Abstract
This study evaluated the effects of aerobic exercise performed both previously and after the induction of diabetes mellitus on changes of renal function and structure in streptozotocin-induced diabetic rats. Female wistar rats were divided into five groups: sedentary control (C + Se); trained control (C + Ex); sedentary diabetic (D + Se); trained diabetic (D + Ex) and previously trained diabetic (D + PEx). The previous exercise consisted of treadmill running for four weeks before the induction of diabetes mellitus. After induction of diabetes mellitus with streptozotocin, the D + PEx, D + Ex and C + Ex groups were submitted to eight weeks of aerobic exercise. At the end of the training protocol, we evaluate the serum glucose, insulin and 17β-estradiol levels, renal function and structure, proteinuria, and fibronectin, collagen IV and transforming growth factor beta 1 (TGF-β1) renal expressions. Induction of diabetes mellitus reduced the insulin and did not alter 17β-estradiol levels, and exercise did not affect any of these parameters. Previous exercise training attenuated the loss of body weight, the blood glucose, the increase of glomerular filtration rate and prevented the proteinuria in the D + PEx group compared to D + Se group. Previous exercise also reduced glomerular hypertrophy, tubular and glomerular injury, as well as the expressions of fibronectin and collagen IV. These expressions were associated with reduced expression of TGF-β1. In conclusion, our study shows that regular aerobic exercise especially performed previously to induction of diabetes mellitus improved metabolic control and has renoprotective action on the diabetic kidney.
Keywords: Diabetes mellitus, exercise training, kidney, proteinuria, TGF-β1
Introduction
Diabetic nephropathy is one of the most important clinical complications of diabetes mellitus (DM) and is the most common cause of end-stage renal disease.1 Although evidences point the female sex as a renoprotective factor against the development of nondiabetic renal diseases,2,3 several studies demonstrated that in DM this renoprotection appears to be lost, possibly due to dysregulation of the production of female sex hormones, which may be present in DM.4–8
Initial stages of diabetic nephropathy are characterized by expansion of extracellular matrix (ECM), glomerular hyperfiltration, and renal hypertrophy associated with microalbuminuria.9–11 However, the progression of the disease leads to severe proteinuria and progressive fall in glomerular filtration rate (GFR).12 Studies demonstrated that early hyperglycemia stimulates the production of ECM components, mainly fibronectin and collagen IV, and this effect appears to be mediated by transforming growth factor beta 1 (TGF-β1) in streptozotocin (STZ)-induced diabetic rats,13,14 as well as, in other models of renal injury.15,16
Currently, no specific therapy is available to inhibit the progression of renal diabetic disease, and although some studies have demonstrated the renoprotective action of pharmacological therapies that target the glycemic and blood pressure controls,17,18 the incidence of diabetic nephropathy continues to increase.1 Regular exercise training is considered an unquestionable component of a healthy lifestyle and widely accepted in the management and prevention of several chronic diseases.19 However, the overall effect of exercise on diabetic nephropathy is still not clear, although it appears to be directly related to the intensity of training. Some studies have shown that exercise training of moderate intensity, besides benefiting metabolic control, improves the kidney function, reduces microalbuminuria, restores oxidative balance and increases nitric oxide (NO) bioavailability in STZ-induced diabetic rats,20–22 spontaneously hypertensive rats23 and humans.24,25 However, the mechanisms underlying the benefits of exercise on the diabetic kidney are poorly understood, and the evidences for the renal effects of previous exercise training in diabetic females are limited.
In this perspective, since the progression of diabetic nephropathy is strongly correlated to the degree of glomerulosclerosis and tubulointerstitial lesions, in this study we aimed to assess the effects of moderate exercise training performed both previously and after the induction of DM on changes of renal function and structure in STZ-induced diabetic female rats.
Materials and methods
Animals
A total of 32 female Wistar rats weighing 180–200 g were housed under controlled environmental conditions (12/12 h light/dark cycle and 24 ± 2℃) with food and water ad libitum. The animals were divided into the following groups: control sedentary (C + Se, n = 6), trained control (C + Ex, n = 6), sedentary diabetic (D + Se, n = 7), trained diabetic (D + Ex, n = 6) and previously trained diabetic (D + PEx, n = 7). This study was approved by the Ethics Committee in Animal Experimentation of the Federal University of Bahia – Multidisciplinary Institute of Health, (protocol 008/2013). All experimental procedures were conducted in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Diabetes mellitus induction
DM was induced by a single intravenous injection of STZ (Alfa Aesar – Ward Hill, MA, USA) (40 mg/kg) diluted in 0.1 M citrate buffer, pH 4.5, in animals weighing 290 to 310 g after 12 h of fasting. Control rats were injected with equivalent amounts of citrate buffer. One week after STZ administration, the diabetes was confirmed by measuring blood glucose levels in a tail vein blood samples with Accu-Chek glucose strips (Roche, Mannheim, Germany). The animals with blood glucose levels higher than 250 mg/dL were considered diabetic.
