Abstract
Saururus chinensis (Lour.) Baill. is a perennial plant distributed throughout Northeast Asia and its roots have been widely used as a traditional medicine for hepatitis, asthma, pneumonia, and gonorrhea. This study was designed to investigate the anti-inflammatory activity of an extract of S. chinensis of the aerial parts (rather than the root), and the signaling pathway responsible for this effect in lipopolysaccharide-stimulated murine macrophages. The subfraction 4 (SCF4) from the n-hexane layer of the ethanol extract of the aerial parts of S. chinensis exhibited the highest nitrite-inhibitory activity. SCF4 significantly inhibited the production of nitrite and the expression of pro-inflammatory mediators via heme oxygenase-1 upregulation. SCF4 caused significant phosphorylation of p38 MAPK and Akt, which subsequently induced the nuclear translocation of p-p65 nuclear factor-κB and Nrf2. SCF4 also suppressed the phosphorylation of signal transducers and activators of transcription 1 (p-STAT1). The heme oxygenase-1 inhibitor zinc protoporphyrin attenuated the inhibitory effect of SCF4 on lipopolysaccharide-stimulated nitrite production and expression of inflammatory mediators, tumor necrosis factor alpha, and p-STAT1. We identified sauchinone as the active compound in S. chinensis extract and SCF4. Sauchinone was shown to significantly inhibit nitrite production and inflammatory mediators expression via heme oxygenase-1 upregulation. These results suggest that S. chinensis extract, SCF4, and its active compound, sauchinone, could be used as an anti-inflammatory agent.
Keywords: Saururus chinensis, sauchinone, anti-inflammation, Heme oxygenase-1, phosphorylation of signal transducers and activators of transcription 1, nuclear factor-κB
Introduction
Saururus chinensis (Lour.) Baill. is a perennial plant that grows throughout China, Korea, and Japan. It is used as a traditional or “folk” medicine for conditions such as hepatitis, edema, pneumonia, jaundice, and gonorrhea.1,2 Previous reports have shown that an extract of S. chinensis roots has various positive effects, including antioxidant, anti-inflammatory, antiviral, antihypertensive, anti-septic, and anti-cancer activities.3–7 Sauchinone, a diastereomeric ligand and a potential anti-inflammatory compound, has been isolated from the n-hexane fraction of S. chinensis root extract.8,9 However, the cellular and molecular mechanisms of the extract of the aerial parts of this plant and its active compounds have not been extensively characterized.
Inflammation is a complex process involving numerous components with functions such as healing injured cells and tissues or eliminating pathogens.10,11 Macrophages play a vital role in the inflammatory process and create a link between innate and adaptive immunity.12,13 When macrophages become activated, they express pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1).14 Macrophages also upregulate the gene expression of pro-inflammatory mediators, including cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which release prostaglandin E2 (PGE2) and nitric oxide (NO), respectively.15 Lipopolysaccharide (LPS) is a pro-inflammatory mediator that triggers strong immune responses in animals. In addition, LPS induces activation of the signal transducer and activator of transcription (STAT) protein and controls the expression of iNOS.16,17
Heme oxygenase-1 (HO-1) is a cytoprotective enzyme that limits the rate of heme degradation. It shows anti-oxidative and anti-inflammatory activity by activating transcription factors, such as heat shock factor, nuclear factor erythroid 2-related factor 2 (Nrf2), and activator protein-1.18,19 Andreadi et al.20 and Naidu et al.21 demonstrated that dietary polyphenols and phorbol myristate acetate activated nuclear factor (NF)-κB, which regulates major pro-inflammatory cytokines through upregulation of HO-1 expression. Therefore, regulation of HO-1 is considered an important therapeutic strategy against inflammatory response.22 HO-1 induction involves various signal transduction pathways, such as extracellular signal-regulated protein kinase (ERK) and c-Jun N-terminal.
The aerial parts of S. chinensis are rich in biomass compared to the roots, which makes the aerial extract more easily obtainable than the root extract. In this study, we provide new insights into the anti-inflammatory effect of S. chinensis aerial extract in LPS-induced RAW264.7 cells and the cellular and molecular mechanisms based on the induction of HO-1 via the activation of MAPK or STAT pathways. We also identified the active compound responsible for the anti-inflammatory effect of S. chinensis.
Materials and methods
Chemicals
LPS (Escherichia coli, serotype 055:B5), pyrrolidine dithiocarbamate (PDTC), zinc protoporphyrin (ZnPP), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO). Sauchinone (high purity) was obtained from Elcom Science Co., Ltd. (Seoul, Korea). Rabbit polyclonal antibodies specific for p38, phosphorylated (p-) p38, c-Jun N-terminal kinases (JNK), Akt, p-Akt, p65, p-p65, iNOS, COX-2, and β-actin, as well as horseradish peroxidase-labeled donkey anti-rabbit and anti-mouse secondary antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Rabbit polyclonal antibodies specific for Nrf2, Lamin A/C, STAT1, p-STAT1, STAT3, and p-STAT3 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal antibody specific for HO-1 was obtained from Enzo Life Sciences Inc. (Farmingdale, NY). The TNF-α enzyme-linked immunosorbent assay kits were purchased from R&D Systems, Inc. (Minneapolis, MN).
Preparation and subfractionation of S. chinensis extract
The dried aerial parts of S. chinensis were ground to a powder and immersed in 80% ethanol (EtOH) at room temperature for three days to extract the active components. The solvent was then evaporated under reduced pressure at a temperature not exceeding 50 ℃, to yield 139 g of S. chinensis extract (SCE). The SCE (100 g) was re-suspended in distilled water (DW, 1 L) and fractionated with n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), n-butanol (BuOH), and DW. The detailed procedure for the activity-guided fractionation of the extracts is shown in Figure 2(a). The n-hexane layer (5 g) was chromatographed on a silica gel column and eluted with 90% methanol:DW (9:1) to obtain seven subfractions. The drop speed into the tubes was controlled at 1 drop every 7–10 s and a new tube used every 10 min. The detailed procedure for the subfractionation of the n-hexane layer is shown in Figure 3(a).
