Abstract
Hypertension frequently results in severe complications in cardiovascular system and histopathological changes in the heart. To better understand the cellular processes and signaling pathways responsible for the proper functioning of the heart, we decided to check whether doxazosin affects the density of structures containing S100A6 and atrial natriuretic factor in the heart of spontaneously hypertensive rats. The aim of this study is to find differences in the density of the structures containing S100A6 and atrial natriuretic factor in the heart of spontaneously hypertensive rats treated with doxazosin compared to untreated animals. Fragments of heart were collected from five spontaneously hypertensive rats and five spontaneously hypertensive rats receiving doxazosin for six weeks (dose 0.1 mg per 1 kg of body weight). On the paraffin sections S100A6 and atrial natriuretic factor peptides were localized in the heart using immunohistochemistry. Positive immunohistochemical reaction for S100A6 was observed in atrial and ventricular cardiomyocytes and in the coronary vasculature. In the heart of hypertensive rats treated with doxazosin the S100A6 immunoreactivity was significantly lower compared to untreated animals. Immunodetection of atrial natriuretic factor in the heart of rats confirmed presence of peptide in atrial myocardium. Delicate atrial natriuretic factor-immunoreactivity was observed also in few ventricular cardiomyocytes. The atrial natriuretic factor-immunosignal was significantly weaker in hearts of hypertensive rats receiving doxazosin compared to spontaneously hypertensive rats untreated. Since we found that doxazosin reduces the levels of S100A6 and atrial natriuretic factor peptides in the heart of spontaneously hypertensive rats, it can be assumed that cardiovascular disorders that occur in hypertension may be associated with disturbances of cellular processes and signaling pathways.
Keywords: S100A6, ANF, heart, rat, spontaneously hypertension, doxazosin
Introduction
Hypertension is one of the most prevalent diseases worldwide. It was estimated that approximately 36–56% of the middle-aged population suffers from elevated blood pressure values.1 The disease frequently results in severe complications in cardiovascular system, such as heart failure, cardiac ischemia or myocardial infarction.2 Hypertension leads to histopathological changes in cardiac tissue including hypertrophy, fibrosis and inflammation, as well as remodelling of heart and vascular wall.3 In addition, abnormalities in myocardial mechanics were observed in patients with hypertension.4 A comparative evaluation of cardiomyocytes contractility in hypertensive and normotensive rats demonstrated that under hypertension conditions cardiac muscle cells contracted much slower and with lower force.5 This pathological state contributes also to more frequent cardiomyocytes apoptosis.6
Function of cardiac muscle cells is strictly dependent on calcium ions, which play a major role in the activation of signal transduction pathways and regulate contractile performance. There are various calcium-binding proteins determining intracellular calcium cycling. Among them, increasing importance is attributed to S100 protein family. S100 family comprises low molecular proteins with two EF-hand type calcium-binding domains. Several members such as S100A1, S100A2, S100A6, and S100B were identified in heart tissue.7–9
Ultrastructural localization of S100A1 in cardiomyocytes demonstrated that this peptide interacts with sarcoplasmic ryanodine receptor and sarco/endoplasmic reticulum Ca2+ ATPase (SERCA 2) – receptors responsible for Ca2+ transients during contraction cycle.8,9 Studies on mice and rabbits indicated that overproduction of S100A1 enhances cardiomyocytes contractility, whereas inactivation of S100A1 gene results in diminished contraction of cardiac muscle cells.10–12 Considering that mice with a deletion of S100A1 gene had elevated systolic blood pressure, it might be assumed that this protein is involved in hypertension-associated impaired functionality in cardiomyocytes.13,14
Another member of the S100 family, S100A6 play a significant role in the regulation of cardiomyocytes differentiation and proliferation.15 It was indicated that S100A6 has a beneficent effect on cardiac muscle cell viability. Most et al.16 show that S100A1 prevents cardiac muscle cells death by activation of extracellular signal-regulated protein kinase 1/2 (ERK1/2). Tsoporis et al.17,18 demonstrated that S100A6 reduced cardiac myocytes loss induced by tumor necrosis factor-alpha (TNF-α) treatment. In consecutive studies, the authors found that S1006 attenuates cardiomyocytes hypertrophy and apoptosis in mice with myocardial infarction.
Beside impairment of myocardium contractility, hypertension also disturbs heart endocrine function. Larochelle et al.19 indicated that patients with essential and renovascular hypertension have elevated atrial natriuretic factor (ANF) concentration in blood. Increased ANF plasma content was also noticed in rats subjected to different hypertension models.20,21 Furthermore, studies of hypertensive animals revealed enhanced ANF content in heart homogenates.20,21 Considering that fluctuation of calcium ions in myocytes changes expression of ANF, it might be suspected that S100 proteins will affect ANF biosynthesis. This assumption was confirmed by Most et al.16 and Pleger et al.14 who demonstrated that S100A1 gene transduction weakened the rise of ANF production occurring after myocardial infarction.