Maximal running test
Before starting the training protocol, all animals were submitted to a three-day adaptation period on the stop treadmill (AVS Projects – SP, Brazil) and five-day adaptation period on the treadmill at a speed of 10 m/min for 5 min. After this period, the animals were submitted to a maximum running test (1 m/min increments every 3 min up to the rat reached exhaustion) on three alternate days, in order to determine the exercise training intensity and the aerobic capacity. This test was performed at the beginning, at the end of the fourth week, and at the end of the training protocol.21
Exercise training protocol
The exercise training duration was 20 min in the first week and increased up to 1 h by the end of the fourth week, with no inclination of the treadmill, five day per week. Similarly, the intensity of exercise was also progressively increased (55–70%) building up to 70% of the maximal running test toward the end of the protocol.21 Only the D + PEx group was submitted to four weeks of aerobic exercise training on the treadmill before inducing DM. After this period, DM was induced in the D + Se, D + Ex and D + PEx groups. One week after induction of DM, the C + Ex, D + Ex and D + PEx groups were submitted to treadmill running for eight weeks, as shown in Figure 1. Forty-eight hours after the last exercise session, the animals were placed in individual metabolic cages in order to collect 24-h urine samples to measure creatinine, urinary flow and proteinuria. Then, the animals were euthanized by decapitation and blood samples were collected to quantify creatinine, insulin and 17β-estradiol levels. The kidneys were removed and weighed for histological and immunohistochemical studies.
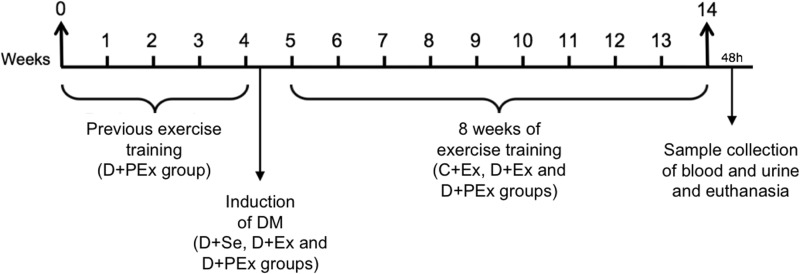
Figure 1.
Experimental design. 0–4, previous exercise training in the D + PEx group (4 weeks); 4–5, induction of DM in the D + Se, D + Ex and D + PEx groups; 5–14, continuation of exercise training in the D + PEx group and initiation of exercise training in the D + Ex and C + Ex groups; 48h, sample collection of blood and urine 48h after the last training session, and euthanasia
Body weight, blood pressure and glycemia
Body weight (BW) and blood pressure were measured weekly. The blood pressure was measured by a programmable tail-cuff sphygmomanometer (LE 5001 Electro-Sphygmomanometer, Panlab, Spain). The animals were submitted to a fasting period of 12 h for measurement of fasting capillary glycemia (FCG) by a digital glucometer (Accu-Check Active glucose strips, Roche, Mannheim, Germany).
Determination of Serum 17β-estradiol and insulin concentrations
The dosage of serum 17β-estradiol was performed by chemiluminescence. The serum insulin was analyzed by radioimmunoassay (Research® Linco, St. Charles, MO, USA). The measurements of plasma insulin were performed by adding 125 I labeled insulin and anti-insulin antibody on plasma samples, remaining in incubation overnight at 4℃. The precipitating reagent was separated by centrifugation to obtain a pellet, whose radiation was read by a gamma counter after removal of the supernatant. The concentration of insulin was achieved by constructing a calibration curve constructed from the calibrator kit reagents.
Renal function
Serum and urine creatinine was measured by colorimetry using Jaffé method (Abbott Diagnostics Kit). The GFR was calculated through the creatinine cleareance. Urinary flow was determined from total volume of urine in 24 h, and the proteinuria was measured by colorimetric method (Abbott Diagnostics Kit). All the biochemical and renal function parameters were analyzed using an automatic biochemical analyzer (Abbott Diagnostics C.4100 – Saint-Laurent, Quebec, Canada).
Renal morphology
Glomerulosclerotic index
Kidney samples from rats of all groups were fixed in methacarn solution (methanol 60%, chloroform 30%, and 10% acetic acid) and processed for paraffin embedding. Histological sections (4 µm) of kidney tissue were stained with the periodic acid-Schiff (PAS) reagent, counterstained with hematoxylin, and examined under light microscopy (Olympus BX51, Japan). The focal segmental glomerulosclerosis (FSGS) was evidenced by segmental increases in glomerular matrix, segmental obliteration or dilation of capillary lumina, and accumulation of hyaline. One hundred glomeruli per section were randomly selected and the FSGS was graded according to Saito et al. (1987) on a scale of 0 to 4 (grade 0 = normal glomeruli; grade 1 = sclerotic area up to 25%; grade 2 = sclerotic area 25–50%; grade 3 = sclerotic area 50–75%; grade 4 = sclerotic area 75–100%). The glomerulosclerotic index (GSI) was calculated using the following formula: GSI = (1xn1) + (2xn2) + (3xn3) + (4xn4)/nT, where nx is the number of glomeruli in each grade of glomerulosclerosis, and nT is the sum of glomeruli evaluated.26
Glomeruli morphometry
To analyze glomerular areas, 4 µm histological sections were stained with hematoxylin-eosin and the outer edges of 30 glomerular tufts of each kidney were traced manually on a video screen. The encircled areas were determined by computerized morphometry through the Image J software. The analysis of all the procedures was performed with the observer blinded to the treatment groups.