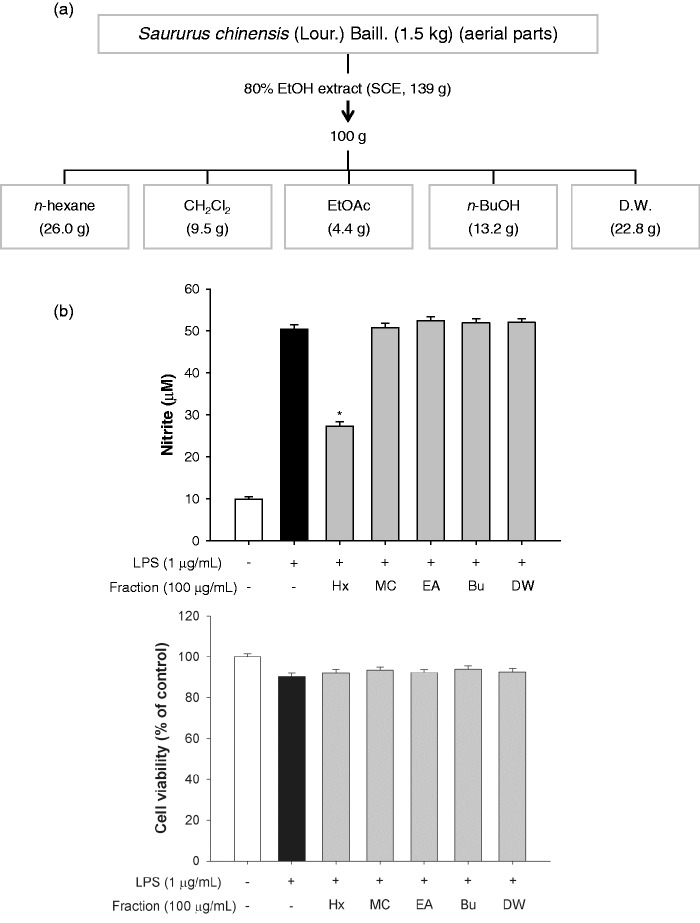
Figure 2.
The n-hexane layer isolated from SCE had an anti-inflammatory effect. (a) Schematic of the fractionation of Saururus chinensis. (b) Determination of the anti-inflammatory fractions by estimation of nitrite production and cell viability following incubation with various fractions for 2 h. Data are the means ± SEM (n = 3), *p < 0.05. Bu: n-BuOH; EA: EtOAc; Hx: n-hexane; MC: dichloromethane; SCE: S. chinensis extract; SEM: standard error of the mean; DW: distilled water
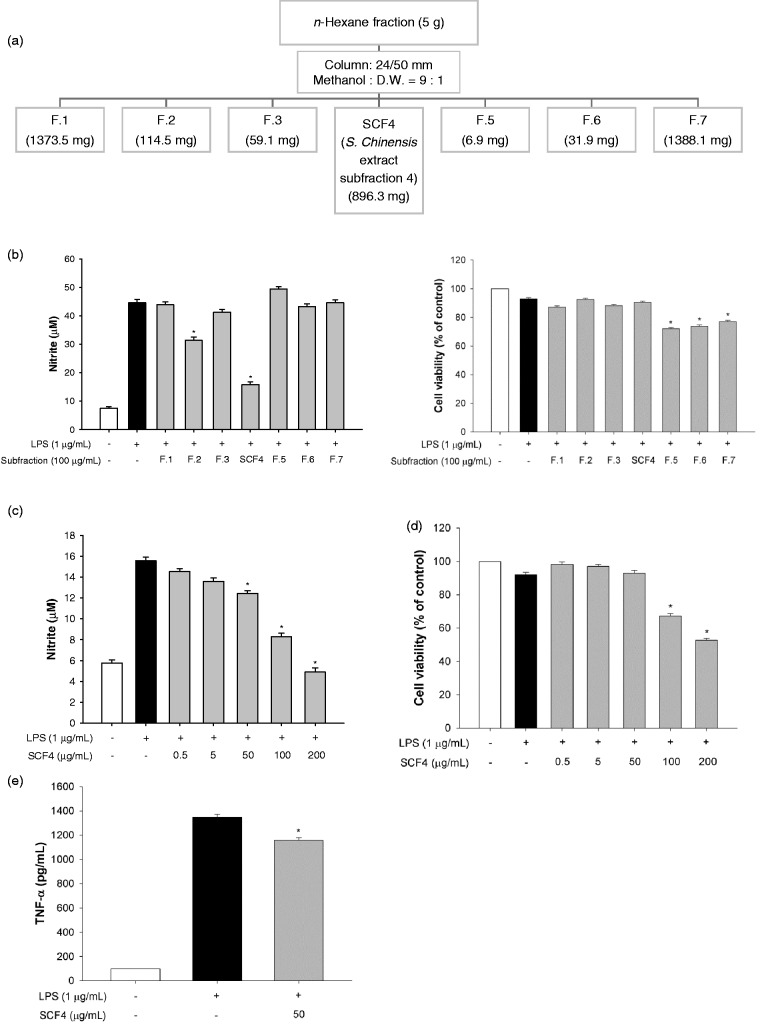
Figure 3.
SCF4 had a key role in the anti-inflammatory activity of the n-hexane layer of the SCE among the seven subfractions. (a) Schematic of the subfractionation of the n-hexane layer of the SCE. (b) Determination of the anti-inflammatory effect of the subfractions by estimation of nitrite production and cell viability after incubation with the fractions for 2 h. (c) Decreased nitrite production by SCF4 at concentrations ranging from 0.5 to 200 μg/mL. (d) SCF4 was not cytotoxic at concentrations ranging from 0.5 to 50 μg/mL. (e) SCF4 reduced LPS-activated TNF-α levels in cells. Data are the means ± SEM (n = 3), *p < 0.05. LPS: lipopolysaccharide; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SCE: S. chinensis extract; SEM: standard error of the mean; TNF: tumor necrosis factor; DW: distilled water
High-performance liquid chromatography analysis
High-performance liquid chromatography (HPLC) analysis of the SCE (1 mg/mL), subfraction 4 of the n-hexane fraction (SCF4, 1 mg/mL), and sauchinone (100 ng/mL, as a standard) was conducted using a C18 column with the temperature set at 30 ℃. The mobile phase consisted of 0.4% aqueous phosphoric acid (A) and acetonitrile (B). The gradient conditions used were similar to previously reported conditions.9 The flow rate was 0.8 mL/min and the injection volume was 10 μL. A detection wavelength of 250 nm was used for the analysis of SCE, SCF4, and sauchinone. The absorption spectra of the samples were recorded between 190 nm and 380 nm.
Cell culture
Macrophage RAW264.7 cells derived from BALB/c mice were obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin at 37 ℃ and exposed to 5% CO2. We performed an endotoxin detection test for each treatment (SCE, subfractions 1–7, and sauchinone) using the Limulus Amebocyte Lysate (LAL).