Because of high mortality of hypertensive patients, researchers and clinicians keep on making every effort to improve the effectiveness of treatment of elevated blood pressure. Doxazosin – an antagonists of α1 adrenoreceptors – is one of the recently launched medication to treat hypertension. It was found that doxazosin decreases the activity of sympathetic nervous system, causes relaxation of vascular smooth muscle, and thus lowers blood pressure in patients with essential hypertension.22 Unfortunately, doxazosin increased the risk of chronic heart failure in patients.23 Moreover, in vitro studies demonstrated that doxazosin induces cardiomyocytes apoptosis.24 Given that cardiomyocytes isolated from mice with deleted gene for α1 adrenoreceptors had faster contraction rates, it might be suspected that block of those receptor by doxazosin could affect cardiac contractility.25 This issue has not been, however, explored so far.
Up to now, there are no reports concerning localization of S100A6 and ANF in heart of hypertensive rats nor influence of doxazosin treatment on proteins distribution in cardiac tissue. For this reason, it appears worth undertaking the immunohistochemical identification and evaluation of, S100A6- and ANF-positive structures in heart of rats with spontaneous hypertension with and without doxazosin treatment.
Materials and methods
Experimental model
Male spontaneously hypertension rats were purchased from Polish Mother’s Memorial Hospital Research Institute in Lodz, Poland. All experimental procedures involving animals and their care were approved by local authorities and conducted in conformity with the national and international laws and Guidelines for the Use of Animals in Biochemical Research.26 Study assumptions, aim, schedule and model of animal treatment were approved by the Senate Committee for Supervision of Experiments on Humans and Animals, Medical University of Białystok Nr 2001/16.
The study was performed on 10 young male Wistar rats (6 weeks of age), whose body weight at the beginning of the experiment was within 180–200 g (the mean body weight: 190 ± 10 g). The animals were kept in lighted and ventilated conditions with room temperature and maintained day and night rhythm. The rats had a free access to standard granulated chow, and drinking water was available but were fasted overnight (16–18 h) before the experiment.
The animals were divided into two equal groups: control groups – five spontaneously hypertensive rats (SHR) (180 ± 6) and experimental group – five SHR treated with doxazosin (0.1 mg/1 kg b. w. in drinking water per day), similar in terms of baseline parameters. After six weeks, the experiment rats were anesthetized by pentobarbital, administered interperitoneally at a dose of 50 mg/kg; then the hearts were immediately collected. Subsequently, the hearts were fixed in Bouin’s fluid for 24 h at +4℃, and processed routinely for embedding in paraffin. Sections were cut at 4 µm in thickness, and processed by immunohistochemistry for S100A6 and ANF detection.
Identification of S100 proteins and ANF by immunohistochemical methods
Immunohistochemistry was performed using the EnVision Plus-HRP Detection Kit (K4011 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) for rabbit antibody. Paraffin-embedded sections were deparaffinized and hydrated in pure alcohols. For antigen retrieval, the sections were subjected to pretreatment in a pressure chamber for 1 min at 21 psi at 125℃, using the Target Retrieval Solution with ph 6.0 (S 2369 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) for S100A6 and using Target Retrieval Solution with pH 9.0 (S 2367, Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) for ANF (S 2367 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup). After being cooled to room temperature, sections were incubated with the Peroxidase Blocking Reagent (S 2001 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) for 10 min, to block endogenous peroxidase activity. Subsequently, sections were incubated with primary antibody for S100A6 (purchased from Nencki Institute of Experimental Biology, produced in-house) and for ANF (polyclonal rabbit ANF antiserum, H-005-24, purchased at the Phoenix Pharmaceuticals, Inc., Mountain View, CA). The antisera for S100A6 and ANF were previously diluted in Antibody Diluent (S 0809 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) in a ratio of 1:500 and 1:2000, respectively. Sections with S100A6 antibody were incubated overnight at 4℃ in a humidified chamber, whereas incubation with ANF–antibody lasted 1 h and was performed at room temperature. The procedure was followed by incubation with secondary antibody (conjugated to horseradish peroxidase-labelled polymer). The bound antibodies were visualized by 1-min incubation with liquid 3,3′–diaminobenzidine substrate chromogen. The sections were finally counterstained in hematoxylin QS (H – 3404, Vector Laboratories; Burlingame, CA), mounted and evaluated under light microscope. Appropriate washing with Wash Buffer (S 3006 Dako Denmark A/S, Produktionsvej 42, DK-2600 Glostrup) was performed between each step. Negative control was carried out by incubating sections with the diluent and normal serum instead of the primary antiserum. All the performed control reactions gave negative results, and positive control was conducted for specific tissue recommended by producers.