Tubulointerstitial Lesions
To evaluate the tubulointerstitial lesions, 4 µm histological sections were stained with hematoxylin-eosin. The tubulointerstitial damages were defined as tubular necrosis, tubular lumen dilation or atrophy, and inflammatory cell infiltrate. We analyzed 30 grid fields (0.087µm2 each) per kidney cortex and the damages were graded according to Shih et al.27 on a scale of 0 to 4 (0 = normal; 0.5 = small focal areas; 1 = involvement of less than 10% of the cortex and outer medullae; 2 = 10–25% involvement of the cortex and outer medullae; 3 = 25–75% involvement of the cortex and outer medullae; 4 = extensive damage involving more than 75% of the cortex and outer medullae).27
Immunohistochemistry studies
The sections were incubated overnight at 4℃ with (1:30) polyclonal anti-TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); (1:500) polyclonal anti-Collagen IV (Abcam, Cambridge, UK) and (1:500) polyclonal anti-Fibronectin (Chemicon, Temecula, CA, USA).28,15 The reaction product was detected with avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA). The color reaction was developed with 3,3-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA), and the sections were then counterstained with methyl green or hematoxylin. Nonspecific protein binding was blocked by incubation with 20% goat serum in PBS for 30 min. Negative controls consisted of replacing the primary antibodies with equivalent concentrations of normal rabbit IgG. The quantification of immunoreactivity of the reactions was obtained using Image-J software and the results were expressed as percentages.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 5 program. Data of urine flow rate and 17β-estradiol were submitted to Kruskal-Wallis nonparametric test with multiple comparisons by Dunn test, and expressed as median and percentile 25 and 75. Pearson’s and Spearman's tests were used for analysis of correlations. The other data were submitted to one-way analysis of variance followed by the Newman-Keuls multiple comparisons test, and are presented as mean ± standard error of the mean (SEM). Statistical significance was defined at a value of P < 0.05.
Results
Body weight, maximal running test, fasting capillary glycemia
All diabetic rats presented reduction in body weight compared to control groups (P < 0.001). However, this reduction was lower in D + PEx group compared to the D + Se group (P < 0.05). The exercise also reduced BW of the C + Ex group compared to the C + Se group (P < 0.05) (Table 1). The previous exercise increased the physical capacity of D + PEx group in the end of the fourth week of training compared to the other experimental groups, as well as regarding its own initial physical capacity (P < 0.01). After induction of DM, exercise training improved physical performance in D + Ex and D + PEx rats compared to the D + Se group (P < 0.001). The exercise training also improved the physical capacity of the C + Ex group compared to the C + Se group (P < 0.05) (Table 1). All the diabetic rats presented increased glycemia compared to the control groups (P < 0.001); however, the D + PEx group presented a discreet reduction of glycemia compared to D + Se group (P < 0.05) (Table 1).
Table 1.
Body weight, maximal running test, fasting capillary glycemia of C + Se, C + Ex, D + Se, D + Ex and D + PEx rats
| C + Se | C + Ex | D + Se | D + Ex | D + PEx | |
|---|---|---|---|---|---|
| Body weight (g) | |||||
| Initial | 192.0 ± 3.0 | 192.5 ± 1.4 | 196.4 ± 2.2 | 188.2 ± 2.4 | 191.9 ± 3.8 |
| After STZ/CB injection | 284.0 ± 11.9 | 278.6 ± 8.1 | 279.5 ± 7.0 | 289.7 ± 6.0 | 279.4 ± 5.0 |
| Final | 357.0 ± 18.5 | 317.7 ± 7.6* | 190.2 ± 9.8*** | 215.6 ± 9.7*** | 231.1 ± 7.7***# |
| Maximal running test (m/min) | |||||
| Initial | 23.5 ± 1.0 | 21.6 ± 1.2 | 22.9 ± 0.3 | 22.4 ± 0.4 | 23.4 ± 0.5 |
| End of week 4 | 23.9 ± 0.5 | 22.7 ± 0.8 | 21.4 ± 0.4 | 20.4 ± 1.1 | 28.7 ± 1.3**###†††$$ |
| Final | 20.4 ± 1.4 | 29.6 ± 1.4*$ | 9.8 ± 3.4**$ | 23.3 ± 1.6### | 28.0 ± 0.7###$$ |
| Fasting capillary glycemia (mg/dl) | |||||
| Initial | 93.25 ± 3.5 | 86.25 ± 3.3 | 94.45 ± 3.2 | 91.50 ± 4.6 | 95.25 ± 2.9 |
| After STZ/CB injection | 94.8 ± 2.0 | 95.3 ± 2.3 | 414.7 ± 14.3*** | 405.6 ± 16.1*** | 403.0 ± 11.2*** |
| Final | 94.4 ± 2.2 | 96.6 ± 2.1 | 584.0 ± 6.9***$$$ | 564.6 ± 22.6***$$$ | 495.2 ± 44.6***#$$$ |
Note: The data are reported as mean ± SEM. After STZ/CB injection, one week after streptozotocin or citrate buffer injection. * P < 0.05, ** P < 0.01 and *** P < 0.001 versus C + Se and C + Ex; # P < 0.05 and ### P < 0.001 versus D + Se; ††† P < 0.001 versus D + Ex; $$ P < 0.01 and $$$ P < 0.001 versus initial data.
Serum hormone levels, systolic blood pressure and kidney weight
All the diabetic groups rats presented drastic reduction in plasma insulin levels compared to the control groups (P < 0.001). However, exercise reduced serum insulin levels in the C + Ex group compared to the C + Se group (P < 0.01) (Table 2). There was no statistical difference in E2 levels between the experimental groups (Table 2). Injection of STZ induced a significant increase in systolic blood pressure in all diabetic groups (P < 0.001) (Table 2). The relative weight of kidneys of the all diabetic rats was higher than the control groups (P < 0.001). However, the exercise attenuated theses effects in the D + Ex and D + PEx groups (P < 0.01) (Table 2).
Table 2.