Nitrite measurement and cell viability
RAW264.7 cells were seeded in a 96-well plate (3 × 104 cells/well) and incubated overnight. The cells were pre-treated for 2 h with one of the following: various concentrations of SCE, the five solvent layers of the SCE (n-hexane, CH2Cl2, EtOAc, n-BuOH, and DW), subfractions 1–7, and sauchinone, after which they were exposed to LPS or control (no LPS) for 22 h. To assess the accumulation of nitrite as an indirect indicator of NO production, 100 μL culture supernatants or standards (sodium nitrite) were added to 100 μL Griess reagent (1% sulfanilamide and 0.1% N-1-napthyl-ethylenediamine dihydrochloride in 2.5% H3PO4). The absorbance was measured at 540 nm after 20-min incubation at room temperature. The nitrite levels of the samples were calculated using a standard curve prepared from known concentrations of sodium nitrite (0, 2, 4, 8, 16, 32, 64, and 128 μM). Lastly, fresh medium and MTT were added to the cells, followed by incubation for 4 h for the MTT assay, and the absorbance was measured at 595 nm.
Measurement of TNF-α levels
RAW264.7 cells were seeded in 96-well plates (3 × 104 cells/well) and incubated overnight. The cells were pre-treated for 2 h with SCF4 (50 μg/mL), followed by treatment with or without LPS for 22 h. The supernatants of the culture media were transferred to e-tubes for determination of the TNF-α levels using an enzyme-linked immunosorbent assay kit following the manufacturer's instructions.
Western blot analysis
Protein samples (40 μg) from the RAW264.7 cells were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel and separated by electrophoresis. The proteins were subsequently transferred to nitrocellulose (NC) membranes and treated with specific primary antibodies (1:1000) overnight. This was followed by detection with horseradish peroxidase-labeled donkey anti-rabbit (1:2000) or anti-mouse (1:2000) secondary antibodies. A monoclonal antibody against mouse β-actin and the rabbit polyclonal antibody specific for Lamin A/C were used as loading controls. The bands were visualized using the EZ-Western Lumi Pico reagents (Daeil Lab Service Co. Ltd., Seoul, Korea) according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean ± standard error of the mean. The group differences were determined using a one-way analysis of variance and Dunnett's test, followed by a modified t-test with the Bonferroni correction for comparisons between individual groups; p < 0.05 was considered significant (Sigma Stat).
Results
Extract of S. chinensis aerial parts suppressed LPS-induced nitrite production in RAW264.7 cells
We first evaluated the anti-inflammatory effect and the cytotoxic potential of the crude extract of the aerial parts of S. chinensis (80% EtOH) by incubating LPS-stimulated RAW264.7 cells with the extract for 2 h and then measuring nitrite production and cell survival rate. The crude extract significantly inhibited nitrite production by 25.6% and 87.2% at concentrations of 20 µg/mL and 200 µg/mL, respectively, compared to the LPS treatment (Figure 1(a)). However, the viability of the extract-treated cells was not affected at concentrations ranging from 2 to 200 µg/mL compared to the viability of LPS-treated or non-treated cells (Figure 1(b)). To identify potential anti-inflammatory effects of S. chinensis, we isolated the solvent fractions (Figure 2(a)). The nitrite production significantly decreased only in the n-hexane fraction-treated cells compared to the LPS-treated cells. There were no differences in cell viability among the groups at a concentration of 100 µg/mL (Figure 2(b)).
Figure 1.
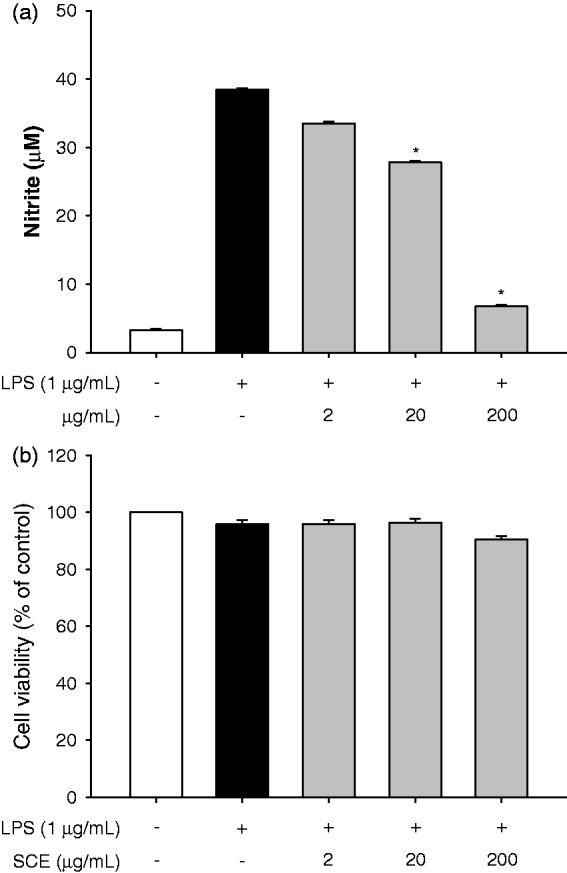
SCE had an anti-inflammatory effect. (a) Determination of anti-inflammatory effect of SCE by estimation of nitrite production following incubation with SCE for 2 h. (b) Determination of cell viability following incubation with SCE for 22 h. Data are the means ± SEM (n = 3), *p < 0.05. SCE: S. chinensis extract; SEM: standard error of the mean; LPS: lipopolysaccharide
To determine the active subfractions, the n-hexane fraction of SCE was further fractionated, as described in the Material and Methods section (Figure 3(a)). Subfraction 4 (SCF4) demonstrated a significant inhibitory effect on LPS-induced nitrite production but did not affect the cell viability at a concentration of 100 µg/mL (Figure 3(b)). SCF4 significantly inhibited nitrite production in a dose dependent manner at concentrations ranging from 50 to 200 µg/mL (Figure 3(c)) and showed cytotoxic effects at concentrations above 100 µg/mL (Figure 3(d)). We also confirmed that SCF4 significantly lowered the LPS-activated TNF-α level by 14.2% at the non-cytotoxic concentration of 50 μg/mL in RAW264.7 cells (Figure 3(e)). For this reason, we selected 0.5 µg/mL, 5 µg/mL, and 50 μg/mL concentrations of SCF4 for use in the subsequent experiment.