Microscopic and quantitative analysis
Five rats were used for each studied group and five specimens of each antibody of each animal were observed and photomicrographed under the Olympus BX41 light microscope, with a digital camera (Olympus DP12) and a standard morphometric program (NIS-Elements Advanced Research software of Nikon) installed on computer.
The results of immunoreactive structure with S10A6 and ANF expression were searched for and observed their location.
From all heart sections, five randomly selected microscopic fields (each field of 0.785 mm2, magnification of 200× (20× the lens and 10× the eyepiece)) were documented. In each analyzed image of heart, the width of 25 randomly selected cardiomyocytes was measured. In software for image analysis was also measured intensity of immunohistochemical reaction with S100A6 and ANF antibody. Intensity of immunohistochemical reaction was determined using 0 to 256 gray scale level, where a completely black pixel got a value of 0, whereas one with a value of 256 is completely white or bright.
Statistical analysis
All presented data were statistically analyzed by means of software computer package Statistica Version 10.0. The corresponding mean values were computed automatically; significant differences were determined by Student’s t-test; p < 0.05 was taken as the level of significance.
Results
The antisera against S100A6 and ANF gave positive results in the hearts of all rats studied; however, the density of S100A6- and ANF-containing structures and intensity of reactions varied between the groups studied.
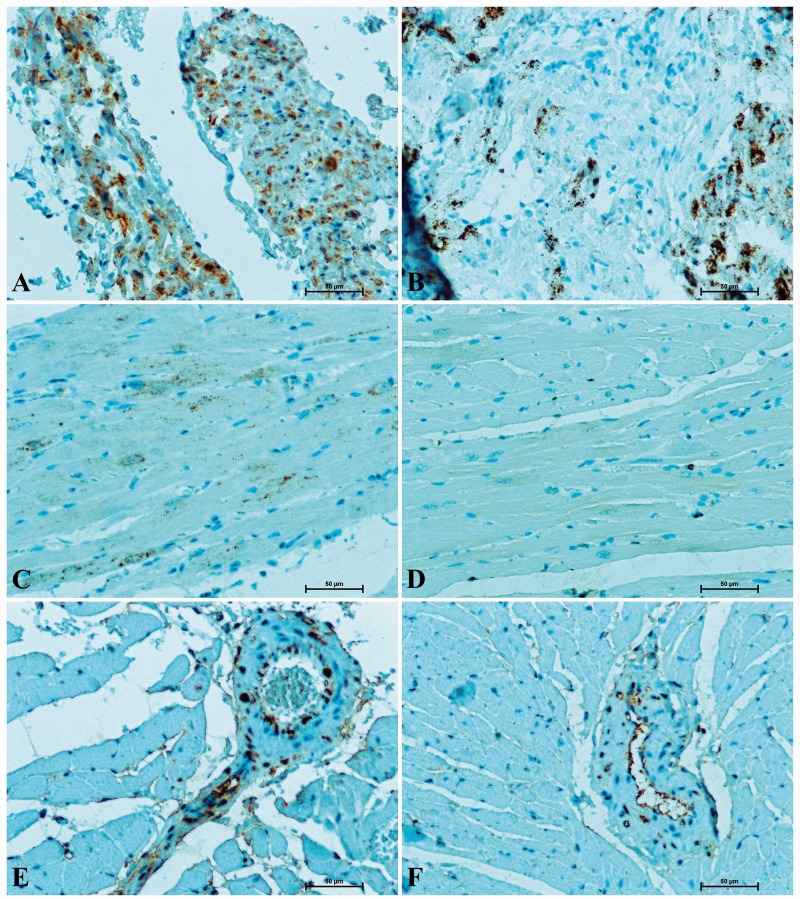
S100A6 protein was identified in atrial and ventricular myocardium. The S100A6-immunoreactivity was more intense in atrial cardiomyocytes than in ventricular ones (Figure 1 (a) to (d)). In S100A6-immunopositive cardiomyocytes, immunosignal was the most intense in the vicinity of the cell nucleus. S100A6 protein was also found in endothelial cells and smooth muscle cells of coronary vasculature (Figure 1 (e) and (f)). In the hearts of hypertensive rats, the immunoreaction in cardiomyocytes was strong (atrium) or moderate (ventricle) (Figure 1(a) and (c)), while in rats which had undergone doxazosin treatment the intensity of S100A6-staining in cardiac muscle cells was weak (Figure 1(b) and (d)). Similarly, in rats receiving doxazosin a less intense S100A6-immunosignal in vessels supplying the heart wall was noticed (Figure 1(f)) when compared to untreated SHR (Figure 1(e)).
Figure 1.