Insulin and 17β-estradiol levels, systolic blood pressure and kidney weight of C + Se, C + Ex, D + Se, D + Ex and D + PEx rats
| C + Se | C + Ex | D + Se | D + Ex | D + PEx | |
|---|---|---|---|---|---|
| Insulin | 2.02 ± 0.19 | 1.39 ± 0.23** | 0.28 ± 0.05*** | 0.12 ± 0.01*** | 0.23 ± 0.07*** |
| E2 | 19.0(15.5;29.0) | 16.9(12.3;24.6) | 15.5(9.8;21.3) | 11.0(9.0;13.0) | 11.0(9.5;15.5) |
| SBP | 104.0 ± 4.0 | 100.4 ± 2.9 | 130.3 ± 3.8*** | 131.9 ± 2.9*** | 124.6 ± 3.0*** |
| KW/BW | 0.27 ± 0.01 | 0.3 ± 0.01 | 0.68 ± 0.03*** | 0.69 ± 0.00***## | 0.59 ± 0.03***##†† |
Note: The data are reported as mean ± SEM, or median and percentile 25 and 75. Insulin (ng/ml); E2, 17β-estradiol (pg/ml); SBP, systolic blood pressure (mmHg); KW/BW, relative weight of kidneys (g/100 g of BW). ** P < 0.01 and *** P < 0.001 versus C + Se and C + Ex; ## P < 0.01 versus D + Se; †† P < 0.01 versus D + Ex.
Renal function
No difference in serum creatinine was observed among the experimental groups. However, the GFR of the D + Se group was higher than the control groups (P < 0.05), whereas exercise was able to reduce it in both the D + PEx and D + Ex groups (P < 0.05). The proteinuria was higher in the D + Se (P < 0.001) and D + Ex (P < 0.05) groups, whereas previous exercise training reduced the proteinuria in D + PEx group compared to the D + Se (P < 0.001) and D + Ex (P < 0.05) groups. DM induced an increase in urinary flow in diabetic animals compared to the control groups (Table 3).
Table 3.
Renal function data of C + Se, C + Ex, D + Se, D + Ex and D + PEx rats
| C + Se | C + Ex | D + Se | D + Ex | D + PEx | |
|---|---|---|---|---|---|
| Pcreat. | 0.48 ± 0.02 | 0.42 ± 0.06 | 0.44 ± 0.02 | 0.50 ± 0.06 | 0.59 ± 0.07 |
| GFR | 0.29 ± 0.06 | 0.27 ± 0.04 | 0.62 ± 0.1* | 0.36 ± 0.03# | 0.34 ± 0.07# |
| UFlow | 4.5(3.8; 5.0) | 1.4(1.4; 4.1) | 71.7(38.2; 93.8)* | 101.6(94.1; 102.2)* | 96.5(70.1; 99.3)* |
| UProt | 6.56 ± 1.11 | 4.16 ± 1.16 | 30.51 ± 5.33*** | 21.12 ± 2.94* | 9.35 ± 1.59###† |
Note: The data are reported as mean ± SEM, or median and percentile 25 and 75. Pcreat., plasma creatinine (mg/dL); GFR, glomerular filtration rate (ml/min/100 g); UFlow, urine flow (µl/min); UProt, proteinuria (mg/24 h). * P < 0.05 and *** P < 0.001 versus C + Se and C + Ex; # P < 0.05 and ### P < 0.001 versus D + Se; † P < 0.05 versus D + Ex.
Renal morphology
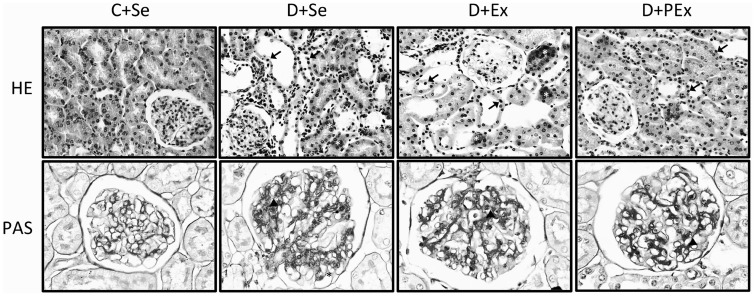
The glomerular tuft area was greater in D + Se group than the control groups (P < 0.01), but exercise prevented this increase in the D + Ex (P < 0.05) and D + PEx (P < 0.01) groups (Table 4). The exercise training also attenuated the increase of the focal segmental glomerulosclerosis in the D + PEx and D + Ex groups compared to the D + Se group (P < 0.01) (Table 4, Figure 2). The tubulointerstitial lesions were more intense in the D + Se (P < 0.001) and D + Ex (P < 0.05) groups, and the previous exercise was able to prevent this effect in the D + PEx group (P < 0.001) (Table 4, Figure 2).
Table 4.