SCF4-induced down-regulation of iNOS and COX-2 protein expression and up-regulated HO-1 protein expression
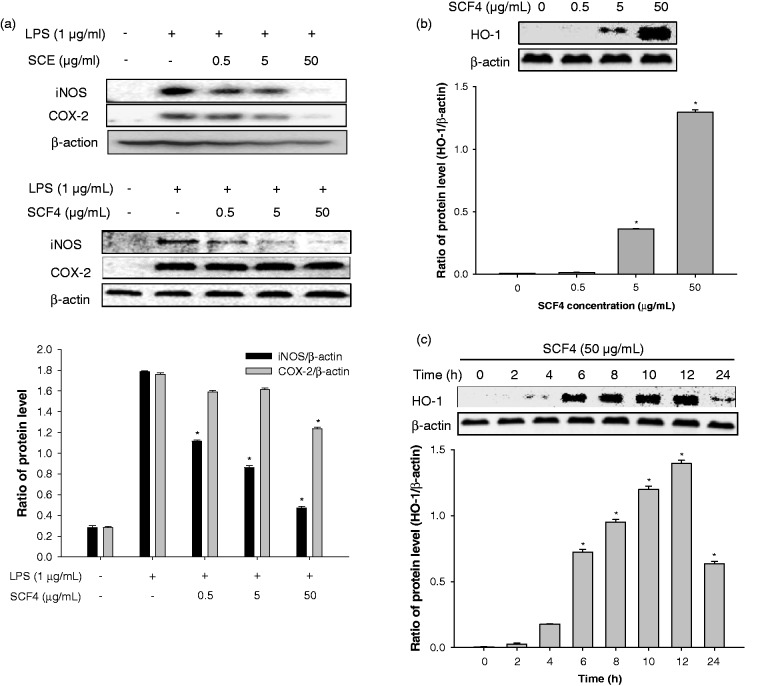
COX-2 and iNOS are the major pro-inflammatory enzymes in LPS-stimulated cells. Therefore, to investigate the extent of inhibition on pro-inflammatory enzymes, we measured iNOS and COX-2 levels in LPS-stimulated RAW264.7 cells following treatments with SCE and SCF4. As shown in Figure 4(a), SCE significantly inhibited iNOS and COX-2 expression, and SCF4 significantly repressed the expression of iNOS at concentrations ranging from 0.5 to 50 μg/mL but repressed the expression of COX-2 only at a concentration of 50 μg/mL. Additionally, we investigated the effects of SCF4 on the expression of HO-1 in RAW264.7 cells, as it also regulates iNOS and COX-2 expression. This was achieved by examining the extent of HO-1 expression following treatment with increasing concentrations of SCF4 at various time points. The induction of HO-1 expression significantly peaked at a concentration of 50 μg/mL (Figure 4(b)). The SCF4-mediated increase in HO-1 expression occurred 4 h following treatment, persisted for up to 12 h, and then gradually diminished after 24 h (Figure 4(c)).
Figure 4.
SCE and SCF4 decreased the expression of pro-inflammatory mediators. (a) Protein expression of pro-inflammatory mediators iNOS and COX-2 by western blot analysis. (b) HO-1 expression was increased dose- and time-dependently following treatment with SCF4. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. COX-2: cyclooxygenase-2; HO-1: heme oxygenase-1; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; SCE: S. chinensis extract; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean
SCF4 increased HO-1 protein expression by NF-κB and Nrf2 nuclear translocation via activation of p38 MAPK and Akt signaling pathways
To determine the signaling pathways involved in the up-regulation of HO-1 by SCF4, we assessed the levels of p-MAPKs (p-p38 and p-JNK) and p-Akt in SCF4-treated RAW264.7 cells. Treatment with SCF4 increased the levels of p-p38 and p-Akt (Figure 5(a)) in a dose-dependent manner but did not significantly affect p-JNK level at concentrations ranging from 0.5 to 50 μg/mL (Figure 5(a)). At a concentration of 50 μg/mL SCF4, the phosphorylation of p38 and Akt significantly peaked at 30 min (6-fold) and 120 min (1.2-fold; Figure 5(b)), respectively.
Figure 5.

SCF4 activated the p38 MAPK and Akt signaling pathways but did not significantly affect the JNK pathway. (a) Dose-dependent effects on protein expression of p38, Akt, and JNK by western blot. (b) Time-dependent effects on protein expression of p38 and Akt by western blot. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. MAPKs: mitogen-activated kinases; PI3K: phosphatidylinositol 3-kinase; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean
Subsequently, we investigated the effects of SCF4 on p65 (a subunit of NF-κB) and Nrf2 induction. The results showed that p65 and Nrf2 were translocated from the cytosol to the nucleus at the highest concentration of SCF4 (50 μg/mL) in a time-dependent manner (Figures 6(a) and (b)).
Figure 6.
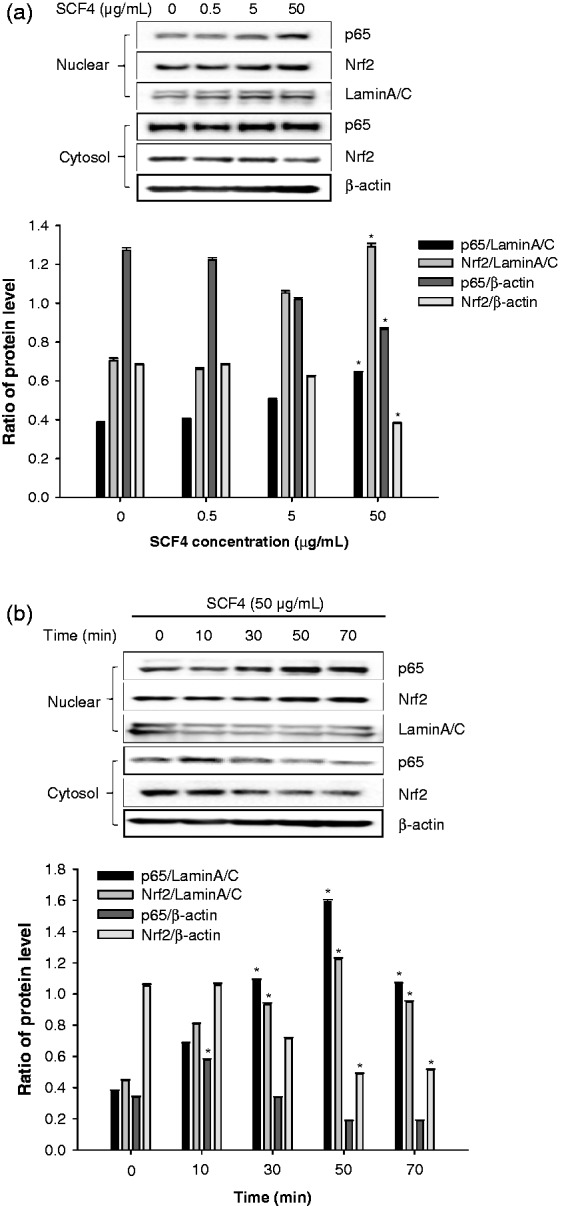
SCF4 activated NF-κB and Nrf2 signaling pathways. (a) Dose-dependent effects on protein expression of p65 and Nrf2 by western blot. (b) Time-dependent effects on protein expression of p65 and Nrf2 by western blot. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2: nuclear factor erythroid 2-related factor 2; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean
SCF4 suppressed LPS-induced pro-inflammatory mediator expression via HO-1 up-regulation
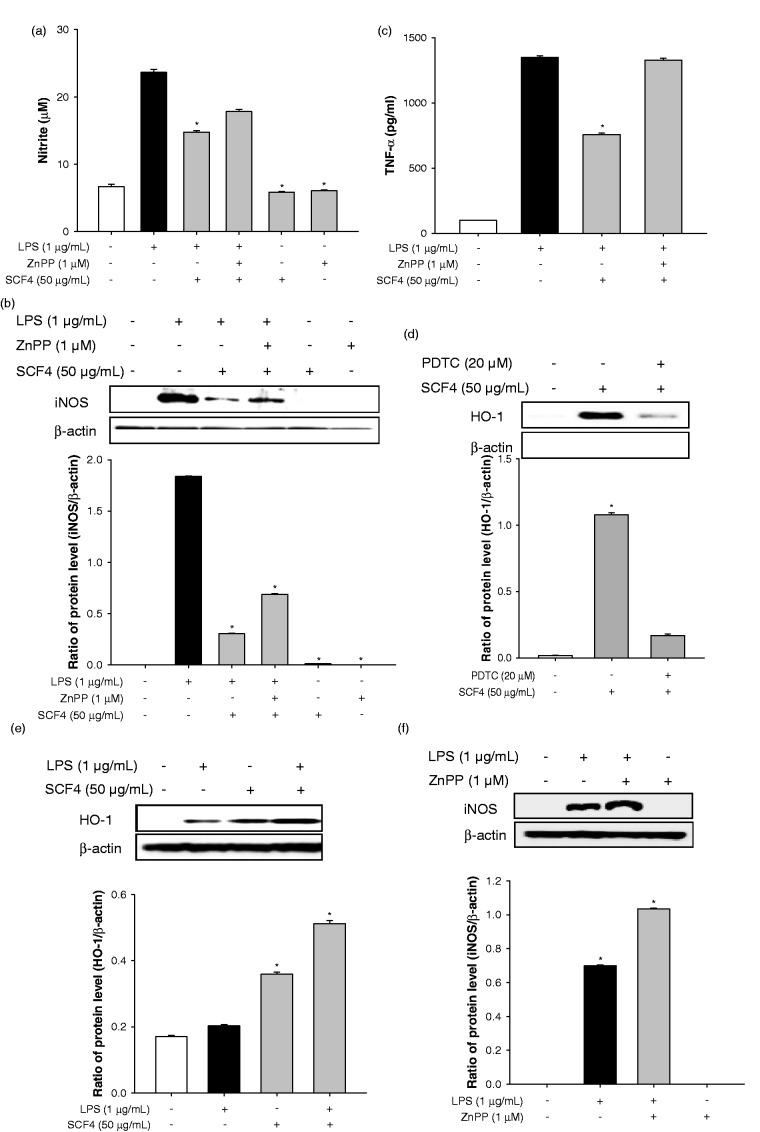