Immunodetection of S100A6 in heart of rat: (A) SHR – strong S100A6-reactivity in the atrial myocardium; (B) SHR with doxazosin treated – less numerous atrial muscle cells stained with anti-S100A6; (C) SHR – immunoreactivity with S100A6 in ventricular cardiomyocytes; (D) SHR receiving doxazosin – considerably weaker S100-immunosignal in ventricular myocardium; (E) rat with spontaneous hypertension – intense reaction in the wall of coronary vasculature; (F) SHR with doxazosin treated – delicate signal for S100A6 in vessels supplying heart wall. (A color version of this figure is available in the online journal.)
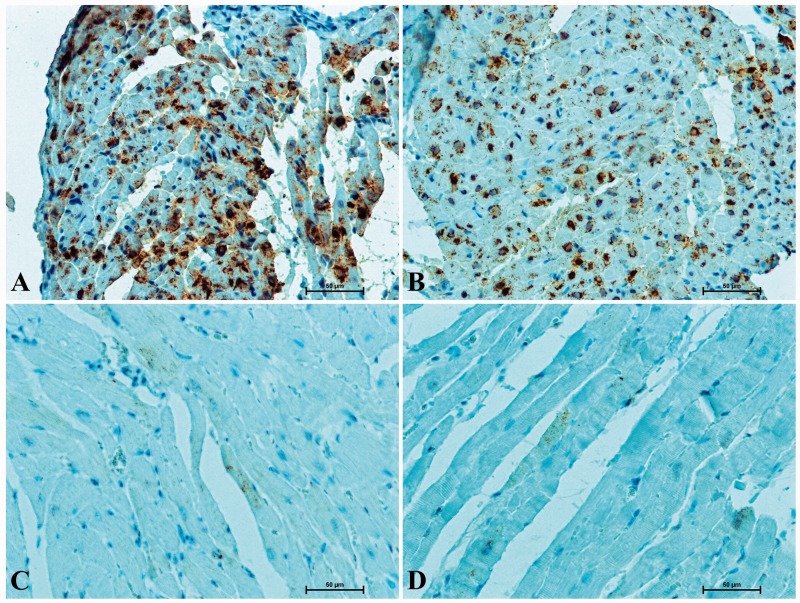
Immunohistochemical detection of ANF in the hearts of rats revealed its presence in atrial cardiomyocytes (Figure 2(a) and (b)). In the cytoplasm of atrial muscle cells darkly stained secretory granules were observed. A weak ANF-immunosignal was also noticed in a number of ventricular cardiomyocytes (Figure 2(c) and (d)). In the heart of SHR, numerous atrial muscle cells displayed strong immunostaining for ANF (Figure 2(a)). In the rats receiving doxazosin the presence of ANF was observed in a smaller number of atrial cardiomyocytes and the intensity of immunohistochemical reaction was significantly lower (Figure 2(b)) when compared to the animals not treated with doxazosin.
Figure 2.
Positive ANF-immunostaining in heart of rat (A) SHR – very strong immunosignal in majority of atrial cardiomyocytes, (B) SHR with doxazosin treated – less numerous atrial muscle cells with ANF-immunoreactivity; (C) SHR – delicate ANF-reaction in some of ventricular cardiomyocytes; (D) SHR receiving doxazosin – single ventricular muscle cells with weak ANF-immunoreactivity. (A color version of this figure is available in the online journal.)
Computer image analysis confirmed weakening of the immunohistochemical reactions for S100A6 and ANF in the hearts of rats treated with doxazosin compared to drug-naive SHR (Table 1).
Table 1.
Intensity of immunohistochemical reaction with S100A6 and ANF antibody and the width (µm) of cardiomyocytes in hearts of control rats, untreated doxazosin SHR and doxazosin treated SHR (mean ± SD)
| Group of rats | Width of cardiomyocytes (µm) | Intensity of IR for S100A6 | Intensity of IR for ANF |
|---|---|---|---|
| SHR rats | 13.5 ± 1.76 | 91.7 ± 20.00 | 59.4 ± 28.30 |
| SHR rats treated with doxazosin | 11.9 ± 2.30 | 144.9 ± 24.48 | 64.7 ± 31.09 |
| p | <0.0001 | <0.0001 | 0.0082 |
IR: immunoreactivity.
Morphometric studies showed a smaller diameter of cardiomyocytes of SHR treated with doxazosin compared to the myocardial cells of hypertensive rats receiving no medication (Table 1).
Discussion
Despite extensive research into the pathogenesis of hypertension and disease-related organ damage, the mechanisms leading to complications in the cardiovascular system of hypertensive patients are still not fully understood.
Myocardial contractile performance and the survival of cardiomyocytes are determined by calcium-binding proteins belonging to the S100 protein family. Recent studies indicate that cardiomyocytes function is also modulated by ANF which reduces the proliferation and contraction ability of these cells. However, it is clear from the review of the available literature that there are no reports evaluating changes in the distribution of S100 proteins and ANF in hypertensive heart disease. Clinical and experimental data show that doxazosin, which has recently been used in hypertension treatment, significantly affects cardiac action but to date no research on whether the drug influences the expression of the aforementioned peptides in cardiac tissue has been conducted.