Tubulointerstitial lesions, glomerulosclerotic index, glomerular tuft area and immunostaining area for fibronectin (FN), collagen IV (coll. IV) and TGF-β1 of C + Se, C + Ex, D + Se, D + Ex and D + PEx rats
| C + Se | C + Ex | D + Se | D + Ex | D + PEx | |
|---|---|---|---|---|---|
| TIL | 0.24 ± 0.04 | 0.17 ± 0.02 | 1.38 ± 0.21*** | 0.93 ± 0.12*# | 0.65 ± 0.08### |
| GSI | 0.13 ± 0.01 | 0.16 ± 0.02 | 1.02 ± 0.16*** | 0.61 ± 0.12**## | 0.52 ± 0.02*## |
| AreaGT | 8007 ± 517.0 | 7833 ± 707.0 | 10667 ± 586.7** | 8989 ± 147.6# | 8259 ± 183.5## |
| FN | 7.45 ± 2.8 | 8.55 ± 3.3 | 25.53 ± 2.2** | 11.93 ± 3.3## | 9.42 ± 3.2## |
| Coll. IV | 2.23 ± 0.4 | 2.65 ± 0.4 | 32.26 ± 2.8*** | 26.07 ± 3.4*** | 18.43 ± 1.5***##† |
| TGF-β1 G | 5.22 ± 0.7 | 3.90 ± 1.0 | 18.18 ± 1.8*** | 12.42 ± 2.0** | 4.64 ± 0.9###†† |
| TGF-β1TI | 0.75 ± 0.3 | 0.69 ± 0.2 | 10.61 ± 1.6*** | 9.17 ± 1.2*** | 3.67 ± 1.1###†† |
Note: The data are reported as mean ± SEM. TIL, tubulointerstitial lesions (score); GSI, glomerulosclerotic index (score); AreaGT, glomerular tuft area (µm2); TGF-βG, glomerular transforming growth factor beta 1; TGF-βTI, tubulointerstitial transforming growth factor beta 1. * P < 0.05, ** P < 0.01 and *** P < 0.001, versus C + Se and C + Ex; # P < 0.05, ## P < 0.01 and ### P < 0.001, versus D + Se; † P < 0.05 and †† P < 0.01 versus D + Ex.
Figure 2.
Representative photomicrographs of tubulointerstitial lesions (HE staining) and glomerular histological changes (PAS staining) of C + Se, D + Se, D + Ex, D + PEx rats. Note in D + PEx reductions in tubulointerstitial lesions (arrow) and reductions in glomerular histological changes (arrowhead). Original magnification × 200 in HE and × 400 in PAS
Immunohistochemical studies
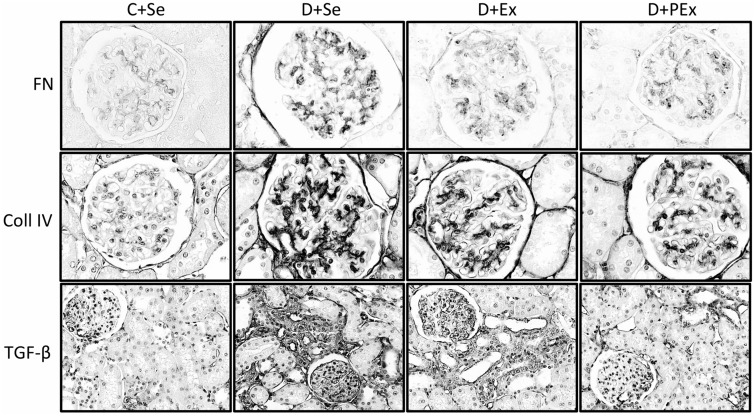
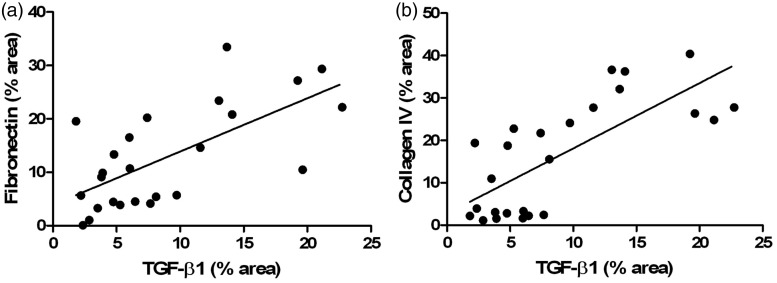
The D + Se group showed increased fibronectin expression in the glomeruli compared to controls groups (P < 0.001), whereas the exercise training was able to attenuate this effect in both D + Ex and D + PEx groups (P < 0.01) (Table 4, Figure 3). All the diabetic groups presented increased expression of collagen IV in glomeruli (P < 0.01), but only the D + PEx group demonstrated lower expression of this protein compared to D + Se (P < 0.001) and D + Ex (P < 0.05) groups (Table 4, Figure 3). Regarding the TGF-β1 expression, there was an extensive immunostaining of this cytokine in both the glomerular mesangium and tubulointerstitial compartment in the D + Se (P < 0.001) and D + Ex (P < 0.01) groups. However, only the previous exercise training was able to attenuate the intensity of immunostaining in D + PEx group compared to D + Se (P < 0.001) and D + Ex (P < 0.01) groups (Table 4, Figure 3). The expressions of fibronectin (r = 0.67; P < 0.001) and collagen IV (r = 0.74; P < 0.0001) were positively correlated to the expression of TGF-β1 (Figure 4), respectively. The photomicrographs of the C + Ex group are not presented due to similarity between the immunohistochemical and histological pattern of D + Se and C + Ex groups.
Figure 3.
Immunolocalization of fibronectin, collagen IV and TGF-β1 in the renal cortex of C + Se, D + Se, D + Ex, D + PEx rats. Note in D + PEx reductions in immunostaining of fibronectin (FN), collagen IV (coll IV) and TGF-β (transforming growth factor beta 1). Original magnification × 400 in FN and coll IV, and × 200 in TGF-β
Figure 4.
Correlation between the expressions of fibronectin and TGF-β1 (a) and collagen IV and TGF-β1 in glomeruli of C + Se, C + Ex, D + Se, D + Ex and D + PEx. n = 5 for all groups. (a) Pearson rank coefficient r = 0.67, P < 0.001, and (b) Spearman rank coefficient r = 0.74, P < 0.0001
Discussion
The present study investigated the renoprotective effects of exercise training on renal function and structure changes in STZ-induced diabetic female rats. Our data demonstrate that aerobic exercise performed prior to induction of DM attenuated the loss of BW, blood glucose, increase of GFR, and considerably reduced proteinuria in diabetic rats. The previous exercise also reduced the glomerular hypertrophy, tubular and glomerular injury, expressions of extracellular matrix proteins and TGF-β1 in glomeruli and renal cortex of these animals.
