. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Wang B, Xu Y. Expression of lysyl oxidase in human osteosarcoma and its clinical significance: a tumor suppressive role of LOX in human osteosarcoma cells. Int J Oncol 2013; 43: 1578–86. [DOI] [PubMed] [Google Scholar]
- 33.Kucharzewska P1, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status ofglioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 2013; 110: 7312–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanos D. Halazonetis, Vassilis Gorgoulis, Jiri Bartek. An oncogene-induced DNA damage model for cancer development. Science 2008; 319: 1352–55. [DOI] [PubMed] [Google Scholar]
- 35.Ding Miao, Zhang Erlong, He Rong, Wang Xingyong. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci 2013; 104: 1401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol 2009; 4: 43–43. [DOI] [PMC free article] [PubMed] [Google Scholar]