These are the first studies concerning the immunohistochemical identification and comparative assessment of the S100A6 and ANF peptides in the hearts of rats with spontaneous hypertension treated and untreated with doxazosin.
Immunolabelling of S100A6 protein in rat hearts gave a positive reaction in atrial and ventricular cardiomyocytes and coronary vasculature in all the studied rats. The present studies demonstrate that the blockage of α1-adrenoceptors with doxazosin in SHR significantly lowered S100A6 immunoreactivity in heart tissue. Our findings, and studies by Tsoporis et al.17,27,28 who showed that the stimulation of the a1-adrenergic receptors caused an increase in the biosynthesis of S100 protein in rat cardiomyocytes, suggest a significant role of a1-adrenoceptors in the regulation of S100A6 gene expression.
The changing hemodynamic conditions in hypertension lead to the remodelling of the heart wall and cardiac hypertrophy. There is evidence that hypertension-associated heart hypertrophy might be invoked by the activation of the α1-adrenergic receptors. During hypertension, the level of norepinephrine (NE), which stimulates α1-adrenoceptors, is significantly increased. Tsoporis et al.28 demonstrated that α1-adrenoceptors agonist phenylephrine (PE) induced the biosynthesis of the skeletal α-actin (skACT) and β-myosin heavy chain (β-MHC) in rat cardiomyocytes, and resulted in an increase in the number of these cells. Other researchers have observed that the inhibition of a1-adrenoceptor with doxazosin attenuated cardiac hypertrophy in rats with ventricular pressure-overload.29 A reduction of ventricular hypertrophy after doxazosin treatment was also observed in patients with essential hypertension.30
Our results confirm a reduction in the diameter of cardiac muscle cells in rats with spontaneous hypertension treated with doxazosin compared to untreated SHR.
Literature data suggest that S100A6 protein might be involved in the adaptive processes limiting progression of heart hypertrophy in hypertension. Tsoporis et al.18,28 stated that S100A6 attenuated cardiomyocytes hypertrophy induced by PE. The weakening of S100A6-reaction in the hearts of SHR receiving doxazosin, demonstrated by the current study, might be related to the properties of the drug associated with the inhibition of the heart cells hypertrophy.
The in vitro studies indicate that doxazosin induces cell apoptosis by the upregulation of Bax, caspase-3, caspase-8 gene and the downregulation of the Bcl-2 gene.31 On the basis of the latest reports it might be assumed that S100A6 is implicated in this process. Joo et al.32 showed that S100A6 protein promoted cell death by increasing caspase-3 level. Słomnicki et al.33 demonstrated that S100A6 increased the transcription of p53 protein and enhanced cell sensitivity to oxidative stress. On the other hand, other researchers have demonstrated the beneficial effect of S100A6 on cardiomyocytes viability. Tsoporis et al.17 noticed that S100A6 prevented apoptosis of cardiomyocytes caused by TNFα. Some authors showed that the induced overexpression of S100A6 protein decreased cardiomyocytes loss in rats with experimental myocardial infarction.18 The decreased intensity of S100A6-immunostaining in the hearts of SHR treated with doxazosin observed in the present study, might be explained by the compensatory mechanism attenuating cardiomyocytes death, or might be one of the mechanisms of the proapoptotic action of doxazosin. This matter requires further research in order to gain a better understanding of the role of S100A6 protein in determining cardiomyocytes viability under physiological and pathological conditions.
Immunohistochemical identification of ANF in the hearts of rats confirmed the presence of the peptide in atrial myocardium. A weak ANF-reaction was also observed in a number of ventricular cardiomyocytes. The ANF-immunosignal was significantly weaker in the hearts of hypertensive rats receiving doxazosin in comparison to untreated SHR. Our findings are consistent with reports by Sakata et al.34 who indicated that the blockage of a1-adrenenoceptor with doxazosin decreased ANF production.
Multiple mechanisms including the increased activity of renin-angiotensin-aldosterone system (RAA) and the activation of the hypothalamic-pituitary-adrenal axis (HPA-axis) are involved in the pathogenesis of hypertension. Both systems participate not only in stress response, vascular resistance regulation and water-mineral balance, but also modulate ANF biosynthesis in the heart.
Experimental studies have demonstrated that the activity of the HPA axis is regulated by a1-adrenoceptors. Shimizu35 stated that agonists (NE and PE) of a1-adrenoceptors induced the secretion of adrenocorticotropic hormone (ACTH), whereas antagonists (phentolamine and phenoxybenzamine) of a1-adrenoceptors had the opposite effect. Similar findings are presented in reports by Feldman and Weidenfeld,36 Handley and Mithani,37 Yorimitsu et al.38 Jager et al.39 stated that doxazosin had a negative effect on aldosterone secretion by adrenocortical cells porcine. Mulatero et al.40 found that an intravenous administration of doxazosin to patients with primary aldosteronism significantly lowered plasma aldosterone concentration. Considering the above, the observed reduction in ANF-immunoreactivity in the hearts of SHR treated with doxazosin may result from the negative action of the drug on HPA axis activity and the secretion of adrenocortical hormones.