SCF4-mediated up-regulation of HO-1 protein expression is instigated by the blockade of LPS-induced iNOS, nitrite, and TNF-α expression. To assess this affect, we estimated the levels of these pro-inflammatory factors in RAW264.7 cells treated with the selective HO-1 inhibitor ZnPP. LPS-induced nitrite production and iNOS protein expression were significantly decreased by SCF4 treatment compared to that observed in LPS-treated cells (Figures 7(a) and (b)). In addition, SCF4-ZnPP co-treatment to some extent recovered LPS-induced nitrite production and iNOS protein expression compared to the SCF4-LPS co-treatment group. On the other hand, SCF4-ZnPP co-treatment totally recovered LPS-induced TNF-α expression compared to the SCF4-LPS treatment group (Figure 7(c)). In addition, treatment with the NF-κB inhibitor PDTC eliminated the SCF4-induced HO-1 expression in RAW264.7 cells (Figure 7(d)). To further ascertain the regulatory mechanism involved, we examined the level of HO-1 expression induced by SCF4 and LPS. Treatment of RAW264.7 cells with SCF4 induced higher HO-1 expression than LPS treatment. Simultaneous treatment of the cells with both SCF4 and LPS induced higher HO-1 expression than that obtained with either substance alone (Figure 7(e)). iNOS expression was higher in RAW264.7 cells co-cultured with ZnPP and LPS than that in cells treated with LPS alone but ZnPP alone did not induce iNOS expression (Figure 7(f)). Our results suggest that different mechanisms regulate SCF4- and LPS-induced HO-1 protein expression in RAW264.7 cells.
Figure 7.
HO-1 inhibitor (ZnPP) blocked the anti-inflammatory effect of SCF4. (a) Nitrite production in LPS-stimulated cells treated with SCF4 in the presence or absence of ZnPP. (b) iNOS expression in LPS-stimulated cells treated with SCF4 in the presence or absence of ZnPP. (c) TNF-α level in LPS-stimulated cells treated with SCF4 in the presence or absence of ZnPP. (d) The HO-1 expression in SCF4-induced cells with or without PDTC. (e) Simultaneous treatment with SCF4 and LPS induced HO-1 expression more than individual treatment of RAW264.7 cells with either substance alone. (f) iNOS expression increased more in RAW264.7 cells co-cultured with ZnPP and LPS than that in cells treated with only LPS. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. HO-1: heme oxygenase-1; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; PDTC: pyrrolidine dithiocarbamate; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean; TNF: tumor necrosis factor; ZnPP: zinc protoporphyrin
SCF4 inhibited STAT1 activation via induction of HO-1
Our results revealed that SCF4 significantly inhibited the phosphorylation of STAT1 at a concentration of 50 μg/mL but did not inhibit p-STAT3 expression (Figure 8(a)). In addition, we determined the inhibitory effects of SCF4-induced HO-1 on STAT1 signaling in LPS-mediated cells by assessing the p-STAT1 levels in ZnPP-treated cells. As shown in Figure 8(b), SCF4 significantly lowered LPS-induced STAT1 phosphorylation while co-treatment with SCF4 and ZnPP essentially abolished this effect.
Figure 8.
HO-1 expression induced by SCF4 blocked the LPS-induced inflammation activated by STAT1 signaling. (a) Phosphorylation of STAT1 decreased following treatment with SCF4. (b) Inhibitory effects of SCF4 on LPS-induced STAT1 signaling were diminished following treatment with ZnPP. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. HO-1: heme oxygenase-1; LPS: lipopolysaccharide; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean; STAT: signal transducer and activator of transcription; ZnPP: zinc protoporphyrin
Sauchinone mediated anti-inflammatory effects via HO-1 upregulation
In this study, SCF4 was obtained by fractionation of the n-hexane layer of SCE. We used HPLC analysis to identify and quantify the individual compounds in SCF4, including sauchinone. Consequently, we speculated that sauchinone could be one of the active compounds in SCF4. Our results demonstrated that sauchinone was present in SCE and SCF4 and was obtained at yields of 6.80 and 41.65 mg/g, respectively (Figure 9(a)). We also investigated whether the sauchinone isolated from S. chinensis had a key role in the anti-inflammatory activity via induction of HO-1. We determined the extent of inhibition of nitrite production, iNOS expression, and induction of HO-1 expression. The results showed that sauchinone significantly induced HO-1 protein expression in a dose-dependent manner, at concentrations ranging from 2.5 to 10 μg/mL (Figure 9(b)). In addition, sauchinone significantly inhibited nitrite production and iNOS expression in LPS-stimulated RAW264.7 cells (Figures 9(c) and (d)). The inhibitory effect of nitrite production (but not iNOS production) was partially abolished by co-treatment with sauchinone and ZnPP (Figures 9(e) and (f)).
