Abstract
Stress is an important condition of modern life. The successful wound healing requires the execution of three major overlapping phases: inflammation, proliferation, and remodeling, and stress can disturb this process. Chronic stress impairs wound healing through the activation of the hypothalamic–pituitary–adrenal axis, and the glucocorticoids (GCs) hormones have been shown to delay wound closure. Therefore, the aim of this study was to investigate the effects of a GC receptor antagonist (RU486) treatment on cutaneous healing in chronically stressed mice. Male mice were submitted to rotational stress, whereas control animals were not subjected to stress. Stressed and control animals were treated with RU486. A full-thickness excisional lesion was generated, and seven days later, lesions were recovered. The RU486 treatment improves wound healing since contraction takes place earlier in RU486-treated in comparison to non-treated mice, and the RU486 treatment also improves the angiogenesis in Stress+RU486 mice when compared to stressed animals. The Stress+RU486 group showed a decrease in inflammatory cell infiltration and in hypoxia-inducible factor-1α and inducible nitric oxide synthase expression; meanwhile, there was an increase in myofibroblasts quantity. In conclusion, blockade of GC receptors with RU486 partially ameliorates stress-impaired wound healing, suggesting that stress inhibits healing through more than one functional pathway.
Keywords: Chronic stress, glucocorticoids, wound healing, antagonist treatment, RU486
Introduction
Wound healing requires the timely orchestration and efficient execution of three major overlapping phases: inflammation, proliferation, and remodeling.1 Many factors, including stress, can disturb this process. Stress impairs the course of the cutaneous healing2–4 through the activation of the hypothalamic–pituitary–adrenal axis (HPA) and consequent secretion of glucocorticoids (GCs) and catecholamines.5 A deregulation of GCs secretion provides one neuroendocrine pathway through which stress alters wound healing,6,7 and GCs have been implicated in the impairment of wound healing in restraint-stressed mice.8
After injury, neutrophils and macrophages leave the peripheral blood to reach the wound site, where the appropriate functions of these cells are crucial to evolution of the healing process. A wide range of molecules are important to regulate and coordinate the inflammatory phase during healing. Hypoxia-inducible factor-1α (HIF-1α) is expressed in skin wounds,9,10 as a mediator of cell response to inflammation11 and nitric oxide (NO) is important to HIF-1α stabilization and function.12 In proliferative and remodeling phases, fibroblasts proliferate and migrate to deposit a collagen-rich matrix in the lesion area. A proportion of fibroblasts differentiate into myofibroblasts, which are responsible for wound contraction.13 Thus, the wound-healing process is affected when stress modulates neutrophil and macrophage recruitment and function14,15 and also dermal fibroblast and myofibroblast activities.16 Nonetheless, how GCs influences the stress response is not completely understood. Therefore, the aim of this study was to investigate the effects of a GC receptor blockade on healing of cutaneous lesions in stressed mice.
Methods
Animals, wounding, and macroscopic analyses
This study was approved by the Ethical Committee for Animal Use of UERJ (CEA/004/2010). Male Swiss mice (25–35 g) were maintained in a room at 22℃ and with 12-h light/dark cycle. A set of animals was chronically submitted to stress.16,17 Briefly, rotational stress was applied daily by spinning the cages at 115 rpm for 15 min every hour, beginning three days before wounding until euthanasia. The GC receptor antagonist treatment was administered every day via intraperitoneal (IP) injection of RU486 (20 mg/kg; mifepristone, Sigma-Aldrich, St. Louis, MO) in β-cyclodextrin (Sigma-Aldrich); control groups received only β-cyclodextrin. The IP injection began one day before wounding and continued until euthanasia. One group of mice was only chronically stressed (Stress; n = 20), whereas another group was stressed and received the RU486 treatment (Stress + RU486; n = 20). One group was not submitted to any stress or RU486 treatment (Control; n = 20), and another group of control mice received only the RU486 treatment (RU486; n = 20). Three days after beginning the stress protocol, mice were anesthetized IP with ketamine (150 mg/kg) and xylazine (15 mg/kg), and a full-thickness excisional wound (1 cm2) was created.
To evaluate wound contraction, immediately after wounding and four, seven, and 14 days later, the lesion’s margins were traced on a transparent plastic sheet placed over the wound; at the same time points, wounds were inspected for re-epithelialization. The wound area was measured using ImageJ software (NIH, Bethesda, MD).