The principal mechanisms stimulating ANF secretion are associated with an increase in sodium concentration in the blood and hypervolemia. It has been documented that α1-adrenoceptors influence the renal blood flow, urine volume, sodium and potassium excretion. Elhawary and Pang41 stated that injecting rats with a1-adrenergic stimulators caused antidiuresis and antinatriuresis, while specific a1-adrenenoceptor antagonists abolished this effect. The enhanced tubular Na+ and water reabsorption after the activation of a1-adrenoceptors has also been demonstrated in dogs42 and rabbits.43 Therefore, it might be assumed that a decrease in the intensity of ANF-immunosignal in the hearts of SHR receiving doxazosin observed in our study may be associated with the action of doxazosin on renal function and fluid volume.
The results of numerous studies point to the important role of inflammation in the pathogenesis of hypertension. It has been also found that several interleukins enhance the production of ANF in atrial myocytes. Considering that a1-adrenenoceptors are expressed in leucocytes, it might be assumed that these receptors are involved in the regulation of the immune response in physiological and pathological conditions. Grisanti et al.,44 documented that the activation of a1-adrenenoceptors stimulates immune cells and secretion of proinflammatory cytokines. Other studies have revealed that prazosin α1-adrenenoceptor antagonist significantly reduces interleukin-6 level in patients subjected to physical exertion.45 Given the above, a weakened ANF-immunoreaction in hypertensive rats receiving doxazosin might be explained by suppression of the immune system after drug treatment.
The α1-adrenoceptor regulates contractile performance of cardiac muscle cells. The α1-adrenergic stimulation significantly increases contraction of cardiomyocytes. The blockage of α1-adrenoceptor inhibits calcium outflow from the endoplasmic reticulum and results in impaired functioning of cardiac muscle cells. It has been indicated that ANF significantly reduces cardiomyocytes contractility. Delaflotte et al.46 and Bilzer et al.47 showed that ANF had the ability to antagonize vasoconstrictive effect of α1-adrenergic agonists in rats. In view of the above, reduction of ANF – immunoreactivity in the hearts of SHR treated with doxazosin – is possibly a result of compensatory mechanisms limiting further deterioration of cardiac function after receiving the medication.
The present report suggests that S100A6 and ANF might be involved in the protection of cardiac cells in hypertension.
Acknowledgments
This work was supported by statutory funds from the Medical University of Bialystok.
Authors’ contributions
IK, ZP, AF, MM participated in the study design; IK, ZP, AF and MM conducted the experiments, data collection and analysis of the data; IK wrote the manuscript; ZP contributed to manuscript preparation and performed statistical analysis of those studies. All authors participated in interpretation of the studies and review and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.WHO Report, 2013. A global brief on Hypertension.
- 2.Schmieder RE. End organ damage in hypertension. Dtsch Arztebl Int 2010; 107: 866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB. Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res 1996; 32: 1096–107. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging 2009; 2: 382–90. [DOI] [PubMed] [Google Scholar]
- 5.Delbridge LM, Connell PJ, Morgan TO, Harris PJ. Contractile function of cardiomyocytes from the spontaneously hypertensive rat. J Mol Cell Cardiol 1999; 28: 723–33. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Bing OH, Long X, Robinson KG, Lakatta EG. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol 1997; 272: 2313–9. [DOI] [PubMed] [Google Scholar]
- 7.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1999; 1450: 191–231. [DOI] [PubMed] [Google Scholar]
- 8.Kraus C, Rohde D, Weidenhammer C, Qiu G, Pleger ST, Voelkers M, Boerries M, Remppis A, Katus HA, Most P. S100A1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy. J Mol Cell Cardiol 2009; 47: 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maco B, Mandinova A, Dürrenberger MB, Schäfer BW, Uhrík B, Heizmann CW. Ultrastructural distribution of the S100A1 Ca2+-binding protein in the human heart. Physiol Res 2001; 50: 567–74. [PubMed] [Google Scholar]