There are evidences suggesting an influence of gender on the incidence and progression of nondiabetic renal disease, and 17β-estradiol have been reported to exert renoprotective actions in both experimental animals and women of fertile age.2–5 However, the influence of gender on diabetic renal disease is controversial. While some studies have demonstrated that diabetic nephropathy progress is faster in males,29,30 others indicate the opposite,31,32 suggesting that the renoprotection conferred to females is lost in the presence of DM, possibly due to a dysregulation of sex hormones.5,6 In this study, DM did not induce changes in 17β-estradiol levels, suggesting that our findings were independent of the levels of this hormone.
Previous exercise training reduced the hyperglycemia and the weight loss, and increased physical capacity in the D + PEx group indicating an improvement in metabolic capacity in these animals, which, at least in part, may have contributed to the attenuation of diabetes pathophysiologic complications investigated in this study. These findings are in accordance with other studies, in which the aerobic training presented beneficial effects over the metabolic profile, with better glycemic control and reduced weight loss in STZ-induced diabetic rats.20,33. Additionally, Silva et al. demonstrated that moderate aerobic exercise performed previously to induction of DM was more effective in improving metabolic profile than exercise performed after disease onset.21 Although some studies have shown the possibility of restoration of insulin production in STZ-induced DM,34,35 our data show that the insulin concentrations were low in diabetic rats, without differences between trained and untrained rats. Differently, the C + Ex group had decreased insulin concentrations compared to C + Se group, possibly due to better sensitivity to this hormone induced by exercise training. Studies have shown that the deformity of skeletal muscle cell owing to muscle contraction is a key signal to stimulate the GLUT-4 translocation independently of insulin, allowing higher sensitivity to this hormone and increased glucose uptake by skeletal muscle.36,37
Renal function changes observed in D + Se group were characterized by increases in GFR, proteinuria and urinary flow. However, the exercise training attenuated the increase in GFR in all diabetic rats and prevented the increase of proteinuria in the D + PEx group. The literature presents conflicting results about the effects of exercise training on the renal function and proteinuria, and evidence suggests that the intensity of physical training program is determinant in the renal response. Thus, some studies showed that intense physical exercise increased albuminuria in both experimental animals38 and humans,39 while moderate-intensity regular exercise prevented albuminuria/proteinuria in STZ-induced diabetic rats.20,21,40 Moderate exercise also prevented reduction in GFR and attenuated albuminuria in spontaneously hypertensive rats23 and in diabetic and cardiopathic human.24,25 Although the mechanisms by which moderate exercise training improves renal function are not fully understood, there are evidences indicating that the better metabolic control, reduction of oxidative stress, and increased production and bioavailability of NO may be involved in this process.20,21,23,40 In fact, aerobic training during eight weeks induced better metabolic control, reduction of thiobarbituric acid reactive substance (TBARS), and increased NO production, delaying the progression of diabetic nephropathy in STZ-induced diabetic rats.20
Our results show increase in systolic blood pressure in all diabetic groups when compared to control groups, regardless of physical exercise. Studies demonstrated reduction in systolic arterial pressure due to impaired autonomic function, while the exercise was able to reverse this effect in type 1 diabetic rats.21,33 On the other hand, in accordance with our study, it was demonstrated that increased blood pressure due to the exacerbated renin-angiotensin-aldosterone system activation in STZ-induced diabetic rats.41,42 Therefore, the increase in systolic arterial pressure observed in this study may be due, at least in part, to increased serum levels of angiotensin II. However, future studies are needed to confirm this finding. The best blood pressure control is also one of the mechanisms by which exercise exerts protective action against the development of renal damage,23 but in our study the mechanisms involved were independent of blood pressure, since there was no difference in parameter between trained and untrained diabetic animals.
Since the progressive accumulation of ECM is strongly correlated to the development of renal failure,10,12 the effects of exercise on this pathological feature of diabetic nephropathy have particular clinical importance. The expansion of mesangial matrix in early stage of diabetic nephropathy may occur by increased accumulation of proteins that are normally present in these structures, such as fibronectin and type IV collagen.43 During tissue remodeling, collagens have numerous binding partners and among those critical for fibrillar assembly include fibronectin, collagen-binding integrins, and latent TGF-binding proteins.44 In renal fibrotic diseases, fibronectin is the first protein to be deposited, constituting a scaffold for the deposition of other proteins and acting as a fibroblast chemoattractant.45 As expected, our results demonstrated increased expression of extracellular matrix proteins in the D + Se group, whereas the exercise prevented the accumulation of fibronectin and only the previous exercise attenuated the accumulation of collagen IV in glomeruli of the D + PEx group. Furthermore, D + PEx group rats also showed reduction in glomerular and tubulointerstitial lesions and hypertrophy. Rodrigues et al. demonstrated that moderate exercise prevented the mesangial expansion and reduced vacuolar degenerative changes in the tubules of STZ-induced diabetic rats.20 Aerobic training was associated with less mesangial volume expansion, and less glomerular basement membrane thickening in a model of obesity-related diabetes46 and in STZ-induced diabetic Sprague–Dawley rats.40 Up to this time, there are no studies evaluating the effect of exercise, especially the previous exercise, on the regulation of ECM proteins, and the mechanisms by which it promotes this probable protective action are still unclear. However, it is reasonable to suppose that the likely increase in NO production induced by exercise could be involved in this process. Studer et al. showed that NO inhibited TGF-β bioactivity and fibronectin synthesis, suppressing the stimulation of mesangial cell and matrix protein synthesis in diabetic rats.47
Our data demonstrate that the increased expression of matrix proteins in the D + Se group was followed by increased expression of TGF-β1, whereas previous exercise also reduced the expression of this cytokine in the D + PEx group. Furthermore, the expressions of fibronectin and collagen IV were positively correlated to the expression of TGF-β1. Several studies have demonstrated the involvement of hypertrophic and pro-sclerotic cytokine TGF-β1 in the progression of diabetic nephropathy, especially mediating ECM accumulation and fibrosis development.8,10,13 The starting stimulation of TGF-β1 in diabetic kidney is principally induced by hyperglycemia,10 glycation of proteins,11 and increased angiotensin II.12 In 1994, Sharma and Ziyadeh showed that an early hyperglycemic state stimulated the production of fibronectin and collagen IV in the kidney of STZ-induced diabetic mice. The authors described that such effect seemed to be mediated by TGF-β1, since the upregulation of this cytokine and its respective receptor were followed by an upregulation of fibronectin and collagen IV genes.13 However, no previous study reported the effects of previous exercise training on the structural and hypertrophic changes induced by DM.