Seven days after wounding, mice were anesthetized and euthanized. Serum samples were frozen at −20℃, half of the lesions were frozen at −70℃, and the other half, along with the adjacent skin, were formalin-fixed and paraffin-embedded.
Biochemical, microscopic, and molecular analyses
To confirm the presence of stress-induced physiological alterations, norepinephrine levels were indirectly estimated by measuring plasmatic normetanephrine levels to demonstrate the overproduction of catecholamines.16,18
The quantity of myofibroblasts was evaluated with mouse anti-alpha-smooth muscle actin (DAKO, Carpinteria, CA). To identify F4/80-positive cells and neutrophils, the sections were immunolabeled with mouse anti-F4/80 (Serotec Inc., Raleigh, UK) and neutrophil marker (Santa Cruz Biotechnology, Santa Cruz, CA), respectively. The primary antibodies were detected using the EnVision System (DAKO), and diaminobenzidine was used as chromogen. No labeling was observed when the primary antibody was omitted. To quantify myofibroblasts, F4/80-positive cells, and neutrophils, and also to quantify the blood vessels—in hematoxylin–eosin stained sections, 10 random fields per animal (15,000 µm2) were analyzed in the granulation tissue using a 40× objective lens. The results were expressed as the average number of positive cells/mm2 or blood vessels/mm2. All quantifications were performed blindly.
The frozen wound fragments were homogenized, and total protein concentration was determined using bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Proteins (20 µg) were resolved by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred to polyvinyl difluoride membranes. Membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich) and probed with anti-HIF-1α, anti-inducible nitric oxide synthase (NOS2), and anti-β-actin antibodies (Santa Cruz Biotechnology) followed by biotin-conjugated antibody and streptavidin-conjugated horseradish peroxidase developed by chemiluminescence (Santa Cruz Biotechnology). Densitometry analysis was performed using ImageJ, and band intensities were normalized to the corresponding band intensities of β-actin (arbitrary units ratio).
Statistical analysis
For comparison between groups, data (mean ± standard error of the mean) were analyzed by using one-way analysis of variance with Newman–Keuls or Kruskal–Wallis with Dunns in GraphPad Prism software (GraphPad Prism 5.0, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
To confirm the stress-induced physiological alterations, plasmatic normetanephrine levels were estimated to demonstrate the overproduction of stress hormones. In Stress and Stress + RU486 groups, the normetanephrine levels were higher than in the non-stressed mice both seven (Control: 12.76 ± 0.97 ng/µl; RU486: 16.41 ± 1.34 ng/µl; Stress: 24.76 ± 1.86 ng/µl; Stress + RU486: 24.54 ± 1.17 ng/µl; p < 0.0001) and 14 days after wounding (data not shown). The normetanephrine levels were similar in Stress and Stress + RU486 groups, indicating that RU486 treatment has no effect over catecholamines secretion.
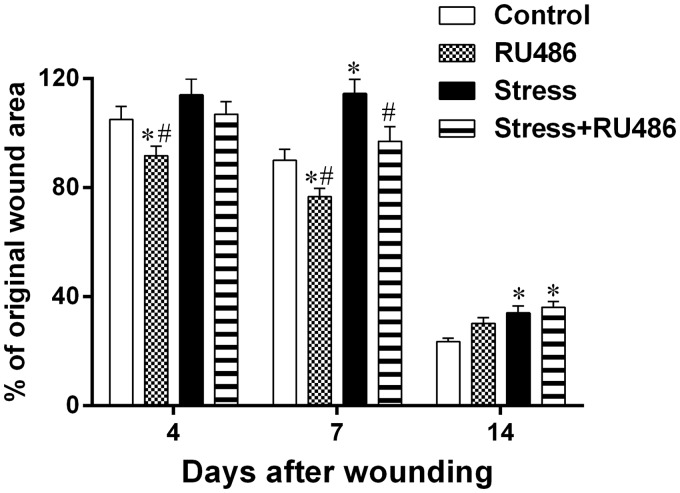
The treatment with RU486 initially ameliorates the wound contraction. Seven days after wounding, the wound area of the Stress + RU486 group was smaller than the wound area of the Stress group. Moreover, 14 days after wounding, the wound areas of both Stress and Stress + RU486 groups were larger than the wound area of the Control group and similar to each other (Figure 1). During wound contraction, re-epithelialization also took place earlier in RU486-treated groups. Eleven days after wounding, more wounds were re-epithelialized in the RU486-treated groups than in their respective control groups (Control: 22.2%; RU486: 61.1%; Stress: 30%; Stress + RU486: 43.75%; p < 0.0001). Fourteen days after wounding, the percentage of re-epithelialization was similar between all of the groups (data not shown). Thus, GC receptor blockade treatment is important to the early response in the wound-healing process.