Figure 9.
The anti-inflammatory effects of sauchinone isolated from SCF4 were mediated by the induction of HO-1. (a) HPLC profile of sauchinone in SCE and SCF4. (b) HO-1 expression increased following treatment with sauchinone. (c) Sauchinone inhibited nitrite production in LPS-stimulated RAW264.7 cells. (d) iNOS expression in LPS-stimulated RAW264.7 cells. (e) Nitrite production in LPS-stimulated cells treated with SCF4 in the presence or absence of ZnPP. (f) iNOS expression in LPS-stimulated cells treated with SCF4 in the presence or not ZnPP. Results of the western blot analysis represent three independent experiments that yielded similar results. Data are the means ± SEM (n = 3), *p < 0.05. HO-1: Heme oxygenase-1; HPLC: high-performance liquid chromatography; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; SCE: S. chinensis extract; SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis; SEM: standard error of the mean; ZnPP: zinc protoporphyrin
Discussion
Recent studies have shown that the root of S. chinensis exhibits anti-inflammatory effects. In particular, sauchinone, a biologically active compound isolated from the root of S. chinensis, inhibits pro-inflammatory responses through increased HO-1 expression via the ERK pathway.9 However, the anti-inflammatory activity of the aerial parts of S. chinensis, and the cellular and molecular mechanisms involved (besides the ERK pathway) have not yet been determined. In the present study, we showed that the aerial parts of S. chinensis also inhibit LPS-induced pro-inflammatory mediators, activate p38 and Akt pathways, lead to nuclear translocation of NF-kB and Nrf2, and induce phosphorylation of the STAT1/3 pathway. Furthermore, we identified HO-1 as one of the major mediators and sauchinone as one of the active compounds responsible for the anti-inflammatory effects of the aerial parts of S. chinensis.
In a previous study, we screened 103 medicinal plants that are frequently used in Korea, China, and Japan to identify those with anti-inflammatory effects; S. chinensis showed promising results. Yoo et al.23 demonstrated that the EtOH extract of the dried aerial parts of S. chinensis suppressed the production of nitrite in LPS-stimulated RAW264.7 macrophages. Our finding that the extract of the aerial parts of S. chinensis inhibit LPS-induced nitrite production in RAW264.7 cells provides further evidence in support of the previous study. Furthermore, we employed thin-layer chromatography (TLC) and HPLC to separate the anti-inflammatory components, isolating and characterizing this activity specifically in SCF4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis.
Macrophages release pro-inflammatory cytokines (e.g. IL-1 or TNF-α), which stimulate iNOS production and contribute to an increase in reactive nitrogen species such as NO. Inhibition of NO production is thought to prevent oxidative damages and inflammatory responses. The enzymes iNOS and COX-2 play crucial roles as pro-inflammatory mediators in the activation of macrophages by inducing NO production and PGE2 expression, respectively.15 Therefore, we determined the levels of iNOS and COX-2 following treatment of cells with SCF4 in order to confirm the inhibitory effects of this subfraction on iNOS and COX-2 expression. Consequently, we believe that the aerial parts of S. chinensis could have anti-inflammatory potential, owing to suppression of the expression of pro-inflammatory mediators under LPS-induced inflammatory stress.
HO-1 is a potent anti-inflammatory and cyto-protective enzyme that is induced in response to numerous stress stimuli, including oxidative stress, presence of heavy metals, cytokines, bacterial compounds, H2O2, and growth factors.24,25 HO-1 overexpression has been shown to have a protective effect in animal models of inflammatory diseases, such as atherosclerosis, cardiac ischemia, and autoimmune neuroinflammation.26–28 These reports have prompted studies focused on identifying pharmacological HO-1 inducers in human models. Previous reports have shown that inflammatory signaling induces HO-1 expression, and the up-regulation of HO-1 inhibits iNOS expression in LPS-stimulated macrophages.29,30 We therefore investigated whether HO-1 is a potential target for the anti-inflammatory effects of SCF4, and tested the signaling pathways related to the expression of HO-1 using a model of inflammatory stimulation. Our data showed that SCF4-mediated HO-1 expression was induced at a concentration of 5 μg/mL, with maximal effect observed at a concentration of 50 μg/mL at 12 h.
This study also assessed the activation of MAPKs and PI3K/Akt signaling cascades, which are known to be the principal mediators of HO-1 expression. SCF4 was found to increase HO-1 expression via phosphorylation and activation of p38 and Akt but not that of p-JNK levels, in LPS-induced inflammatory cells. In the inflamed state, NF-κB and Nrf2 activation is required for HO-1 gene or protein expression and is regulated by kinases of the MAPK and PI3K/Akt signaling pathways.31,32 SCF4 induced the nuclear translocation of p65 (a subunit of the NF-κB transcription complex) and Nrf2 in the presence of inflammatory stimuli. Taken together, these results indicate that signaling mechanisms related to NF-κB and Nrf2 phosphorylation via p38 MAPK and Akt activation are responsible for SCF4-induced HO-1 up-regulation. Consistent with our data, several previous studies have reported that S. chinensis could regulate the induction of HO-1 expression through various intracellular signaling pathways, including MAPK. The methanol extract of the roots of S. chinensis increased the production of HO-1 and Nrf2 in LPS-treated primary mast cells.33 Jeong et al.34 also showed that sauchinone, an active lignan isolated from the roots of S. chinensis, upregulated HO-1 expression through the p38 MAPK-Nrf2/ARE-dependent pathway in t-butyl hydroperoxide-damaged HepG2 cells. However, Li et al.9 found that sauchinone induced HO-1 expression via the ERK pathway in LPS-stimulated RAW264.7 cells but did not affect the p38 MAPK and JNK pathways. The differences in the results can be attributed to the extract preparation method, the plant part used for extraction, and differences in experimental conditions (i.e. cell types, use of LPS or ZnPP).