- 10.Kettlewell S, Most P, Currie S, Koch WJ, Smith GL. S100A1 increases the gain of excitation-contraction coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell Cardiol 2005; 39: 900–10. [DOI] [PubMed] [Google Scholar]
- 11.Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Börries M, Niroomand F, Pieske B, Janssen PM, Eschenhagen T, Karczewski P, Smith GL, Koch WJ, Katus HA, Remppis A. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci USA 2001; 98: 13889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remppis A, Most P, Löffler E, Ehlermann P, Bernotat J, Pleger S, Börries M, Reppel M, Fischer J, Koch WJ, Smith G, Katus HA. The small EF-hand Ca2+ binding protein S100A1 increases contractility and Ca2+ cycling in rat cardiac myocytes. Basic Res Cardiol 2002; 97: 56–62. [DOI] [PubMed] [Google Scholar]
- 13.Desjardins JF, Pourdjabbar A, Quan A, Leong-Poi H, Teichert-Kuliszewska K, Verma S, Parker TG. Lack of S100A1 in mice confers a gender-dependent hypertensive phenotype and increased mortality after myocardial infarction. Am J Physiol Heart Circ Physiol 2009; 296: 1457–65. [DOI] [PubMed] [Google Scholar]
- 14.Pleger ST, Harris DM, Shan C, Vinge LE, Chuprun JK, Berzins B, Pleger W, Druckman C, Völkers M, Heierhorst J, Øie E, Remppis A, Katus HA, Scalia R, Eckhart AD, Koch WJ, Most P. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res 2008; 102: 786–94. [DOI] [PubMed] [Google Scholar]
- 15.Au KW, Kou CY, Woo AY, Chim SS, Fung KP, Cheng CH, Waye MM, Tsui SK. Calcyclin binding protein promotes DNA synthesis and differentiation in rat neonatal cardiomyocytes. J Cell Biochem 2006; 98: 555–66. [DOI] [PubMed] [Google Scholar]
- 16.Most P, Pleger ST, Völkers M, Heidt B, Boerries M, Weichenhan D, Löffler E, Janssen PML, Eckhart AD, J Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest 2004; 114: 1550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsoporis JN, Izhar S, Parker TG. Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-alpha. J Biol Chem 2008; 283: 30174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsoporis JT, Izhar S, Desjardins JF, Leong-Poi H, Parker TG. Conditional cardiac overexpression of S100A6 attenuates myocyte hypertrophy and apoptosis following myocardial infarction. Curr Pharm Des 2014; 20: 1941–9. [DOI] [PubMed] [Google Scholar]
- 19.Larochelle P, Cusson JR, Gutkowska J, Schiffrin EL, Hamet P, Kuchel O, Genest J, Cantin M. Plasma atrial natriuretic factor concentrations in essential and renovascular hypertension. Br Med J (Clin Res Ed) 1987; 294: 1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An MR, Chung YJ, Kang DG, Nam SC, Lee J. Augmented expression of cardiac atrial natriuretic peptide system in hypertensive rats. J Korean Med Sci 1999; 14: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf K, Kurtz A, Pfeifer M, Höcherl K, Riegger GA, Krämer BK. Different regulation of left ventricular ANP, BNP and adrenomedullin mRNA in the two-kidney, one-clip model of renovascular hypertension. Pflugers Arch 2001; 442: 212–7. [DOI] [PubMed] [Google Scholar]
- 22.Kyprianou N, Vaughan TB, Michel MC. Apoptosis induction by doxazosin and other quinazoline alpha1-adrenoceptor antagonists: a new mechanism for cancer treatment? Naunyn Schmiedebergs Arch Pharmacol 2009; 380: 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis BR, Cutler JA, Furberg CD, Wright JT, Farber MA, Felicetta JV, Stokes JD. ALLHAT Collaborative Research Group. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the antihypertensive and lipid-lowering treatment to prevent Heart Attack Trial. Ann Intern Med 2002; 137: 313–20. [DOI] [PubMed] [Google Scholar]
- 24.González-Juanatey JR, Iglesias MJ, Alcaide C, Piñeiro R, Lago F. Doxazosin induces apoptosis in cardiomyocytes cultured in vitro by a mechanism that is independent of alpha1-adrenergic blockade. Circulation 2003; 107: 127–31. [DOI] [PubMed] [Google Scholar]
- 25.Hein L, Schmitt JP. Alpha(1)-adrenoceptors in the heart – friend or foe? J Mol Cell Cardiol 2003; 35: 1183–5. 4. [DOI] [PubMed] [Google Scholar]
- 26.Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemost 1987; 58: 1078–84. [PubMed] [Google Scholar]
- 27.Tsoporis JN, Marks A, Van Eldik LJ, O'Hanlon D, Parker TG. Regulation of the S100B gene by alpha 1-adrenergic stimulation in cardiac myocytes. Am J Physiol Heart Circ Physiol 2003; 284: 193–203. [DOI] [PubMed] [Google Scholar]