Figure 1.
Analysis of wound contraction. Three days after beginning of the stress protocol, a full-thickness excisional lesion (1 cm2) was generated in the dorsal skin. The wound area was measured immediately after wounding (d0) and seven (d7) and 14 (d14) days later. Wound contraction was estimated based on lesion area at d7 and d14 in relation to lesion area at d0 (% of original wound area). Data are expressed as mean ± SEM (*vs. Control group; #vs. Stress group)
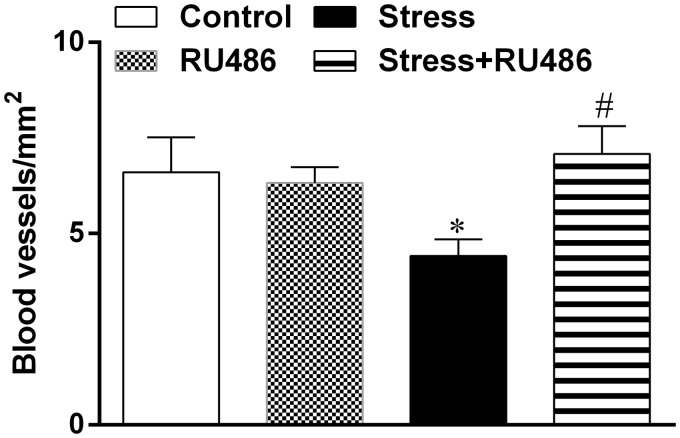
Once it was demonstrated that GCs have potent anti-inflammatory properties,7 we evaluated the influence of RU486 on early inflammatory stage of wound healing. Seven days after wounding, it was observed an infiltration of inflammatory cells on wounds of all mice. Angiogenesis was intense and similar between control groups, but the Stress group showed a decrease in blood vessels number that was reversed by RU486 treatment (Figure 2). The inflammatory infiltrate (neutrophils and F4/80-positive macrophages) was similar in the wound area of Control and RU486 groups. The Stress group showed an increase in inflammatory infiltrate, and the Stress + RU486 group demonstrated a decrease in those cells when compared to the Stress group (Table 1). It is noteworthy that neutrophils and macrophages had a distinct distribution. In the deep region, neutrophils were more abundant in the Stress group, and the RU486 treatment affected the neutrophil density in both RU486 and Stress + RU486 groups. Meanwhile, macrophages were more abundant in the Stress group in the superficial region when compared to Control, Stress, and Stress + RU486 groups. Fusiform fibroblasts were present in low number and diffusely distributed in the granulation tissue of all groups. As expected, myofibroblasts were more concentrated in the margins mainly in the superficial region of granulation tissue. In this region, the number of myofibroblasts was diminished in the Stress and RU486 groups when compared to the Control group, but RU486 treatment restores the myofibroblast density in the Stress + RU486 group (Table 1).
Figure 2.
Angiogenesis in granulation tissue. Three days after beginning of the stress protocol, a full-thickness excisional lesion (1 cm2) was generated on the dorsal skin. Histological sections of the wounds—recovered seven days after wounding—were stained with hematoxylin-eosin and the presence of blood vessels was evaluated to quantify the angiogenesis in the granulation tissue
Table 1.