Further investigation found that the HO-1 inhibitor ZnPP was able to block SCF4-induced decreases in levels of nitrite and TNF-α, as well as intracellular decreases in iNOS protein. This led to the conclusion that anti-inflammatory activity may be related to HO-1 over-expression induced by SCF4. Our data also showed that SCF4-induced HO-1 upregulation was significantly reversed in the presence of the NF-κB inhibitor, PDTC. Taken together, HO-1 is one of the major targets of SCF4 and various pro-inflammatory mediators able to inhibit HO-1 up-regulation by SCF4.
Another pivotal inflammatory signaling pathway is the JAK-STAT pathway.35 Several studies have shown that LPS induces iNOS expression via activation of the JAK-STAT pathway.14,15 The STAT family comprises seven members: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. Among these proteins, STAT1 and STAT3 are suggested to be critical regulators of the inflammatory response. Despite their structural similarities, STAT1 and STAT3 act as antagonists and mutually inhibit each another.36 STAT1 is an important transcription factor that induces inflammatory mediators, such as iNOS and COX2. Conversely, STAT3 plays a role in IL-10-mediated anti-inflammatory signaling. Therefore, to investigate whether SCF4 is involved in the JAK-STAT signaling pathway, we examined its effect on p-STAT1/3 expression in LPS-stimulated RAW264.7 cells. Our results showed that LPS treatment increased the levels of p-STAT1 and p-STAT3 in the cells, whereas SCF4 significantly suppressed the phosphorylation of STAT1 and inversely increased the phosphorylation of STAT3. We also co-treated the LPS-stimulated cells with ZnPP and SCF4 to assess how HO-1 inhibition changes this effect. The ZnPP treatment attenuated the decrease of the p-STAT1 level, indicating that the anti-inflammatory activity of SCF4 in LPS-damaged cells is associated with decreased expression of iNOS and COX-2 through STAT1-related signaling via HO-1 up-regulation. Our data are consistent with those of previous studies showing that the phosphorylation of STAT1 is responsible for the anti-inflammatory effect in cells treated with several herbs.37,38
Finally, we identified the active compound responsible for the anti-inflammatory effect of the aerial parts of S. chinensis. Previously, sauchinone has been isolated from the n-hexane layer of S. chinensis roots and reported to induce HO-1 expression via the ERK signaling pathway in RAW264.7 cells.21 SCF4 (extracted from S. chinensis aerial parts) used in this study contained more than 4% sauchinone, which is similar to the amount found in the roots of the plant. To examine the relationship between the active compound and the anti-inflammatory activity, we tested sauchinone isolated from the aerial parts of S. chinensis in RAW264.7 cells. Sauchinone, similar to SCF4, was found to inhibit iNOS expression and nitrite production and increase HO-1 expression (Figures 9(b), (c), and (d)). Furthermore, sauchinone-induced nitrite production was attenuated in the presence of the HO-1 inhibitor ZnPP.
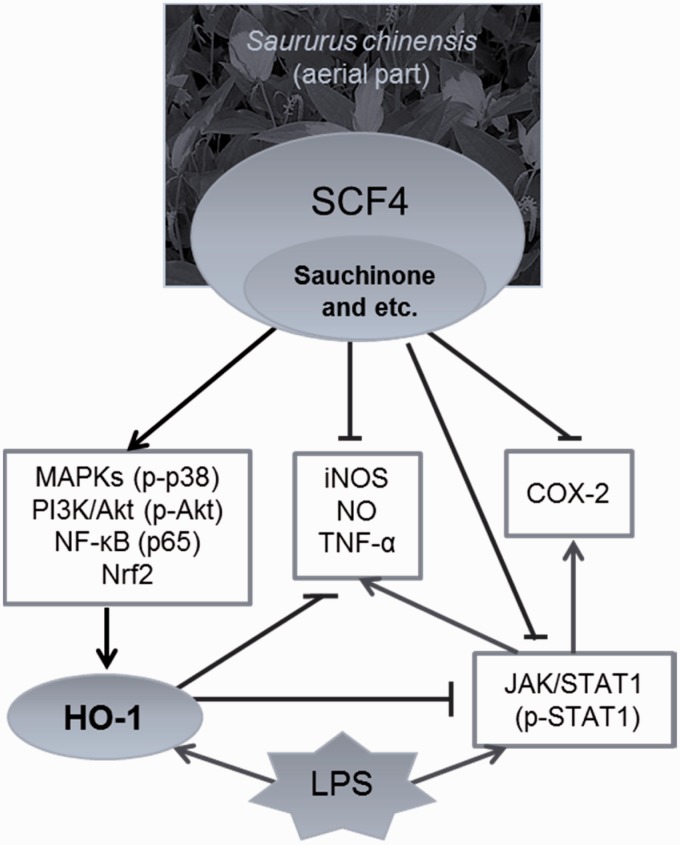
This is the first study to report the anti-inflammatory effect of the aerial parts of S. chinensis through HO-1 up-regulation via MAPK and STAT1 activation. The data suggested that sauchinone and SCF4 obtained from the extract of S. chinensis up-regulated HO-1 expression in LPS-stimulated RAW264.7 cells. This induction of HO-1 expression by sauchinone and SCF4 was mediated via the MAPK, PI3K/Akt, NF-κB, and STAT1 signaling pathways. Sequentially, the sauchinone- and SCF4-induced HO-1 expression inhibited the production of LPS-induced inflammatory mediators (Figure 10). Therefore, we suggest that sauchinone and SCF4 possess abundant potential as potent anti-inflammatory pharmacological compounds.
Figure 10.
A model illustrating the anti-inflammatory effect SCF4 via HO-1 induction. SCF4: subfraction 4 of the n-hexane layer of the EtOH extract of the aerial parts of S. chinensis
Authors' contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; XM and YJJ conducted the experiments, XM and SCK wrote the manuscript, and YMC and IK participated in data interpretation. XM and IK contributed equally to this work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the Gachon University research fund of 2014.