In conclusion, our data show that regular exercise training of moderate intensity performed previously to induction of DM improved metabolic control and attenuated the renal function and structure changes. Our study also shows that previous exercise reduced the expression of collagen IV, and this effect was associated with reduced expression of TGF-β1. Taken together, these findings suggest that the regular previous exercise training is effective in protecting the kidney against the progression of diabetic nephropathy in STZ-induced diabetic female rats.
Acknowledgments
We thank the financial support of Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We also thank to Samira I Sousa, Jussara A Silva, Jéssica Dias, Cláudia S Souza, Emanuele P Santos, and Paulo HB Lima, students in our laboratory, for taking part in some aspects of these studies.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; LSBA and TJS conceived and designed the experiments; LSBA, FAS, VBC, CEFA, BAD, RAV and MVO performed the experiments; LSBA, TJS, TMC, ACMM, RAV and ACS analyzed the data; TMC, ACMM, RAV and ACS contributed reagents/materials/analysis tools; LSBA, TJS and RAV contributed to the writing of the manuscript.
References
- 1.Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J 2014; 38: 252–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Torres I, Roque P, El Hafidi M, Diaz-Diaz E, Baños G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Radic Res 2009; 43: 761–71. [DOI] [PubMed] [Google Scholar]
- 3.Amaral LS, Silva JA, Trindade TM, Ribas WB, Macedo CL, Coimbra TM, Soares TJ. Renal changes in the early stages of diet-induced obesity in ovariectomized rats. Physiol Res 2014; 63: 723–32. [DOI] [PubMed] [Google Scholar]
- 4.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 2000; 11: 319–29. [DOI] [PubMed] [Google Scholar]
- 5.Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol 2005; 288: F399–405. [DOI] [PubMed] [Google Scholar]
- 6.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med 2005; 2: 227–37. [DOI] [PubMed] [Google Scholar]
- 7.Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis 2013; 20: 390–95. [DOI] [PubMed] [Google Scholar]
- 8.Dixon A, Maric C. 17β-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-protein expression and signaling. Am J Physiol Renal Physiol 2007; 293: F1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma K, McGowan TA. TGF-beta in diabetic kidney disease: role of novel signaling pathways. Cytokine Growth Factor Rev 2000; 11: 115–23. [DOI] [PubMed] [Google Scholar]
- 10.Volpini RA, Silva CGA, Costa RS, Coimbra TM. Effect of enalapril and losartan on the events that precede diabetic nephropathy in rats. Diabetes Metab Res Rev 2003; 19: 43–51. [DOI] [PubMed] [Google Scholar]
- 11.Bangstad HJ, Osterby R, Dahl-Jørgensen K, Berg KJ, Hartmann A, Nyberg G. Early glomerulopathy is present in young, type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 1993; 36: 523–29. [DOI] [PubMed] [Google Scholar]
- 12.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 2014; 5: 1–13. DOI: 10.3389/fendo.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-beta1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol 1994; 267: F1094–1101. [DOI] [PubMed] [Google Scholar]
- 14.Ziyadeh FN, Sharma K, Ericksen M, et al. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by activation of transforming growth factor-beta. J Clin Invest 1994; 93: 536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonçalves JG, de Bragança AC, Canale D, Shimizu MH, Sanches TR, Moysés RM, Andrade L, Seguro AC, Volpini RA. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. Plos ONE 2014; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wensing LA, Campos AH. TBX3, a downstream target of TGF-β1, inhibits mesangial cell apoptosis. Exp Cell Res 2014; 328: 340–50. [DOI] [PubMed] [Google Scholar]
- 17.Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: New strategies are needed to retard progressive renal function decline. Semin Nephrol 2012; 32: 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agha A, Bashir K, Anwar E. Use of losartan in reducing microalbuminuria in normotensive patients with type-2 diabetes mellitus. Nepal Med Coll J 2007; 9: 79–83. [PubMed] [Google Scholar]
- 19.Smith AC, Burton JO. Exercise in kidney disease and diabetes: time for action. J Ren Care 2012; 38: 52–58. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues AM, Bergamaschi CT, Araújo RC, Mouro MG, Rosa TS, Higa EM. Effects of training and nitric oxide on diabetic nephropathy progression in type I diabetic rats. Exp Biol Med 2011; 236: 1180–7. [DOI] [PubMed] [Google Scholar]