Effects of RU486 treatment on the distribution of granulation tissue cells seven days after wounding
| Measurement | Groups | Granulation tissue (S) | Granulation tissue (D) | Granulation tissue (total) |
|---|---|---|---|---|
| Neutrophils/mm2 | Control | 853 ± 140 | 344 ± 71 | 637 ± 99 |
| RU486 | 1402 ± 176 | 132 ± 60*# | 767 ± 156 | |
| Stress | 967 ± 62 | 566 ± 53* | 792 ± 46* | |
| Stress + RU486 | 921 ± 54 | 202 ± 50# | 598 ± 52# | |
| F4/80-positive macrophages/mm2 | Control | 153 ± 32 | 567 ± 43 | 360 ± 35 |
| RU486 | 209 ± 35# | 640 ± 49 | 421 ± 36 | |
| Stress | 423 ± 37* | 598 ± 45 | 505 ± 30* | |
| Stress + RU486 | 80 ± 17# | 262 ± 35*# | 169 ± 21*# | |
| α-SMA-positive myofibroblasts/mm2 | Control | 350 ± 41 | 25 ± 12 | 196 ± 29 |
| RU486 | 141 ± 27* | 15 ± 11 | 81 ± 17* | |
| Stress | 157 ± 27* | 38 ± 11 | 102 ± 16* | |
| Stress + RU486 | 257 ± 39# | 59 ± 16 | 161 ± 24 |
Data shown as mean ± SEM.
S: superficial region; D: deep region; α-SMA: alpha-smooth muscle actin; SEM: standard error of the mean.
vs. Control group; #vs. Stress group.
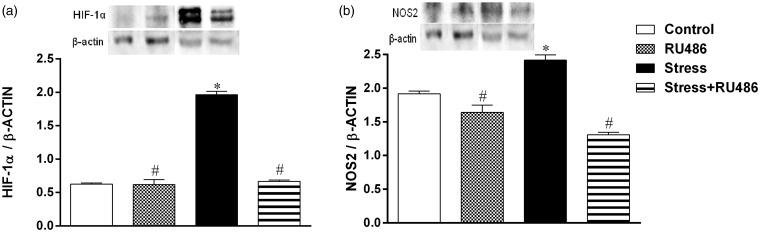
Neutrophils and macrophages can regulate the expression of the HIF-1α which, in turn, regulates the expression of a wide set of genes, including NOS2.19 To evaluate the expression of these proteins in a stressful inflammatory condition, western blot analyses were performed. In the Stress group, the HIF-1α and NOS2 expressions were increased in comparison to both Control and RU486 groups, and the RU486 treatment could reverse this effect (Figure 3).
Figure 3.
HIF-1α and NOS2 expression in lesion tissue. Three days after beginning of the stress protocol, a full-thickness excisional lesion (1 cm2) was generated on the dorsal skin. Seven days after wounding, lesion was collected and proteins were resolved by SDS-PAGE and immunoblotted for HIF-1α, NOS2, and β-actin. The densitometry of the blots is expressed in arbitrary units to show the HIF-1α/β-actin ratio (a) and the NOS2/β-actin ratio (b). Data are expressed as mean ± SEM (*vs. Control group; #vs. Stress group)
NOS2: inducible nitric oxide synthase; HIF-1α: hypoxia-inducible factor-1α
Discussion
A meta-analytical study using diverse wound-healing models and outcomes found that across studies, there was an average correlation of −0.42 between psychological stress and wound healing.20 So, the relationship between stress and wound repair is not only significant, but also clinically relevant.21 Stress cause the release of several stress hormones—primarily GCs (through the HPA) and catecholamines (through the SNS),22–24 resulting in simultaneously elevated plasma concentrations of GCs, norepinephrine, and epinephrine.25 Elevated plasma levels of normetanephrine and corticosterone were previously detected in restraint8,26 and rotational stress models.17,27 And the blockade of GCs receptors—including with RU486 treatment—aid no change to the high plasma corticosterone levels in restraint-stressed animals26,28,29 and to the high plasma normetanephrine levels in our model.
Stress induces a downregulation of the early inflammatory response in wound healing by an increase in serum GCs levels.6,7 We recently demonstrated that in stressed mice, the blockade of beta adrenoceptors restores normal wound healing, highlighting the activation of beta-adrenergic signaling in mediating negative effects of stress in our model.16,30,31 It was previously demonstrated that the blockade of GCs receptors in restraint-stressed animals resulted in healing rates that were similar to those of control-punch biopsy wounds.8 The systemic administration of RU486 normalized healing of tape stripping-induced wounds22,32 and of punch biopsy wounds in the face of ongoing stress.8,26 In our full-thickness excisional lesion, we observed that the systemic administration of RU486 accelerates the initial healing rate, four and seven days after wounding. At 14 days after wounding, the wound size was similar between Stress groups, but larger than those in Control. It was recently demonstrated that systemic blockade of GC by RU486 treatment restores normal wound-healing kinetics in a frustration model of stress,26 which differs in time and intensity of stress induction. Thus, the blockade of GCs receptors was not capable of completely restoring healing in our model of chronic stress and full-thickness excisional wound, suggesting that lesion size and the stress model—mostly due to stress persistence—are important for the full recovery of wounds.