References
- 1.Chiu NY, Chang KH. The illustrated medicinal plants of Taiwan, Taipei, Taiwan: SMC Publishing Inc, 1998. [Google Scholar]
- 2.Jung JY, Lee KY, Lee MY, Jung D, Cho ES, Son HY. Antioxidant and antiasthmatic effects of saucerneol D in a mouse model of airway inflammation. Int Immunopharmacol 2011; 11: 698–705. [DOI] [PubMed] [Google Scholar]
- 3.Park HJ, Kim RG, Seo BR, Ha J, Ahn BT, Bok SH, Lee YS, Kim HJ, Lee KT. Saucernetin-7 and saucernetin-8 isolated from Saururus chinensis inhibit the LPS-induced production of nitric oxide and prostaglandin E2 in macrophage RAW264.7 cells. Planta Med 2003; 69: 947–50. [DOI] [PubMed] [Google Scholar]
- 4.Cui H, Xu B, Wu T, Xu J, Yuan Y, Gu Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication. J Nat Prod 2014; 77: 100–10. [DOI] [PubMed] [Google Scholar]
- 5.Ryu SY, Oh KS, Kim YS, Lee BH. Antihypertensive, vasorelaxant and inotropic effects of an ethanolic extract of the roots of Saururus chinensis. J Ethnopharmacol 2008; 118: 284–9. [DOI] [PubMed] [Google Scholar]
- 6.Seo CS, Lee YK, Kim YJ, Jung JS, Jahng Y, Chang HW, Song DK, Son JK. Protective effect of lignans against sepsis from the roots of Saururus chinensis. Biol Pharm Bull 2008; 31: 523–6. [DOI] [PubMed] [Google Scholar]
- 7.Lee YJ, Kim J, Yi JM, Oh SM, Kim NS, Kim H, Oh DS, Bang OS, Lee J. Anti-proliferative neolignans from Saururus chinensis against human cancer cell lines. Biol Pharm Bull 2012; 35: 1361–6. [DOI] [PubMed] [Google Scholar]
- 8.Park G, Kim HG, Sim Y, Sung SH, Oh MS. Sauchinone, a lignan from Saururus chinensis, protects human skin keratinocytes against ultraviolet B-induced photoaging by regulating the oxidative defense system. Biol Pharm Bull 2013; 36: 1134–9. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Lee DS, Choi HG, Kim KS, Kang DG, Lee HS, Jeong GS, Kim YC. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol Pharm Bull 2011; 34: 1566–71. [DOI] [PubMed] [Google Scholar]
- 10.Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol 1997; 37: 517–54. [DOI] [PubMed] [Google Scholar]
- 11.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol 2012; 92: 529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara N, Kobayashi K. Macrophages in Inflammation. Curr Drug Targets 2005; 4: 281–6. [DOI] [PubMed] [Google Scholar]
- 13.Barton CH, Biggs TE, Baker ST, Bowen H, Atkinson PG. Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens. J Leukoc Biol 1999; 66: 757–62. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. Proinflammatory cytokines. Chest 2000; 118: 503–8. [DOI] [PubMed] [Google Scholar]
- 15.Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, Kim GJ, Kim HM, Um JY, Ko SG. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kappaB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol 2007; 110: 490–7. 2007. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Albani P, Santangelo R, Torrisi L, Nicoletti VG, de Vellis J, Giuffrida Stella AM. JAK/STAT signaling pathway mediates cytokine-induced iNOS expression in primary astroglial cell cultures. J Neurosci Res 2001; 65: 417–24. [DOI] [PubMed] [Google Scholar]
- 17.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol 2002; 3: 392–8. [DOI] [PubMed] [Google Scholar]
- 18.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 2007; 36: 166–74. [DOI] [PubMed] [Google Scholar]
- 19.Rushworth SA, Chen XL, Mackman N, Ogborne RM, O'Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol 2005; 175: 4408–15. [DOI] [PubMed] [Google Scholar]
- 20.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol 2006; 69: 1033–40. [DOI] [PubMed] [Google Scholar]
- 21.Naidu S, Wijayanti N, Santoso S, Kietzmann T, Immenschuh S. An atypical NF-kappa B-regulated pathway mediates phorbol ester-dependent heme oxygenase-1 gene activation in monocytes. J Immunol 2008; 181: 4113–23. [DOI] [PubMed] [Google Scholar]
- 22.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol 2006; 39: 479–91. [DOI] [PubMed] [Google Scholar]
- 23.Yoo HJ, Kang HJ, Jung HJ, Kim K, Lim CJ, Park EH. Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Saururus chinensis extract. J Ethnopharmacol 2008; 120: 282–6. [DOI] [PubMed] [Google Scholar]
- 24.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 2010; 50: 323–54. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 2003; 24: 449–55. [DOI] [PubMed] [Google Scholar]
- 26.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, Agarwal A, Lusis AJ, Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res 2007; 100: 1703–11. [DOI] [PubMed] [Google Scholar]
- 27.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001; 89: 168–73. [DOI] [PubMed] [Google Scholar]
- 28.Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest 2007; 117: 438–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen TY, Sun HL, Yao HT, Lii CK, Chen HW, Chen PY, Li CC, Liu KL. Suppressive effects of Indigofera suffruticosa Mill extracts on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Food Chem Toxicol 2013; 55: 257–64. [DOI] [PubMed] [Google Scholar]
- 30.Hu CM, Liu YH, Cheah KP, Li JS, Lam CS, Yu WY, Choy CS. Heme oxygenase-1 mediates the inhibitory actions of brazilin in RAW264.7 macrophages stimulated with lipopolysaccharide. J Ethnopharmacol 2009; 121: 79–85. [DOI] [PubMed] [Google Scholar]
- 31.Park EJ, Kim YM, Park SW, Kim HJ, Lee JH, Lee DU, Chang KC. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem Toxicol 2013; 55: 386–95. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Park da J, Kim YH, Kim Y, Kim SG, Shon KJ, Choi YW, Lee SJ. Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of Schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immunol Lett 2011; 139: 93–101. [DOI] [PubMed] [Google Scholar]
- 33.Ryu HS, Lee HK, Kim JS, Kim YG, Pyo M, Yun J, Hwang BY, Hong JT, Kim Y, Han SB. Saucerneol D inhibits dendritic cell activation by inducing heme oxygenase-1, but not by directly inhibiting toll-like receptor 4 signaling. J Ethnopharmacol 2015; 166: 92–101. [DOI] [PubMed] [Google Scholar]
- 34.Jeong GS, Lee DS, Li B, Byun E, Kwon DY, Park H, Kim YC. Protective effect of sauchinone by upregulating heme oxygenase-1 via the P38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med 2010; 76: 41–7. [DOI] [PubMed] [Google Scholar]
- 35.Osna N, Elliott K, Chaika O, Patterson E, Lewis RE, Khan MM. Histamine utilizes JAK-STAT pathway inregulating cytokine production. Int Immunol 2001; 1: 759–62. [DOI] [PubMed] [Google Scholar]
- 36.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell–mediated hepatitis: regulation by SOCS. J Clin Invest 2002; 110: 1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Park SY, Kim EK, Ryu EY, Kim YH, Park G, Lee SJ. Acanthopanax senticosus has a heme oxygenase-1 signaling-dependent effect on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. J Ethnopharmacol 2012; 142: 819–28. [DOI] [PubMed] [Google Scholar]
- 38.Jeong SJ, Lim HS, Seo CS, Jin SE, Yoo SR, Lee N, Shin HK. Anti-inflammatory actions of herbal formula Gyejibokryeong-Hwan regulated by inhibiting chemokine production and STAT1 activation in HaCaT cells. Biol Pharm Bull 2015; 38: 425–34. [DOI] [PubMed] [Google Scholar]