- 28.Tsoporis JN, Marks A, Haddad A, O'Hanlon D, Jolly S, Parker TG. S100A6 is a negative regulator of the induction of cardiac genes by trophic stimuli in cultured rat myocytes. Exp Cell Res 2005; 303: 471–81. [DOI] [PubMed] [Google Scholar]
- 29.Perlini S, Palladini G, Ferrero I, Tozzi R, Fallarini S, Facoetti A, Nano R, Clari F, Busca G, Fogari R, Ferrari AU. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension 2005; 46: 1213–8. [DOI] [PubMed] [Google Scholar]
- 30.Monsalve P, Vera O, Pérez Acuña F, Medina O, Ostojich K, López B, Torres N, Lugo de Franco V, Fonseca R. Echocardiographic assessment of doxazosin on left ventricular mass in patients with essential hypertension. Am Heart J 1991; 121: 356–61. [DOI] [PubMed] [Google Scholar]
- 31.Garrison JB, Kyprianou N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death receptor-mediated pathway. Cancer Res 2006; 66: 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joo JH, Yoon SY, Kim JH, Paik SG, Min SR, Lim JS, Choe IS, Choi I, Kim JW. S100A6 (calcyclin) enhances the sensitivity to apoptosis via the upregulation of caspase-3 activity in Hep3B cells. J Cell Biochem 2008; 103: 1183–97. [DOI] [PubMed] [Google Scholar]
- 33.Słomnicki ŁP, Nawrot B, Leśniak W. S100A6 binds p53 and affects its activity. Int J Biochem Cell Biol 2008; 41: 784–90. [DOI] [PubMed] [Google Scholar]
- 34.Sakata Y, Yamamoto K, Masuyama T, Mano T, Nishikawa N, Kuzuya T, Miwa T, Hori M. Ventricular production of natriuretic peptides and ventricular structural remodeling in hypertensive heart failure. J Hypertens 2001; 19: 1905–12. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K. Effect of alpha 1- and alpha 2-adrenoceptor agonists and antagonists on ACTH secretion in intact and in hypothalamic deafferentated rats. Jpn J Pharmacol 1984; 36: 23–33. [DOI] [PubMed] [Google Scholar]
- 36.Feldman S, Weidenfeld J. Involvement of amygdalar alpha adrenoceptors in hypothalamo-pituitary-adrenocortical responses. Neuroreport 1996; 7: 3055–7. [DOI] [PubMed] [Google Scholar]
- 37.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear'-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 1984; 327: 1–5. [DOI] [PubMed] [Google Scholar]
- 38.Yorimitsu M, Okada S, Yamaguchi-Shima N, Shimizu T, Arai J, Yokotani K. Role of brain adrenoceptors in the corticortopin-releasing factor-induced central activation of sympatho-adrenomedullary outflow in rats. Life Sci 2008; 82: 487–94. [DOI] [PubMed] [Google Scholar]
- 39.Jager LP, de Graaf GJ, Widjaja-Greefkes HC. Effects of alpha1-antagonists on production and release of aldosterone and other steroid hormones by porcine adrenocortical cells in vitro. Can J Physiol Pharmacol 1998; 76: 676–83. [DOI] [PubMed] [Google Scholar]
- 40.Mulatero P, Rabbia F, Milan A, Paglieri C, Morello F, Chiandussi L, Veglio F. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension 2002; 40: 897–902. [DOI] [PubMed] [Google Scholar]
- 41.Elhawary AM, Pang CC. Alpha 1b-adrenoceptors mediate renal tubular sodium and water reabsorption in the rat. Br J Pharmacol 1994; 111: 819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osborn JL, Holdaas H, Thames MD, DiBona GF. Renal adrenoceptor mediation of antinatriuretic and renin secretion responses to low frequency renal nerve stimulation in the dog. Circ Res 1983; 53: 298–305. [DOI] [PubMed] [Google Scholar]
- 43.Hesse IF, Johns EJ. The role of alpha-adrenoceptors in the regulation of renal tubular sodium reabsorption and renin secretion in the rabbit. Br J Pharmacol 1985; 84: 715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grisanti LA, Perez DM, Porter JE. Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr Top Membr 2011; 67: 113–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzeo RS, Donovan D, Fleshner M, Butterfield GE, Zamudio S, Wolfel EE, Moore LG. Interleukin-6 response to exercise and high-altitude exposure: influence of alpha-adrenergic blockade. J Appl Physiol 2001; 91: 2143–9. [DOI] [PubMed] [Google Scholar]
- 46.Delaflotte S, Auguet M, Pirotzky E, Clostre F, Braquet P. Effects of atrial natriuretic factor (ANF) on phenylephrine-triggered intra- and extracellular calcium dependent contraction in rat aorta. J Auton Pharmacol 1989; 9: 211–8. [DOI] [PubMed] [Google Scholar]
- 47.Bilzer M, Paumgartner G, Gerbes AL. Prolonged antagonism of alpha 1-adrenergic vasoconstriction in the rat liver by atrial natriuretic peptide. Gastroenterology 1995; 108: 803–11. [DOI] [PubMed] [Google Scholar]