The healing process is a cascade, and success in the later stages of wound repair depends to a large extent on initial events. Inflammation is crucial for repair, and a delayed inflammatory phase could compromise the success of wound healing. RU486, as a known GC receptor antagonist, should ameliorate the negative effects of stress on wound healing. Almost all immune cells have receptors for stress hormones and specifically neutrophils and macrophages express GCs receptors,24 so cell recruitment could be modulated by GCs receptor blockade.33 Indeed, the higher number of neutrophils and F4/80-positive macrophages present in the Stress group was diminished in Stress + RU486. It was described that stress led to an increased neutrophil accumulation that is only reduced after stress removal.1 We recently demonstrated that stress maintained neutrophil and macrophage mobilization in a wound, leading to disturb the granulation tissue formation and delayed wound closure,16,17 as also demonstrated herein.
Immune modulation by stress hormones might proceed through two pathways: directly, through binding of the hormone to its receptor; or indirectly, by deregulation of the production of cytokines, such as interferon-γ, interleukin-1 (IL-1), IL-6, and tumor-necrosis factor-alpha (TNF-α).23 It has been demonstrated that proinflammatory cytokines induce HIF-1 activity, which, in turn, upregulates proinflammatory cytokines expression, establishing a positive feedback loop.19 HIF-1α is expressed in skin wounds,9,10 suggesting that the same protein mediates both response to hypoxia and their ability to participate in inflammation.11 In the current study, mice from the Stress group exhibited an increase HIF-1α expression that is totally abolished in Stress + RU486 group. Additionally, the NOS2 expression observed in Control and RU486 groups is also higher in the Stress group and decreased in the Stress + RU486 group. It was also previously described an increase in NOS2 expression in stressed animals on the first days post-wounding.34 Altogether, these results are consistent with previous observations that NOS2-derived NO is important to HIF-1α stabilization and function.12 To definitively elucidate the NO and NOS2 role in wound healing during stress, the blockade of NO production and action should be performed.
To our knowledge, this is the first description of the roles of HIF-1α and NOS2 in skin wound healing regulated by stress. As neutrophils and macrophages are predominating in the first days following injury and are increased in the Stress group, HIF-1α accumulation in macrophages appears rational. It is also reasonable that the downstream changes observed in HIF-1α and NOS2 expression could represent compensatory responses to the delayed wound healing. We clearly demonstrated that GCs are implicated in the stress regulation of these molecules, since RU486 treatment promotes the blockade of GC peripheral action and abolished this response. We suggest that regulation of specific transcriptional factors soon after injury and their sustained expression until the later stages of the repair process are crucial for wound healing in stress response.
Acknowledgements
The authors would like to thank Denyse Ane Sales Santos for technical support. This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant 302918/2012-4), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (grant E-26/111.787/2012), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (grant PNPD2154/2009).