- 21.Silva KAS, Luiz RS, Rampaso RR, Abreu NP, Moreira ED, Mostarda CT. Previous exercise training has a beneficial effect on renal and cardiovascular function in a model of diabetes. Plos ONE 2012; 7: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurdak H, Sandikci S, Ergen N, Dogan A, Kurdak SS. The effects of regular aerobic exercise on renal functions in streptozotocin induced diabetic rats. J Sports Sci Med 2010; 9: 294–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal D, Elks CM, Reed SD, Mariappan N, Majid DS, Francis J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid Redox Signal 2012; 16: 139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarevic G, Antic S, Vlahovic P, Djordjevic V, Zvezdanovic L, Stefanovic V. Effects of aerobic exercise on microalbuminuria and enzymuria in type 2 diabetic patients. Ren Fail 2007; 29: 199–205. [DOI] [PubMed] [Google Scholar]
- 25.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 2009; 169: 2116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int 1987; 32: 691–9. [DOI] [PubMed] [Google Scholar]
- 27.Shih W, Hines WH, Neilson EG. Effects of cyclosporin A on the development of imune-mediated interstitial nephritis. Kidney Int 1988; 33: 1113–8. [DOI] [PubMed] [Google Scholar]
- 28.Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 2000; 57: 167–82. [DOI] [PubMed] [Google Scholar]
- 29.Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of end-stage renal disease in people with diabetes in the United States population: Do we know the cause? Kidney Int 2005; 67: 1684–91. [DOI] [PubMed] [Google Scholar]
- 30.Sibley SD, Thomas W, de Boer I, Brunzell JD, Steffes MW. Gender and elevated albumin excretion in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) cohort: Role of central obesity. Am J Kidney Dis 2006; 47: 223–32. [DOI] [PubMed] [Google Scholar]
- 31.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990; 39: 1116–24. [DOI] [PubMed] [Google Scholar]
- 32.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carroll TA, Stratton I, Gale EA. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Diab Care 1999; 22: 495–502. [DOI] [PubMed] [Google Scholar]
- 33.Souza SB, Flues K, Paulini J, Mostarda C, Rodrigues B, Souza LE. Role of exercise training in cardiovascular autonomic dysfunction and mortality in diabetic ovariectomized rats. Hypertension 2007; 50: 786–91. [DOI] [PubMed] [Google Scholar]
- 34.Desgraz R, Bonal C. Herrera PL. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab 2011; 22: 34–43. [DOI] [PubMed] [Google Scholar]
- 35.Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014; 514: 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguri M, Adachi H, Ohno T, Oshima S, Kurabayashi M. Effect of a single bout of moderate exercise on glucose uptake in type 2 diabetes mellitus. J Cardiol 2009; 53: 8–14. [DOI] [PubMed] [Google Scholar]
- 37.Frøsig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity 2009; 17: S15–20. [DOI] [PubMed] [Google Scholar]
- 38.Gündüz F, Kuru O, Sentürk UK. Effect of nitric oxide on exercise-induced proteinuria in rats. J Appl Physiol 2003; 95: 1867–72. [DOI] [PubMed] [Google Scholar]
- 39.Christensen CK. Abnormal albuminuria and blood pressure rise in incipient diabetic nephropathy induced by exercise. Kidney Int 1984; 25: 819–23. [DOI] [PubMed] [Google Scholar]
- 40.Albright AL, Mahan JD, Ward KM, Sherman WM, Roehrig KL. Diabetic nephropathy in an aerobically trained rat model of diabetes. Med Sci Sports Exerc 1995; 27: 1270–77. [PubMed] [Google Scholar]
- 41.Peeri M, Habibian M, Azarbayjani MA, Hedayati M. Protective effect of aerobic exercise against L-NAME-induced kidney damage in rats. Arh Hig Rada Toksikol 2013; 64: 229–35. [DOI] [PubMed] [Google Scholar]
- 42.Goyal BR, Mesariya P, Goyal RK, Mehta AA. Effect of telmisartan on cardiovascular complications associated with streptozotocin diabetic rats. Mol Cell Biochem 2008; 314: 123–31. [DOI] [PubMed] [Google Scholar]
- 43.Musial DC, da Silva Júnior ED, da Silva RM, Miranda-Ferreira R, Lima-Landman MT, Jurkiewicz A, García AG, Jurkiewicz NH. Increase of angiotensin-converting enzyme activity and peripheral sympathetic dysfunction could contribute to hypertension development in streptozotocin-induced diabetic rats. Diab Vasc Dis Res 2013; 10: 498–504. [DOI] [PubMed] [Google Scholar]
- 44.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 2003; 14: 1358–73. [DOI] [PubMed] [Google Scholar]
- 45.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 2010; 21: 1819–34. [DOI] [PubMed] [Google Scholar]
- 46.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 1996; 7: 2495–508. [DOI] [PubMed] [Google Scholar]
- 47.Ward KM, Mahan JD, Sherman WM. Aerobic training and diabetic nephropathy in the obese Zucker rat. Ann Clin Lab Sci 1994; 24: 266–77. [PubMed] [Google Scholar]
- 48.Studer RK, De Rubertis FR, Craven PA. Nitric oxide suppresses increases in mesangial cell protein kinase C, transforming growth factor beta, and fibronectin synthesis induced by thromboxane. J Am Soc Nephrol 1996; 7: 999–1005. [DOI] [PubMed] [Google Scholar]