Author contributions
TFA and AMAC designed the study. All authors participated in interpretation of the studies, analysis of the data, and review of the manuscript. TFA and AMAC wrote the manuscript.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Tymen SD, Rojas IG, Zhou X, Fang ZJ, Zhao Y, Marucha PT. Restraint stress alters neutrophil and macrophage phenotypes during wound healing. Brain Behav Immun 2013; 28: 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet 1995; 346: 1194–6. [DOI] [PubMed] [Google Scholar]
- 3.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, Isseroff RR. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med 2009; 6: 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vileikyte L. Stress and wound healing. Clin Dermatol 2007; 25: 49–55. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA 2007; 298: 1685–7. [DOI] [PubMed] [Google Scholar]
- 6.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996; 8: 548–56. [DOI] [PubMed] [Google Scholar]
- 7.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83: 835–70. [DOI] [PubMed] [Google Scholar]
- 8.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun 1998; 12: 64–73. [DOI] [PubMed] [Google Scholar]
- 9.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen 2007; 15: 636–45. [DOI] [PubMed] [Google Scholar]
- 10.Owings RA, Boerma M, Wang J, Berbee M, Laderoute KR, Soderberg LS, Vural E, Jensen MH. Selective deficiency of HIF-1alpha in myeloid cells influences secondary intention wound healing in mouse skin. In Vivo 2009; 23: 879–84. [PubMed] [Google Scholar]
- 11.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003; 112: 645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herr B, Zhou J, Drose S, Brune B. The interaction of superoxide with nitric oxide destabilizes hypoxia-inducible factor-1alpha. Cell Mol Life Sci 2007; 64: 3295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008; 453: 314–21. [DOI] [PubMed] [Google Scholar]
- 14.Khanfer R, Phillips AC, Carroll D, Lord JM. Altered human neutrophil function in response to acute psychological stress. Psychosom Med 2010; 72: 636–40. [DOI] [PubMed] [Google Scholar]
- 15.Mizobe K, Kishihara K, Ezz-Din El-Naggar R, Madkour GA, Kubo C, Nomoto K. Restraint stress-induced elevation of endogenous glucocorticoid suppresses migration of granulocytes and macrophages to an inflammatory locus. J Neuroimmunol 1997; 73: 81–9. [DOI] [PubMed] [Google Scholar]
- 16.Romana-Souza B, Otranto M, Vieira AM, Filgueiras CC, Fierro IM, Monte-Alto-Costa A. Rotational stress-induced increase in epinephrine levels delays cutaneous wound healing in mice. Brain Behav Immun 2010; 24: 427–37. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida TF, Romana-Souza B, Machado S, Abreu-Villaca Y, Monte-Alto-Costa A. Nicotine affects cutaneous wound healing in stressed mice. Exp Dermatol 2013; 22: 524–9. [DOI] [PubMed] [Google Scholar]
- 18.Pisano JJ. A simple analysis for normetanephrine and metanephrine in urine. Clin Chim Acta 1960; 5: 406–14. [DOI] [PubMed] [Google Scholar]
- 19.Dehne N, Brune B. HIF-1 in the inflammatory microenvironment. Exp Cell Res 2009; 315: 1791–7. [DOI] [PubMed] [Google Scholar]
- 20.Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res 2009; 67: 253–71. [DOI] [PubMed] [Google Scholar]
- 21.Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Crit Care Nurs Clin North Am 2012; 24: 201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, Feingold KR. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1657–62. [DOI] [PubMed] [Google Scholar]
- 23.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5: 243–51. [DOI] [PubMed] [Google Scholar]
- 24.Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol 2008; 252: 16–26. [DOI] [PubMed] [Google Scholar]
- 25.De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav 1989; 45: 789–95. [DOI] [PubMed] [Google Scholar]
- 26.Youm JK, Park K, Uchida Y, Chan A, Mauro TM, Holleran WM, Elias PM. Local blockade of glucocorticoid activation reverses stress- and glucocorticoid-induced delays in cutaneous wound healing. Wound Repair Regen 2013; 21: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perissin L, Zorzet S, Rapozzi V, Carignola R, Angeli A, Giraldi T. Seasonal effects of rotational stress on Lewis lung carcinoma metastasis and T-lymphocyte subsets in mice. Life Sci 1998; 63: 711–9. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JK, DeVries AC, Kigerl KA, Dahlman JM, Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun 2009; 23: 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B. Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology 2006; 147: 4430–7. [DOI] [PubMed] [Google Scholar]
- 30.Romana-Souza B, Otranto M, Almeida TF, Porto LC, Monte-Alto-Costa A. Stress-induced epinephrine levels compromise murine dermal fibroblast activity through beta-adrenoceptors. Exp Dermatol 2011; 20: 413–9. [DOI] [PubMed] [Google Scholar]
- 31.Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamines blockade. Exp Dermatol 2010; 19: 821–9. [DOI] [PubMed] [Google Scholar]
- 32.Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol 2000; 278: R367–72. [DOI] [PubMed] [Google Scholar]
- 33.Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y, Tanapat P, Moday H, Rhee R, McEwen BS. Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell Immunol 1998; 186: 45–54. [DOI] [PubMed] [Google Scholar]
- 34.Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun 2005; 19: 217–22. [DOI] [PubMed] [Google Scholar]